Key Points
Question
Does disability progression among patients with secondary progressive multiple sclerosis treated with the monoclonal anti-CD20 antibody rituximab differ from that among such patients never treated with rituximab?
Findings
In this cohort study of 88 propensity score–matched patients, those treated with rituximab had a significantly lower Expanded Disability Status Scale score for up to 10 years of follow-up and significantly delayed confirmed progression compared with matched controls. No associations between confirmed progression and individual patient baseline characteristics were identified.
Meaning
Therapeutic options for patients with secondary progressive multiple sclerosis are limited; however, these findings suggest that B-cell–depleting therapy may be beneficial.
Abstract
Importance
Therapeutic options for patients with secondary progressive multiple sclerosis (SPMS) are limited.
Objective
To analyze disability progression in patients with SPMS treated with rituximab compared with matched control patients never treated with rituximab.
Design, Setting, and Participants
This retrospective cohort study analyzed data obtained from patients with SPMS at 3 multiple sclerosis centers located in Basel and Lugano, Switzerland, and Amsterdam, the Netherlands, from 2004 to 2017. Patients were included for analysis if they had received a diagnosis of SPMS, were treated (57 eligible; 54 included) or never treated (504 eligible; 59 included) with rituximab, and had at least 1 follow-up visit. The variables used for propensity score matching were sex, age, Expanded Disability Status Scale (EDSS) score, and disease duration. Follow-up duration was up to 10 years, with a mean (SD) of 3.5 (2.6) years for rituximab-treated patients and 5.4 (2.4) years for controls in the total cohort and a mean (SD) of 3.5 (2.7) years for rituximab-treated patients and 4.8 (2.2) years for controls in the matched cohort.
Exposures
Comparing EDSS score progression in patients with SPMS (treated with rituximab vs not treated with rituximab) using propensity score matching.
Main Outcomes and Measures
The primary end point was progression of EDSS score after baseline, and the secondary end point was time to confirmed disability progression.
Results
After 1:1 propensity score matching, 44 matched pairs (88 patients) were included in the analysis. At baseline, patients treated with rituximab had a mean (SD) age of 49.7 (10.0) years, mean (SD) disease duration of 18.2 (9.4) years, and mean (SD) EDSS score of 5.9 (1.4), and 26 (59%) were women, whereas controls had a mean (SD) age of 51.3 (7.4) years, mean (SD) disease duration of 19.4 (8.7) years, and mean (SD) EDSS score of 5.70 (1.29), and 27 (61%) were women. In the covariate-adjusted analysis of the matched set, patients with SPMS who were treated with rituximab had a significantly lower EDSS score during a mean (SD) follow-up of 3.5 (2.7) years (mean difference, −0.52; 95% CI, −0.79 to −0.26; P < .001). Time to confirmed disability progression was significantly delayed in the rituximab-treated group (hazard ratio, 0.49; 95% CI, 0.26-0.93; P = .03).
Conclusions and Relevance
In this study, patients with SPMS treated with rituximab had a significantly lower EDSS score for up to 10 years of follow-up and a significantly delayed confirmed progression compared with matched controls, suggesting that B-cell depletion by rituximab may be therapeutically beneficial in these patients. A prospective randomized clinical trial with a better level of evidence is needed to confirm the efficacy of rituximab in such patients.
This cohort study uses propensity score–matching analyses to examine disability progression in matched pairs of patients with secondary progressive multiple sclerosis who were treated with rituximab vs those who were never treated with rituximab.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS).1 The majority of patients present with a relapsing-remitting (RRMS) course of disease, followed by a progressive phase termed secondary progressive MS (SPMS).2 Current disease-modifying treatments target the inflammatory pathology and only indirectly the neurodegenerative pathology of the disease, and their therapeutic effects in (secondary) progressive MS have been very limited.
Data from animal models, human neuropathologic studies, and clinical trials suggest a prominent role for B cells in the pathogenesis of MS, both in RRMS and SPMS. The progressive phase is characterized by a compartmentalized inflammatory process that persists beyond a relatively intact blood-brain barrier. B-cell follicle-like structures are found in the meninges as a correlate of this CNS restricted inflammation.1,3 Potential drugs for progressive forms of MS should, therefore, be able to pass the blood-brain barrier and should be able to target proinflammatory mediators and mechanisms directly in the brain or should influence the immune axis between the peripheral immune system and the CNS.3 Recent studies postulate that there is a B-cell exchange across the blood-brain barrier. Rituximab, a monoclonal CD20 antibody, might affect the B-cell population within the CNS through depletion of the peripheral B-cell compartment.4,5 Rituximab is detectable in low concentrations within cerebrospinal fluid after intravenous administration, opening the possibility of a direct effect on CNS resident B cells.6
In a phase 2 trial, rituximab reduced clinical activity and inflammatory brain lesions in RRMS.7 Another trial tested rituximab in primary progressive multiple sclerosis (PPMS).8 A post hoc analysis showed that rituximab affected disability progression in patients of younger age and those with contrast-enhancing lesions.
More recently the humanized monoclonal anti-CD20 antibody ocrelizumab significantly reduced the percentage of patients with confirmed disability progression (CDP) compared with interferon beta-1a in RRMS and significantly reduced CDP compared with placebo in PPMS.9,10 In 2014, the fully humanized monoclonal anti-CD20 antibody ofatumumab also demonstrated efficacy in a phase 2 clinical study of patients with RRMS.11
In a recently published Swedish study, the authors concluded that treatment with rituximab was safe after a follow-up of up to 2 years.12 Owing to the limited treatment options for SPMS and the extrapolation of results in RRMS and PPMS, rituximab was used off-label for the treatment of SPMS. The present retrospective cohort study uses propensity score matching to compare disease progression between patients who were treated with rituximab and patients who had never been treated with rituximab.
Methods
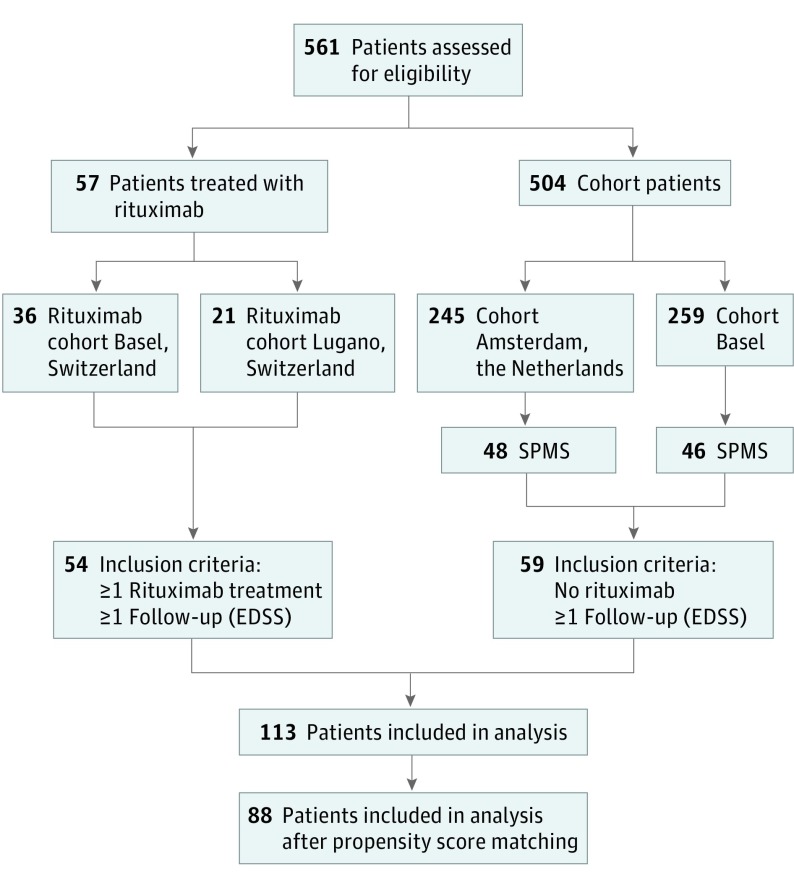
Patients with SPMS, defined according to established criteria,13 were eligible for this retrospective study if they had been treated with rituximab off-label at the MS Centers in Basel or Lugano, Switzerland; had received at least 1 dose of rituximab; had had at least 1 clinical follow-up visit; and had provided informed consent (see Figure 1 for the study flowchart). Patients were treated with rituximab based on the decision of the treating neurologist that the disease was progressive and that no other treatment was available. The study was approved by the local ethics committees of Lugano (comitato etico cantonale, Bellinzona) and Basel (Ethikkommission Nordwestschweiz und Zentralschweiz [EKNZ]) in Switzerland. Informed consent was obtained in written (Lugano) or verbal (by telephone interview, Basel) format.
Figure 1. Study Flowchart.
EDSS indicates Expanded Disability Status Scale; SPMS, secondary progressive multiple sclerosis.
In total, 54 rituximab-treated patients with SPMS were compared with 59 patients with SPMS who had never been treated with rituximab (control group) and were part of an observational cohort study conducted at the MS Center Basel, University of Basel, and the MS Center Amsterdam (at University Medical Center Amsterdam, the Netherlands). That study, including the informed consent procedure, was approved by the local ethics committees of Basel (EKNZ), and Amsterdam (Medical Ethical Committee VUmc), and all patients provided written informed consent.
In a second step, the rituximab-treated and the control groups were matched 1:1 using propensity scores, resulting in 44 matched patient pairs. The matching variables were sex, age, Expanded Disability Status Scale (EDSS) score, and disease duration at baseline.
For all patients, (at least) yearly standardized clinical assessments were performed, including a full neurologic examination (Neurostatus-EDSS). In addition, routine magnetic resonance imaging (MRI) findings for new T2 lesions and gadolinium-enhancing lesions were analyzed at baseline. Because MRIs at baseline were available for only 33 patients (approximately 60% of the total cohort), MRI findings could not be used for the matching process. Magnetic resonance imaging during follow-up was performed in only a minority of patients, and the images were therefore not analyzed for the present study.
Main Outcome Measures
The primary objective of the present study was to compare disease progression as assessed by the EDSS score after baseline in patients with SPMS who were treated with rituximab vs those who were not treated with rituximab. The EDSS score was determined annually for up to 10 years after baseline (Table 1). For patients treated with rituximab, EDSS scores acquired during treatment and for up to 1 year after the end of treatment were analyzed.
Table 1. Baseline Characteristics and Follow-up Duration for the Total Cohort.
| Characteristica | Group Receiving Off-label Rituximab Treatment | Control Group | P Valueb |
|---|---|---|---|
| Before propensity score matching | |||
| No. of patients | 54 | 59 | |
| Age, y | |||
| Mean (SD) | 49.0 (9.6) | 53.5 (8.0) | .008 |
| Median (range) | 49.0 (23.0-71.0) | 54.0 (37.0-70.0) | |
| Female, No. (%) | 32 (59) | 33 (56) | .85 |
| Disease duration, y | |||
| Mean (SD) | 18.6 (9.3) | 18.8 (9.0) | .97 |
| Median (range) | 18.0 (3.0-40.0) | 18.0 (3.0-45.0) | |
| EDSS BL | |||
| Mean (SD) | 6.02 (1.32) | 5.21 (1.47) | .002 |
| Median (range) | 6.25 (2.50-8.50) | 5.50 (2.00-8.00) | |
| Follow-up, mo | |||
| Mean (SD) | 42.3 (31.4) | 64.3 (29.4) | <.001 |
| Median (range) | 33.9 (6.6-111.4) | 59.9 (12.2-123.5) | |
| Follow-up, y | |||
| Mean (SD) | 3.5 (2.6) | 5.4 (2.4) | |
| Median (range) | 2.8 (0.6-9.3) | 5.0 (1.0-10.3) | |
| After propensity score matching | |||
| No. of patients | 44 | 44 | |
| Age, y | |||
| Mean (SD) | 49.7 (10.0) | 51.3 (7.4) | .36 |
| Median (range) | 50.0 (23.0-71.0) | 50.5 (37.0-65.0) | |
| Female, No. (%) | 26 (59) | 27 (61) | >.99 |
| Disease duration, y | |||
| Mean (SD) | 18.2 (9.4) | 19.4 (8.7) | .45 |
| Median (range) | 17.0 (3.0-40.0) | 19.5 (3.0-35.0) | |
| EDSS BL | |||
| Mean (SD) | 5.93 (1.40) | 5.70 (1.29) | .28 |
| Median (range) | 6.00 (2.50-8.50) | 6.00 (2.50-8.00) | |
| Follow-up, mo | . | ||
| Mean (SD) | 41.8 (32.2) | 57.7 (26.5) | 002 |
| Median (range) | 29.8 (6.6-111.4) | 54.5 (12.2-112.3) | |
| Follow-up, y | |||
| Mean (SD) | 3.5 (2.7) | 4.8 (2.2) | |
| Median (range) | 2.5 (0.6-9.3) | 4.5 (1.0-9.4) |
Abbreviation: EDSS BL, Expanded Disability Status Scale score after baseline.
Number and percentage of patients with measurements are given for categorical variables; mean (SD) and median (range) are given for continuous and ordinal variables.
Derived from Fisher exact tests (categorical) and from Wilcoxon rank sum tests (Mann-Whitney tests, continuous). For standardized differences between groups before and after propensity score matching, see eTable 1 in the Supplement.
One secondary objective was to analyze the time to CDP in rituximab-treated patients with SPMS vs matched control patients. Confirmed progression was defined as an increase in the EDSS score 12 or more months after baseline, which was confirmed by a second examination conducted 12 months later. The increase had to be at least 1.5 steps for an EDSS score of 0, 1 step for scores between 1 and 5, and 0.5 steps for scores of 5.5 or greater.
The other secondary objective was to compare baseline characteristics of patients in the rituximab-treated group with confirmed progression with those of patients without confirmed progression in the same group.
Statistical Analysis
An initial comparison of baseline characteristics (age, sex, EDSS score, and disease duration) between the 2 groups showed that patients treated with rituximab were significantly younger and had a higher grade of disability (Table 1). To improve the balance of baseline characteristics between the 2 groups, propensity score matching as implemented in the R package nonrandom software was used to create a 1:1 matched data set of 44 rituximab-treated patients and 44 controls.14 Density plots of the distribution of propensity scores per cohort before and after matching are shown in eFigure 1 in the Supplement. Standardized differences between cohorts before and after propensity score matching were calculated (eFigure 2 and eTable 1 in the Supplement).
Statistical analysis was performed using the total cohort as well as using the matched cohort. Although matching improved the balance, considerable differences in baseline characteristics remained between the matched groups. Thus, all baseline characteristics were included as covariates in the analysis of the total cohort as well as in the analysis of the matched cohort. The primary end point, EDSS score after baseline, was analyzed using a linear mixed-effects model to estimate effect sizes together with 95% CIs. To account for multiple measurements per patient, a random intercept was added for each patient. As explanatory variables, the model included age, sex, disease duration, baseline EDSS score, treatment (rituximab vs control), time after baseline, and the interaction between treatment and time after baseline. All continuous explanatory variables (age, disease duration, and time after baseline) and baseline EDSS scores were centered by subtracting the respective median value for better interpretation of model intercepts.
We used a Bayesian approach to graphically display the fitted values together with their credible intervals (estimated by the corresponding model) for the primary end point. Posterior distributions of the fitted values were calculated using the sim function in the arm package of R.15 The fitted values and credible intervals are displayed for a female “model patient” (the present data set included more women than men) with median values of the baseline EDSS score, baseline age, and disease duration (to represent the data set as accurately as possible).
Time to confirmed progression was analyzed as a secondary end point. Because there were 9 patients with 2 confirmed progression events (all in the control group), we used recurrent event analysis by marginal means and rates models, allowing the accounting of multiple events per patient. Age, sex, disease duration, baseline EDSS score, and treatment were used as explanatory variables. A “cluster” term in the model was used to compute a robust variance for the model, accounting for nonindependent events from the same patient. Moreover, we compared rituximab-treated patients with or without confirmed progression regarding baseline characteristics and rituximab treatments. Because the number of rituximab-treated patients with confirmed progression was small (n = 12), only bivariate associations between confirmed progression and individual patient characteristics were assessed. Associations between confirmed progression and categorical variables (eg, sex) were assessed using the Fisher exact test. Associations between confirmed progression and continuous variables were assessed using a Wilcoxon rank sum test.
All statistical analyses were performed with the statistical software environment R, version 3.4.4 (R Core Team 2018).16 A 2-sided P < .05 was considered statistically significant.
Results
Table 1 shows the baseline characteristics for 54 rituximab-treated patients and 59 control patients who were not treated with rituximab before and after matching. Before matching, patients from the rituximab group were significantly younger and had a higher grade of disability. After matching, there were no significant differences between the groups; however, P values should be compared with caution because group sizes were reduced. At baseline after matching, patients treated with rituximab had a mean (SD) age of 49.7 (10.0) years, mean (SD) disease duration of 18.2 (9.4) years, and mean (SD) EDSS score of 5.93 (1.40), and 26 (59%) were women, whereas controls had a mean (SD) age of 51.3 (7.4) years, mean (SD) disease duration of 19.4 (8.7) years, and a mean (SD) EDSS score of 5.70 (1.29), and 27 (61%) were women. Standardized differences before and after matching showed improved balance for the covariates EDSS score and age after matching (eFigure 2 and eTable 1 in the Supplement). Analysis of MRI findings at baseline, which were not available for all patients (total rituximab group, 33 [61%]; matched rituximab group, 27 [61%]; total control group, 35 [59%]; matched control group, 23 [52%]), revealed that 7 patients (26%) in the matched rituximab group and 0 patients in the matched control group had gadolinium-enhancing lesions. Baseline MRIs for the matched rituximab group were performed a mean (SD) of 84.9 (57.8) days before baseline (median, 77 days; range, 0-198 days), whereas baseline MRIs in the control group were performed at baseline. Of 8 patients treated with rituximab (rituximab total group) with an MRI finding of active disease at baseline, only 1 patient developed CDP during the study period. In the rituximab group (matched cohort), 18 patients (41%) had received no immunomodulatory treatment during the year before baseline, whereas in the control group, a slightly higher proportion (21 patients; 48%;) was untreated (for a detailed treatment summary see eTable 2 in the Supplement).
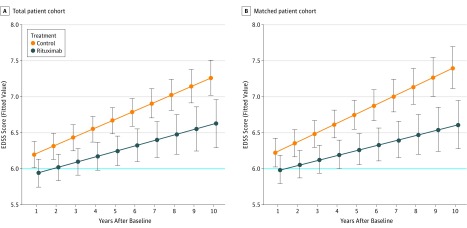
The analysis of the primary end point showed that rituximab treatment significantly reduced progression of disability as measured by yearly EDSS scores for a follow-up of up to 10 years (mean [SD] follow-up, 3.5 [2.6] years) in the total patient cohort (estimate, −0.45; 95% CI, −0.71 to −0.20; P < .001) as well as in the matched cohort (mean [SD] follow-up, 3.5 [2.7] years; estimate, −0.52; 95% CI, −0.79 to −0.26; P < .001). Figure 2 shows progression of EDSS scores over time for both cohorts (total and matched) as estimated by the statistical models.
Figure 2. Progression of Expanded Disability Status Scale (EDSS) Score Over Time for Both Cohorts (Total and Matched).
Fitted values of the EDSS scores together with Bayesian 95% credible intervals 1 to 10 years after baseline for the total cohort (A) and the matched cohort (B). The fitted values represent a female model patient of median age at baseline (A, 51 years; B, 50 years), median disease duration (A, 18 years; B, 18.5 years), and with median baseline EDSS score (A, 6.0; B, 6.0). Median baseline EDSS score is shown as a horizontal blue line. The mean treatment difference (during the follow-up period) between rituximab and control of −0.45 (95% CI, −0.71 to −0.20), estimated by the statistical model on the total set, can be read from panel A at 5.5 years after baseline. Likewise, the mean treatment difference of −0.52 (95% CI, −0.79 to −0.26) for the matched cohort can be read from panel B.
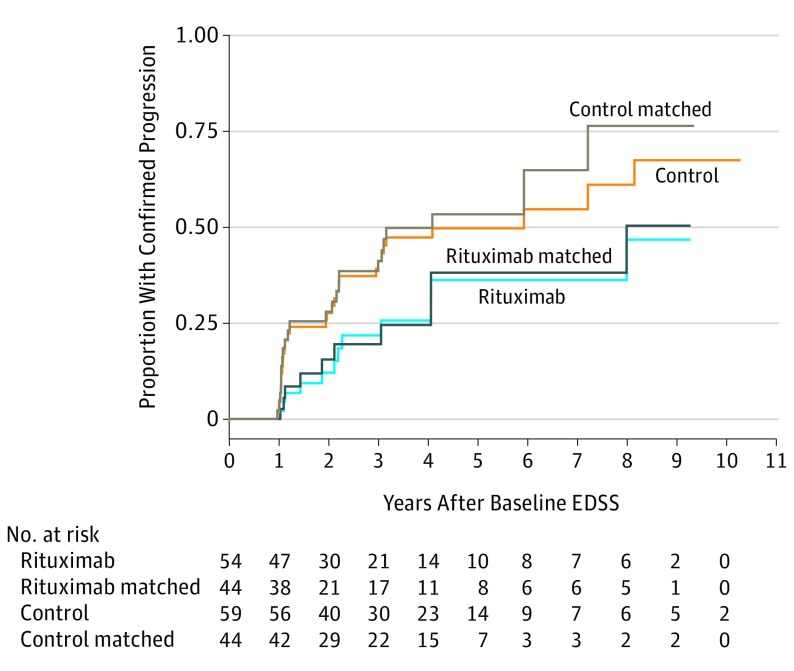
With respect to the secondary end point, time to confirmed progression was significantly longer in the rituximab-treated group than in the control group for the total cohort (hazard ratio, 0.48; 95% CI, 0.26-0.91; P = .02) and the matched cohort (hazard ratio, 0.49; 95% CI, 0.26-0.93; P = .03). Figure 3 shows the Kaplan-Meier plots for time to first confirmed progression.
Figure 3. Kaplan-Meier Curve for Time to First Confirmed Progression.
EDSS indicates Expanded Disability Status Scale.
In the rituximab-treated group, baseline characteristics as well as number and dose of rituximab treatments for 12 patients with or 42 without progression were compared to identify potential predictors for treatment response. Table 2 shows the categorical (sex and reason for rituximab) and continuous (age, EDSS score at baseline, disease duration, number of rituximab cycles, total cumulative dose of administered rituximab, duration of treatment, and treatment intensity) patient characteristics for rituximab-treated patients with or without confirmed progression. Patients with confirmed progression had higher cumulative doses of rituximab and more treatment cycles but for a longer period of time; thus, the treatment intensity (defined as the total amount of rituximab administered in milligrams divided by the duration of treatment in months) remained similar compared with patients without confirmed progression. In summary, no associations between confirmed progression and individual patient baseline characteristics could be identified.
Table 2. Characteristics of Patients Administered Rituximab With or Without Confirmed Progression.
| Characteristica | Rituximab With Confirmed Progression | Rituximab Without Confirmed Progression | P Valueb |
|---|---|---|---|
| Categorical | |||
| No. of patients | 12 | 42 | |
| Female, No. (%) | 7 (58) | 25 (60) | >.99 |
| Reason for rituximab, No. (%) | |||
| Progression | 11 (92) | 26 (62) | .16 |
| Relapses | 1 (8) | 3 (7) | |
| Progression and relapses | 0 | 3 (7) | |
| Other | 0 | 0 (24) | |
| Relapses under treatment, No. (%) | 1 (8) | 5 (12) | >.99 |
| Continuous | |||
| No. of patients | 12 | 42 | |
| Age, y | |||
| Mean (SD) | 49.7 (10.6) | 48.8 (9.4) | .95 |
| Median (range) | 48.0 (33.0-71.0) | 49.0 (23.0-70.0) | |
| Disease duration, y | |||
| Mean (SD) | 19.0 (10.6) | 18.6 (9.0) | .83 |
| Median (range) | 19.5 (3.0-40.0) | 18.0 (3.0-39.0) | |
| Rituximab, No. of cycles | |||
| Mean (SD) | 6.00 (2.09) | 4.29 (2.99) | .04 |
| Median (range) | 6.50 (3.0-9.00) | 3.50 (1.00-11.00) | |
| Rituximab, cumulative dose, mg | |||
| Mean (SD) | 9.54 (3.86) | 6.00 (3.85) | .007 |
| Median (range) | 8.50 (5.00-15.00) | 5.00 (1.00-16.00) | |
| Rituximab, treatment duration, mo | |||
| Mean (SD) | 60.9 (27.2) | 42.1 (30.7) | .03 |
| Median (range) | 49.5 (32.0-110.0) | 34.5 (6.0-107.0) | |
| Rituximab, treatment intensity, mg/moc | |||
| Mean (SD) | 161.03 (32.10) | 160.32 (50.85) | .88 |
| Median (range) | 156.05 (131.82-250.00) | 160.26 (67.31-333.33) | |
| Ordinal | |||
| EDSS BL | |||
| Mean (SD) | 5.71 (1.41) | 6.11 (1.30) | .35 |
| Median (range) | 6.00 (3.00-7.50) | 6.50 (2.50-8.50) |
Abbreviation: EDSS BL, Expanded Disability Status Scale score after baseline.
Number and percentage of patients with measurements are given for categorical variables; mean (SD) and median (range) are given for continuous variables.
Derived from Fisher exact tests (categorical) and from Wilcoxon rank sum tests (Mann-Whitney tests, continuous).
Defined as the total dose in milligrams of rituximab administered divided by the duration of treatment in months.
At the cutoff time for this analysis, 29 of 54 patients (54%) were still being treated with rituximab with no complications. In 5 cases (9%), complications were reported: 1 patient had leukocytoclastic vasculitis in both legs (confirmed by biopsy) after the first infusion (as reported previously17), 1 patient had a segmental herpes zoster infection, and 3 had 1 or more pneumonia events or urinary tract infections.
Two patients died during the follow-up period: 1 died of a spontaneous intracerebral hemorrhage 3 years after stopping rituximab treatment (rituximab therapy was stopped because the disease was stabilized), and 1 died of pneumonia 4 years after rituximab treatment had been stopped.
Discussion
To date, no prospective clinical trial has been performed to evaluate the efficacy of B-cell depletion in SPMS. The main goal of the present study was to compare disease progression in patients with SPMS treated with rituximab with that in matched control patients in a comprehensive real-world cohort. In the rituximab group, we observed a significant reduction of EDSS score progression after baseline, the study’s primary end point. This reduction in disability progression as assessed annually by the EDSS score for up to 10 years was significant in both the total (mean follow-up, 3.5 years) and the propensity score–matched (mean follow-up, 3.5 years) cohorts. The time to confirmed progression was also significantly delayed in the rituximab-treated group compared with the control group for the total and matched cohorts. These findings suggest that rituximab administration may be associated with a beneficial therapeutic effect in patients with SPMS.
Approximately 75% of untreated and 50% of treated individuals in our cohorts developed clinically significant confirmed progression for the 10-year period (Figure 3). This is in line with other cohorts in which the rates of progression during a 3- and a 10-year follow-up were 15% and 75%, respectively.18,19,20
The baseline characteristics of our patient cohort closely resembled those of patients included in the recently completed clinical trial examining the efficacy and safety of siponimod in SPMS (EXPAND).21 The EXPAND study showed a significant reduction of disability progression in the treatment group compared with that in the placebo group. The percentages of patients with a 6-month CDP were for placebo and siponimod 28% and 20% (year 2) and 30% and 23% (year 3), respectively. This is comparable to a CDP in 25% of matched controls and in 12.5% of rituximab-treated patients at year 2 and in 37.5% of matched controls and in 25% of rituximab-treated patients at year 3 in our cohort. Another study looking at the efficacy of natalizumab for reducing disability progression in participants with SPMS (ASCEND)22 did not meet the primary end point of reducing CDP. In a retrospective, propensity score–matched comparison of patients with SPMS from the MSBase registry who did not show an effect of immunomodulatory treatment (mainly interferon beta and glatiramer acetate, no patients treated with rituximab were included in the matched data set analysis) on disease progression, the rate of 6-month confirmed disease progression after 3 years was 23% (J.L., written communication, September 28, 2018).23 These studies, along with our small retrospective analysis, suggest that only a few anti-inflammatory treatments may be associated with a beneficial outcome in patients with SPMS. All analyzed compounds described above influence B-cell biology albeit by different mechanisms of action. The differential response to these treatments may provide clues to understanding which parts of the B-cell response are pathogenic in SPMS and which patients might benefit from such treatments.
It is crucial to identify patient characteristics that indicate a higher chance of treatment response. To identify potential prognostic markers for therapeutic response, baseline characteristics within the rituximab-treated group, comparing patients with or without confirmed progression, were tested. There was no significant difference in any tested category. However, MRI scans were available in only 60% of patients; therefore, these results should be interpreted with caution. Of 8 patients treated with rituximab (rituximab total group) with an MRI finding of active disease at baseline, only 1 patient developed CDP during the study period, whereas 12 of 54 patients in the rituximab total group showed CDP during the present study. This might indicate that patients with active disease respond better to this kind of treatment. In general, gadolinium-enhancing lesions at baseline are a risk factor for CDP in PPMS.10,24 The imbalance of increased MRI lesion activity in the rituximab group of our cohort might therefore favor the control group and underestimate the treatment effects associated with administration of rituximab. Whether MRI lesion activity is also associated with treatment response must be evaluated in larger cohorts with standardized MRI protocols.
There were no major safety concerns during the treatment period, but complications were documented in 5 cases (9.%), mainly related to infections.
Limitations
This study has some limitations. First, it was a retrospective analysis of clinical data and not a prospective, controlled, randomized clinical trial. The present study design has a higher risk of confounding the treatment effect associated with rituximab administration with other patient documented or unknown characteristics and, as a consequence, a biased estimate of the association. In fact, review of the baseline characteristics indicated that patients in the rituximab group tended to be younger, had a higher EDSS score, and showed more MRI lesion activity. It is conceivable that these patients were more likely to be treated with rituximab.
In addition, that a higher proportion of the control patients had not been treated with disease-modifying treatments in the year before baseline (49% and 48% in the total and matched groups, respectively, compared with 39% and 41% of the rituximab-treated patients) could indicate a bias toward a less aggressive disease course in the control group that may contribute to underestimating the effects associated with administration of rituximab. To mitigate confounding effects of known disease characteristics, we used propensity score–based matching in combination with covariate adjustment in the statistical models. As shown by the standardized differences before and after matching, the differences between the groups were smaller after propensity score matching. Furthermore, it was reassuring that the significant association observed in the propensity score–matched comparison was also significant in the total group analysis.
Conclusions
Patients with SPMS who were treated with rituximab had a significantly lower EDSS score up to 10 years after the initial assessment and significantly delayed progression compared with patients with SPMS who had never been treated with rituximab. A prospective randomized clinical trial is needed to show efficacy in this patient group with a higher level of evidence.
eTable 1. Standardized Differences
eTable 2. Treatment
eFigure 1. Density Plot of Propensity Scores
eFigure 2. Standardized Differences Between Groups
References
- 1.Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17(2):210-218. doi: 10.1111/j.1750-3639.2007.00064.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343(20):1430-1438. doi: 10.1056/NEJM200011163432001 [DOI] [PubMed] [Google Scholar]
- 3.Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14(2):183-193. doi: 10.1016/S1474-4422(14)70256-X [DOI] [PubMed] [Google Scholar]
- 4.von Büdingen HC, Kuo TC, Sirota M, et al. B cell exchange across the blood-brain barrier in multiple sclerosis. J Clin Invest. 2012;122(12):4533-4543. doi: 10.1172/JCI63842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palanichamy A, Apeltsin L, Kuo TC, et al. Immunoglobulin class-switched B cells form an active immune axis between CNS and periphery in multiple sclerosis. Sci Transl Med. 2014;6(248):248ra106. doi: 10.1126/scitranslmed.3008930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubenstein JL, Combs D, Rosenberg J, et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003;101(2):466-468. doi: 10.1182/blood-2002-06-1636 [DOI] [PubMed] [Google Scholar]
- 7.Hauser SL, Waubant E, Arnold DL, et al. ; HERMES Trial Group . B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676-688. doi: 10.1056/NEJMoa0706383 [DOI] [PubMed] [Google Scholar]
- 8.Hawker K, O’Connor P, Freedman MS, et al. ; OLYMPUS trial group . Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460-471. doi: 10.1002/ana.21867 [DOI] [PubMed] [Google Scholar]
- 9.Hauser SL, Bar-Or A, Comi G, et al. ; OPERA I and OPERA II Clinical Investigators . Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221-234. doi: 10.1056/NEJMoa1601277 [DOI] [PubMed] [Google Scholar]
- 10.Montalban X, Hauser SL, Kappos L, et al. ; ORATORIO Clinical Investigators . Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209-220. doi: 10.1056/NEJMoa1606468 [DOI] [PubMed] [Google Scholar]
- 11.Sorensen PS, Lisby S, Grove R, et al. Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase 2 study. Neurology. 2014;82(7):573-581. doi: 10.1212/WNL.0000000000000125 [DOI] [PubMed] [Google Scholar]
- 12.Salzer J, Svenningsson R, Alping P, et al. Rituximab in multiple sclerosis: a retrospective observational study on safety and efficacy. Neurology. 2016;87(20):2074-2081. doi: 10.1212/WNL.0000000000003331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lublin FD, Reingold SC; National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis . Defining the clinical course of multiple sclerosis: results of an international survey. Neurology. 1996;46(4):907-911. doi: 10.1212/WNL.46.4.907 [DOI] [PubMed] [Google Scholar]
- 14.Stampf S. Package “nonrandom”: stratification and matching by the propensity score. https://rdrr.io/cran/nonrandom/. Published February 20, 2015. Accessed November 21, 2018.
- 15.Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. New York, NY: Cambridge University Press; 2007. [Google Scholar]
- 16.R Core Team R: a language and environment for statistical computing. The R project for statistical computing. Vienna, Austria. https://www.R-project.org/. Published 2018. Accessed November 27, 2018.
- 17.Kandula P, Kouides PA. Rituximab-induced leukocytoclastic vasculitis: a case report. Arch Dermatol. 2006;142(2):246-247. doi: 10.1001/archderm.142.2.246 [DOI] [PubMed] [Google Scholar]
- 18.Bove R, Chitnis T, Cree BA, et al. ; SUMMIT Consortium . SUMMIT (Serially Unified Multicenter Multiple Sclerosis Investigation): creating a repository of deeply phenotyped contemporary multiple sclerosis cohorts. Mult Scler. 2018;24(11):1485-1498. doi: 10.1177/1352458517726657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Healy BC, Engler D, Gholipour T, Weiner H, Bakshi R, Chitnis T. Accounting for disease modifying therapy in models of clinical progression in multiple sclerosis. J Neurol Sci. 2011;303(1-2):109-113. doi: 10.1016/j.jns.2010.12.024 [DOI] [PubMed] [Google Scholar]
- 20.Cree BA, Gourraud PA, Oksenberg JR, et al. ; University of California, San Francisco MS-EPIC Team . Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol. 2016;80(4):499-510. doi: 10.1002/ana.24747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kappos L, Bar-Or A, Cree BAC, et al. ; EXPAND Clinical Investigators . Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391(10127):1263-1273. doi: 10.1016/S0140-6736(18)30475-6 [DOI] [PubMed] [Google Scholar]
- 22.US National Library of Medicine. A clinical study of the efficacy of natalizumab on reducing disability progression in participants with secondary progressive multiple sclerosis (ASCEND in SPMS). https://clinicaltrials.gov/ct2/show/NCT01416181. Accessed November 21, 2018.
- 23.Lorscheider J, Jokubaitis VG, Spelman T, et al. ; MSBase Study Group . Anti-inflammatory disease-modifying treatment and short-term disability progression in SPMS. Neurology. 2017;89(10):1050-1059. doi: 10.1212/WNL.0000000000004330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevenson VL, Ingle GT, Miller DH, Thompson AJ. Magnetic resonance imaging predictors of disability in primary progressive multiple sclerosis: a 5-year study. Mult Scler. 2004;10(4):398-401. doi: 10.1191/1352458504ms1055oa [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Standardized Differences
eTable 2. Treatment
eFigure 1. Density Plot of Propensity Scores
eFigure 2. Standardized Differences Between Groups