Key Points
Question
Does in vivo [18F]flortaucipir retention in Alzheimer disease (AD) reflect regional tau pathology?
Findings
In this case study of a patient with early-onset AD caused by a PSEN1 mutation, the retention of [18F]flortaucipir in vivo was correlated with both neuritic tau pathology and total tau burden but not with β-amyloid pathology.
Meaning
[18F]Flortaucipir uptake reflects the total tau burden in AD and could thus be considered an in vivo marker of tau pathology.
This case study investigates the association of regional in vivo retention of the tau positron emission tomography ligand [18F]flortaucipir with the density of tau neuropathology in the corresponding brain regions in a patient with early-onset Alzheimer disease caused by a PSEN1 mutation.
Abstract
Importance
In Alzheimer disease (AD), tau filaments form neuronal inclusions in neurites (neuropil threads) and in somata (neurofibrillary tangles), and neurite tau pathology constitutes the most common pathology. Positron emission tomography (PET) ligands have been developed to detect in vivo tau pathology in AD. However, the association of AD tau pathology post mortem with in vivo tau PET retention has not been established. Therefore, there is a need to investigate the associations of tau PET with postmortem tau pathology in AD.
Objective
To study the association of regional in vivo retention of the tau PET ligand [18F]flortaucipir (previously known as AV1451) with the density of tau neuropathology in the corresponding brain regions in a patient with AD.
Design, Setting, and Participants
The patient was a man in his 40s with AD caused by a PSEN1 mutation. Between May 2015 and December 2016, he underwent 2 [18F]flortaucipir PET scans at Lund University Hospital, Lund, Sweden. Postmortem analysis was performed 12 months after the last PET scan. Tau pathology was assessed using phosphorylated tau (AT8) immunohistochemistry and Gallyas silver staining. In addition to the regional total tau pathology burden, the density of tau-positive neurites and intrasomal tau tangles were quantified using a stereology-based method. Further, β-amyloid–containing plaques were detected using 4G8 immunohistochemistry. Data were analyzed between January 2018 and August 2018.
Main Outcomes and Measures
Regional standardized uptake value ratios of [18F]flortaucipir were compared with the amount of tau pathology in the corresponding brain areas.
Results
In this patient, the clinical disease symptoms progressed rapidly in life, paralleled with an annual increase of tau PET retention of 20% to 40% in many cortical regions. Compared with postmortem immunohistochemistry, regional in vivo uptake of [18F]flortaucipir was correlated with the density of tau-positive neurites (AT8: rs = 0.87; P < .001; Gallyas: rs = 0.92; P < .001), intrasomal tau tangles (AT8: rs = 0.65; P = .01; Gallyas: rs = 0.84; P < .001), and total tau burden (AT8: rs = 0.84; P < .001; Gallyas: rs = 0.82; P < .001). No correlations between [18F]flortaucipir and β-amyloid pathology were found.
Conclusions and Relevance
These results indicate that [18F]flortaucipir PET retention is a robust in vivo measure of the total AD tau burden.
Introduction
Several tau positron emission tomography (PET) radiotracers have been recently developed for detection of tau pathology in Alzheimer disease (AD).1 Among these, [18F]flortaucipir (previously known as AV1451) has been the most widely used tau tracer thus far. [18F]Flortaucipir is believed to primarily detect the mixed 3-repeat/4-repeat tau pathology typical of AD, and the in vivo uptake of [18F]flortaucipir is increased in patients with AD.2,3,4,5,6,7 Using in vitro autoradiography, it has been shown that [18F]flortaucipir is colocalized with tau-immunostained aggregates in ex vivo AD brain tissue.8,9,10 Further, the in vivo retention of [18F]flortaucipir has been shown to be correlated with AD-like tau neuropathology in a patient with a p.R406W mutation in the MAPT gene.11 However, to our knowledge, there are no reports describing correlations between in vivo [18F]flortaucipir retention and postmortem tau neuropathology in patients with AD. Further, the cortical neuropil thread tau pathology constitutes the majority (80% to 90%) of the total tau pathology,12 and it remains unclear whether [18F]flortaucipir detects the total tau load in the brain or whether it mainly reflects intrasomal neurofibrillary tangles (NFTs). Therefore, we quantified the regional tau and β-amyloid (Aβ) pathology in a patient with early-onset AD caused by a mutation in the PSEN1 gene and correlated the intensity of the pathology with the retention of [18F]flortaucipir in corresponding brain regions of the PET scan. In addition to the total tau burden, we also quantified both the number of neurites (neuropil threads) and the number of intrasomal tau tangles shown to be positive on phosphorylated tau (AT8) immunohistochemistry and Gallyas silver staining using a method based on modified unbiased stereology.
Methods
Study Participant
All procedures were reviewed and approved by the Regional Ethical Review Board of Lund and the regional radiation safety committee. Written informed consent was obtained from the patient prior to enrollment in the study. The clinical characteristics of the patient have been described in detail before.6 In brief, the patient was diagnosed with early-onset AD caused by a PSEN1 mutation (Thr116Asn) and fulfilled the Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised)13 criteria for dementia combined with the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria for AD.14 The postmortem neuropathological examination confirmed the AD diagnosis.
Magnetic Resonance Imaging and PET
Imaging procedures for this patient have been described in detail previously.6 In brief, [18F]flortaucipir PET scans were performed on a Discovery 690 PET scanner (GE Healthcare) in LIST mode after a bolus injection of 370 MBq of [18F]flortaucipir. The first [18F]flortaucipir PET scan was performed 32 months ante mortem, and the follow-up scan was performed 12 months ante mortem. Low-dose computed tomography scans for attenuation correction were performed immediately prior to the PET scans. Positron emission tomography data from 80 minutes to 100 minutes postinjection were reconstructed into 5-minute frames. A [18F]flutemetamol PET scan was obtained 34 months prior to death. Images were acquired as 4 × 5-minute frames 90 to 110 minutes after injection of 185 MBq of [18F]flutemetamol on a Gemini TF PET/CT scanner (Philips). The PET images were processed through a pipeline developed in-house to obtain standardized uptake value ratio (SUVR) images, with the inferior cerebellar gray matter as the reference region for flortaucipir15 and the pons as the reference region for [18F]flutemetamol.16 Regional analyses of [18F]flortaucipir and [18F]flutemetamol images were performed using PMOD software version 3.711 (PMOD Technologies). For correlational PET pathology analyses, the positions of the areas processed for neuropathological analysis were delineated manually on the magnetic resonance imaging (MRI) image during the neuropathological processing of the brain. The exact positions of the areas assessed for pathological tau and Aβ density could then be identified by comparing the coronal tissue sections with the coronal MRI slices, and the regions of interest (ROIs) on the MRI were placed accordingly. Region of interest volumes were small to accurately match the neuropathology (0.14-0.51 cm3) but were extended into adjacent slices to create a more stable readout of the PET signal. A SUVR image from the second [18F]flortaucipir PET scan was coregistered to the MRI using the Fusion tool in PMOD, and the regional SUVR values were retrieved. Voxelwise annual percentage change representations were calculated by normalizing SUVR images to Montreal Neurological Institute templates and subtracting the baseline scan volume from the follow-up scan and dividing by the baseline scan volume using FSLmaths (http://www.fmrib.ox.ac.uk/fsl). The resulting voxels were then multiplied by 100 and divided by the interscan interval (1.67 years). Images are presented as surface projections on human PALS-B12 brain templates (Van Essen Laboratory) using Caret software version 5.65 (Van Essen Laboratory).17 There was no strong choroid plexus signal in this patient that could interfere with the interpretation of the hippocampal retention.
Neuropathology and Quantitative Assessment of Tau Pathology
The brain was fixed per immersion and perfusion in 4% formaldehyde solution, cut in bihemispheric coronal slices and small regional samples, subsequently embedded in paraffin and sectioned, and then stained. Neurodegenerative structural changes were assessed as to regional extent, local-regional severity of neuronal degeneration and loss, NFT formation and plaque appearance, microvacuolization and reactive gliosis, and intensity and density of protein pathology with regard to tau and Aβ appearance. Tau pathology in the cortical compartment was generally severe, with abundant aggregates seen in all layers and numerous tau-positive neurites and tau-positive tangle-filled neurons in between. Further analyses of tau pathology were performed on paraffin-embedded coronal sections, including the hippocampus, the inferior temporal gyrus (1 sample in the middle part and 1 in the posterior aspect of the gyrus), the temporal pole, the frontal pole, the orbitofrontal gyrus (1 sample in the medial part and 1 in the lateral), the anterior cingulum (1 in the inferior anterior part and 1 in the upper anterior part), the parasagittal parietal cortex, occipital cortex (calcarine sulcus), the putamen, the pons, and the cerebellum. To ensure accurate positioning of the brain regions sampled for neuropathology on the MRI and PET, the positions were indicated on the patient MRI using PMOD at the same time as the regions were being dissected. Immunohistochemistry for AT8 (AT8 antibody, 1:200 dilution; DAKO), Aβ (clone 4G8 antibody, 1:500 dilution; BioSite/BioLegend), and α-synuclein (LB509 antibody, 1:200 dilution; Invitrogen) were performed on 4-μm-thick tissue sections and Gallyas silver stain on 5-μm-thick tissue sections. To achieve antigen retrieval for immunohistochemistry, all sections were microwave pretreated in 10mM citrate buffer with a pH of 6.0 and a temperature of 100°C for 10 minutes. An automated immunostainer (Dako Autostainer Plus Staining System; DAKO) was used for the staining procedure using ChemMate Kit Peroxidase/3,3′-diaminobenzidine (DAKO).
One section per region was analyzed for density of tau-positive neurites and intrasomal tau aggregates with a modified unbiased stereology-based systematic random sampling approach11 using Stereo Investigator (MBF Bioscience) on a Zeiss Axio microscope (Zeiss) with an automated stage. The number of tau-positive neurites (neuropil threads) per micrometer was determined in each region (median [range] ROI area, 15 [9-36] mm2). A total of approximately 80 counting sites per region (neurites crossing two 60 + 60 μm lines) was analyzed at ×40 magnification, with a median (range) of 1780 (0-3009) total hits per region. Intrasomal tau pathology per millimeter squared was assessed in the same regions, with a total of approximately 80 counting frames (200 × 200 μm) at ×20 magnification. Tau was considered intrasomal when tau-positive fibrils were surrounding or adjacent to a cell nucleus or when present in aggregates larger than approximately 6 μm (the size of the cross marker in the software).
To assess the total tau or Aβ burden, high-resolution images (2400 × 1800 pixels) were obtained from representative cortical regions in the AT8-stained, Gallyas-stained, and 4G8-stained sections. Images were acquired at ×10 magnification with a BX53 microscope with an attached DP73 CCD camera (Olympus Corporation). Images were captured under standardized settings using CellSens Dimension software (Olympus Corporation) and were converted to 8-bit black-and-white images using ImageJ software version 1.51s (https://imagej.nih.gov). A threshold to eliminate background was determined using the cerebellar cortex (the threshold was set to include points of intensity less than 150 [scale, 1-255]). For Gallyas images, the threshold had to be set individually in each image since the background intensity of the silver stain varied; the threshold was set to eliminate the background and allow for only silver stain to be labeled. Images were then binarized using the threshold, and the percent area immunostained was determined using the Analyze Particle tool in ImageJ software.
Statistical Analysis
Correlations of PET SUVR values with the total tau burden (percentage of positive area) of tau pathology, with the density of tau-positive neurites, and with the density of intrasomal pathology were analyzed with nonparametric Spearman rank order correlation using Prism 7 (GraphPad). P values were 2-tailed, and P values less than .05 were considered statistically significant.
Results
Clinical Characteristics
The clinical characteristics of this patient have been published in detail previously.6 In brief, the patient presented with a gradual onset of anterograde episodic memory loss, impaired attention, and perceptual-motor function at age 38 years. The patient was otherwise healthy. The Aβ1-42 level in cerebrospinal fluid and [18F]flutemetamol imaging were both abnormal. The patient was diagnosed with early-onset AD, and a mutation was found in the PSEN1 gene (Thr116Asn). The disease progressed rapidly, but even late in the disease, he had preserved insight regarding his cognitive decline. He died of pneumonia at age 46 years.
[18F]Flortaucipir PET
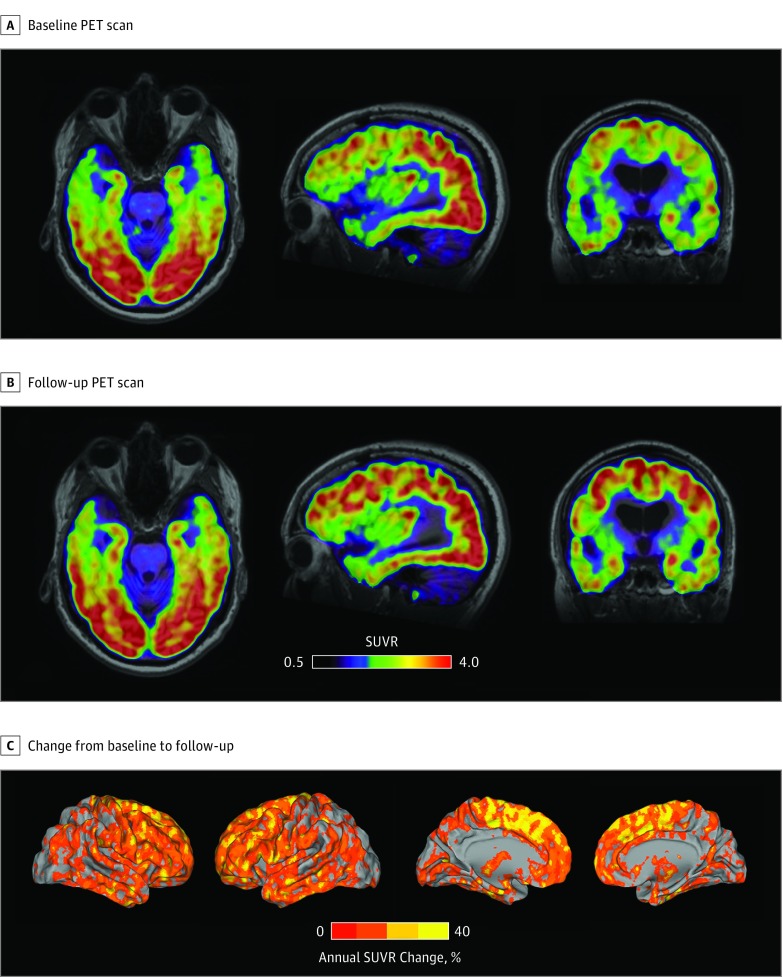
At age 43 years, the [18F]flortaucipir PET showed a substantial retention of the radioligand in the posterior parts of the brain, including the posterior temporal, parietal, and occipital cortices (Figure 1A). A follow-up [18F]flortaucipir scan performed 20 months later indicated that the tau pathology had spread to frontal regions and to the anterior aspects of the temporal lobes (Figure 1B). Annual changes in [18F]flortaucipir retention were highest in the anterior aspects of the brain, reaching levels of annual SUVR increase of 20% to 40% (Figure 1C).
Figure 1. Baseline and Follow-up [18F]Flortaucipir Positron Emission Tomography (PET) and Annual Change in Standardized Uptake Value Ratio (SUVR).
A, SUVR images from the baseline PET scan acquired 32 months prior to the patient’s death. B, SUVR images from the follow-up PET scan acquired 12 months before the patient’s death. C, The annual percentage of SUVR change from the baseline to the follow-up scan.
Neuropathology
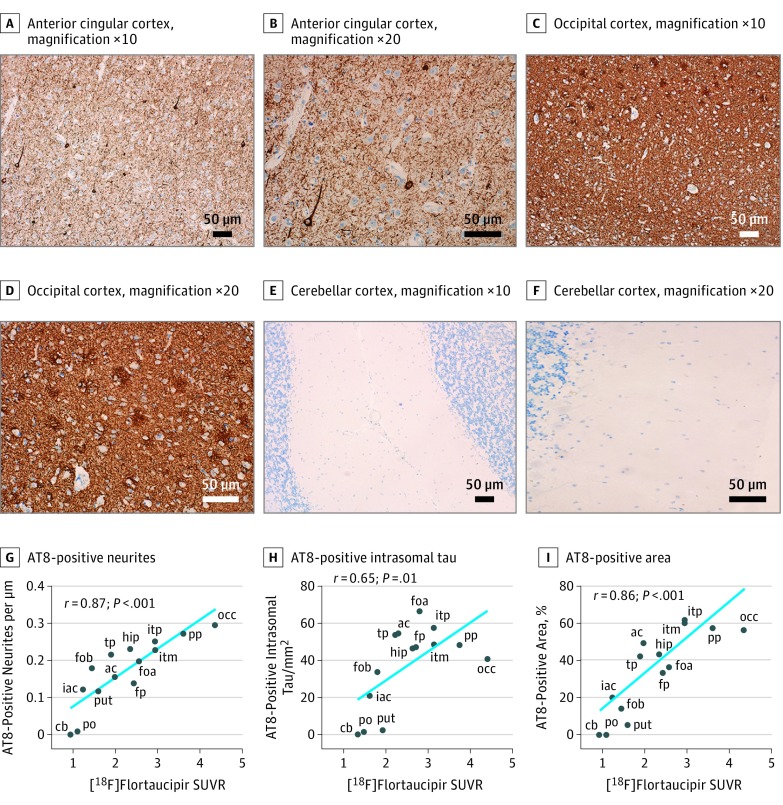
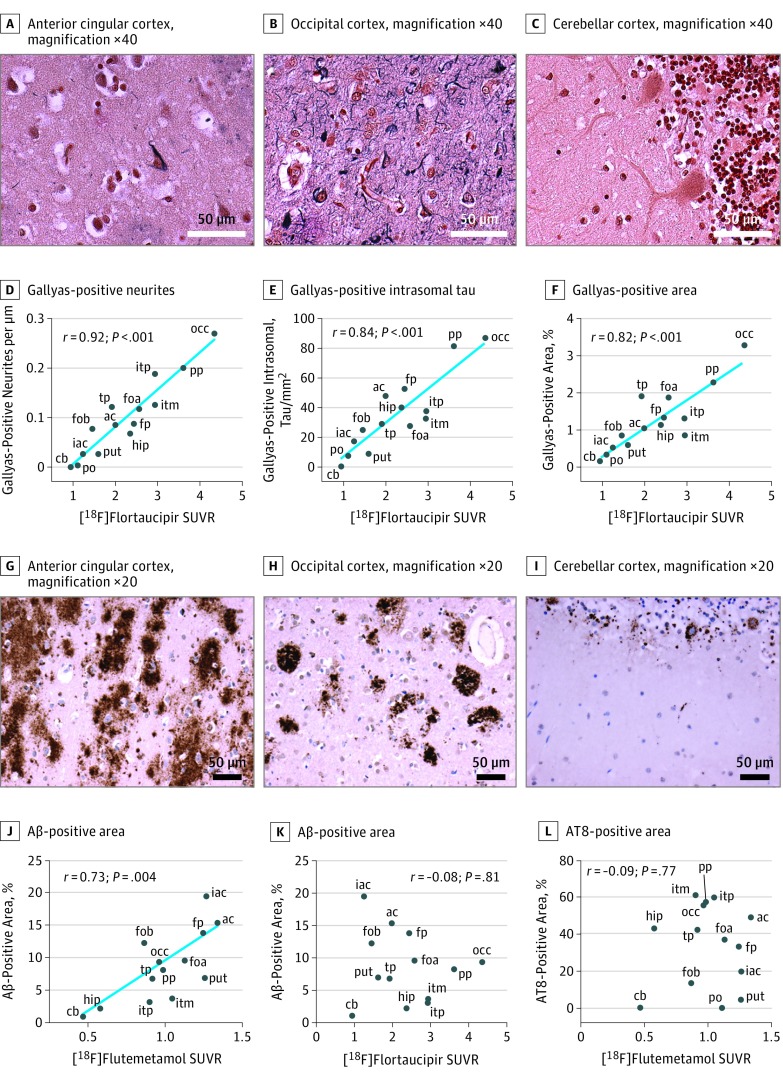
The patient died 12 months after the follow-up [18F]flortaucipir scan. The brain was examined neuropathologically, and the AD diagnosis was confirmed. Tau-positive neurites (neuropil threads) and intrasomal tau tangles were detected using both AT8 immunohistochemistry (Figure 2A-F) and Gallyas staining (Figure 3A-C). The highest density of tau pathology was found in the occipital cortex, where an intense labeling of tau-positive neurites and intrasomal tau tangles could be seen. Using AT8 immunohistochemistry (which is more sensitive to neuritic tau than Gallyas staining), the tau-positive neurites far outnumbered the intrasomal tau aggregates in all studied regions, and this was especially prominent in the occipital cortex. Parietal and inferior temporal cortices were also heavily affected by tau pathology, both in the form of neuritic and intrasomal tau. Even the frontal lobes were affected by tau pathology, but the density of pathology was less pronounced. Only scarce tau pathology could be detected in the pons outside of the locus coeruleus, and the cerebellar gray matter was not affected by tau pathology. 4G8 Immunohistochemistry revealed a substantial density of Aβ-containing plaques throughout the cortex, especially in the frontal lobe and anterior cingulate (Figure 3G-I), but LB509 immunohistochemistry revealed no α-synuclein pathology in any of the studied regions.
Figure 2. Neuropathological Assessment of Phosphorylated Tau (AT8) Pathology and Correlations With Positron Emission Tomography (PET) Data.
Panels A-F depict AT8 immunohistochemistry of the anterior cingular cortex at ×10 (A) and ×20 (B) magnification, the occipital cortex at ×10 (C) and ×20 (D) magnification, and the cerebellar cortex at ×10 (E) and ×20 (F) magnification. Scale bars indicate 50 μm. Panels G-I show the correlations of [18F]flortaucipir standardized uptake value ratio (SUVR) values with AT8 tau-positive neurite density (G), AT8-positive intrasomal tau tangles (H), and the overall density of AT8 tau immunohistochemistry (I). ac indicates anterior cingulum (dexter); cb, cerebellar gray matter; foa, fronto-orbital cortex (sinister), sample a; fob, fronto-orbital cortex (sinister), sample b; fp, frontal pole (sinister); hip, hippocampus (sinister); iac, inferior anterior cingulum (dexter); itm, inferior temporal (sinister), midway along an anteroposterior axis; itp, inferior temporal (sinister), posterior aspect; occ, occipital cortex (sinister; calcarine fissure); po, pons; pp, parasagittal parietal cortex (dexter); put, putamen (sinister); tp, temporal pole (sinister). The blue line indicates linear regressions.
Figure 3. Correlation of Gallyas Silver Stain and β-Amyloid (Aβ) Pathology With Positron Emission Tomography (PET) Retention.
Panels A-C depict Gallyas silver staining of the anterior cingular cortex (A), the occipital cortex (B), and the cerebellar cortex (C), all at ×40 magnification. Scale bars indicate 50 μm. Panels D-F show the correlations of [18F]flortaucipir standardized uptake value ratio (SUVR) values with Gallyas-positive neurite density (D), Gallyas-positive intrasomal tau tangles (E), and the overall density of tau positivity using the Gallyas silver stain (F). Panels G-I depict 4G8 immunohistochemistry to detect Aβ in the anterior cingular cortex (G), the occipital cortex (H), and the cerebellar cortex (I), all at ×20 magnification. Scale bars indicate 50 μm. J, Correlation of Aβ density with [18F]flutemetamol SUVR. K, Correlation of Aβ density with [18F]flortaucipir SUVR. L, Correlation of phosphorylated tau (AT8)–positive area with [18F]flutemetamol SUVR. ac indicates anterior cingulum (dexter); cb, cerebellar gray matter; foa, fronto-orbital cortex (sinister), sample a; fob, fronto-orbital cortex (sinister), sample b; fp, frontal pole (sinister); hip, hippocampus (sinister); iac, inferior anterior cingulum (dexter); itm, inferior temporal (sinister), midway along an anteroposterior axis; itp, inferior temporal (sinister), posterior aspect; occ, occipital cortex (sinister; calcarine fissure); po, pons; pp, parasagittal parietal cortex (dexter); put, putamen (sinister); tp, temporal pole (sinister). The blue line indicates linear regressions.
Correlation of [18F]Flortaucipir Retention With Neuropathology
The density of tau-containing neurites was assessed in AT8-immunostained and Gallyas-stained sections using a stereology-based setup. The density was found to be highly correlated with [18F]flortaucipir retention in the corresponding brain regions (AT8: rs = 0.87; P < .001; Gallyas: rs = 0.92; P < .001) (Figure 2G and Figure 3D). Very similar results were obtained with an alternative approach where the total tau-staining density was determined from cortical images of the analyzed regions (AT8: rs = 0.86; P < .001; Gallyas: rs = 0.82; P < .001) (Figure 2I and Figure 3F) and using a final approach where the neuropathological grade was rated by 3 independent raters (r = 0.85; P < .001) (eMethods and eFigure 1 in the Supplement).
To determine whether the PET retention correlated with the density of intrasomal tau tangles, the presence of these were assessed. Also, for intrasomal tau, we found correlations, albeit slightly less pronounced, with [18F]flortaucipir retention (AT8: rs = 0.65; P = .01; Gallyas: rs = 0.84; P < .001) (Figure 2H and Figure 3E). To control for possible artifacts caused by the small ROI size used in the flortaucipir PET scans, we created larger ROIs by expanding the volumes sampled to 1.5 to 4 centimeters cubed. This approach resulted in highly similar results (eFigure 2 in the Supplement).
Correlation of [18F]Flutemetamol Retention With Neuropathology
The density of Aβ staining showed a significant correlation with [18F]flutemetamol retention (rs = 0.73; P = .004) (Figure 3J) but no correlation with [18F]flortaucipir retention (rs = −0.08; P = .81) (Figure 3K). Similarly, we found no correlation of tau pathology with [18F]flutemetamol SUVRs (AT8 neurites: rs = −0.31; P = .28; AT8 intrasomal tau: rs = 0.13; P = .65; AT8 positive area percentage: rs = −0.09; P = .77 [Figure 3L]; Gallyas neurites: rs = −0.07; P = .82; Gallyas intrasomal tau: rs = 0.05; P = .88; Gallyas positive area percentage: rs = −0.06; P = .84).
Discussion
In the present study, we demonstrate a direct correlation of tau pathology post mortem with region-matched [18F]flortaucipir retention in vivo in a patient with severe early-onset AD caused by a mutation in the PSEN1 gene. We found that the regional signal obtained with PET was strongly correlated with both the total tau burden as well as the density of tau-positive neurites (neuropil threads) in the brain, using both AT8 immunohistochemistry and Gallyas silver staining to assess tau pathology. In an autoradiography study,8 it was suggested that flortaucipir (ie, AV1451) preferentially binds to NFT (intrasomal) tau and less to neuritic tau. The correlation with intrasomal tau tangles in this study was slightly weaker than the correlations with neuritic or total tau, indicating that the flortaucipir retention in vivo is closely related to the total tau burden in the brain and not solely to the intrasomal tau pathology. In another autoradiography experiment,10 only a weak correlation (r2 = 0.24) was found between flortaucipir binding and AT8 immunohistochemistry, in sharp contrast to the results presented here, possibly indicating that autoradiography ex vivo does not capture the whole span of tau pathology. The neuritic tau pathology (neuropil threads) has been suggested to constitute 80% to 90% of the total tau pathology,12 and if that pathology is not reflected using autoradiography, a major component of the pathology will be lost. The presence of NFTs in the cortex of patients with AD correlates with disease duration and disease severity,18 but neuritic tau pathology seems to precede NFT pathology19; the cell loss in AD has been shown to exceed the NFT pathology,20 indicating that other pathologies than NFTs are also detrimental to neuronal function and survival in AD, eg, neuritic tau pathology, Aβ burden, and astrocytic gliosis.
With the advent of tau PET, it has become clear that the spread of tau pathology (as visualized with flortaucipir PET) overall seems to follow the pattern suggested by the Braak staging,21 even though the early deposition of tau pathology might occur in more widespread areas of the cerebral cortex than was initially suggested by the Braak stages.22 The Braak staging provides an assessment of tau pathology along an ordinal scale, where different predefined brain regions that represent the different Braak stages (stages I-VI) are determined to be either positive or negative. This staging system does not take into account the density or magnitude of the tau pathology within the different positive brain regions. This is in contrast to tau PET imaging, which, according to the present study, reliably provides an assessment of the density of AD-like tau pathology along a continuous scale. Therefore, correlational analyses of tau pathology vs tau PET should preferably be performed using quantitative measures of the density of tau pathology rather than correlations with Braak stages alone.
[18F]Flortaucipir has been shown to detect pathological tau aggregates ex vivo.8,9,10 In an autoradiography experiment using [18F]flortaucipir-incubated tissue sections dipped in a photographic nuclear emulsion, the silver grains, derived from the radioactive decay, colocalized with immunohistochemistry for tau-paired helical filaments in NFTs and neurites.9 This constitutes a very strong argument for flortaucipir binding to tau aggregates. Similarly, our finding that flortaucipir PET correlates strongly with tau pathology indicates that most of the signal in in vivo PET is also derived from tau pathology. However, to exclude the possibility that flortaucipir interacts with Aβ pathology in vivo, we quantified Aβ pathology and correlated it with [18F]flortaucipir retention. We found no correlation of Aβ pathology with flortaucipir, nor did we find any correlation of tau pathology with [18F]flutemetamol retention. As expected, and in line with previous reports,23,24 we found a correlation of Aβ pathology with region-matched [18F]flutemetamol PET retention.
Limitations
This study had limitations. One limitation of this study is that the study is based on just a single patient, and results should be interpreted with this in mind. Moreover, the distribution pattern of pathology is different in this PSEN1 mutation carrier compared with patients with late-onset AD, with more tau pathology being present in the occipital and posterior cortices of the participant in this study. Even so, we do not believe that this should affect the correlation between tau PET and tau pathology or the main conclusion of the study. Another limitation is the delay between last [18F]flortaucipir PET scan and postmortem analysis. The rapid progression of the tau pathology and the interval between the follow-up scan and the postmortem analysis may in part be an explanation of some of the mismatch seen in the correlations in Figure 2 and Figure 3. Correlating PET data with neuropathological data is challenging because of the difference in resolution between the different methods used. Neuropathology gives high-resolution and detailed data in small and often relatively few areas, whereas PET imaging gives easily quantifiable data from the whole brain but with a relatively low resolution. We have tried to address these differences by (1) sampling in multiple cortical regions, (2) using an exact matching of the areas sampled for neuropathology and PET, and (3) using both smaller ROIs that are more exactly located compared with the stained postmortem tissue and larger ROIs that are less affected by local variations in the PET retention.
Conclusions
To conclude, we found that there are strong correlations between in vivo [18F]flortaucipir retention and postmortem total tau burden, tau-positive neurites, and intrasomal tau tangles. We found no correlations of Aβ pathology with [18F]flortaucipir or of tau pathology with [18F]flutemetamol. Our results validate the use of [18F]flortaucipir PET to detect and quantify tau pathology in vivo. These results further stress the usefulness of [18F]flortaucipir in evaluation of therapies aiming to remove AD-like tau aggregates from the brain.
eMethods.
eFigure 1. Correlation of neuropathological rating with [18F]flortaucipir retention.
eFigure 2. Correlation of AT8 immunohistochemistry with [18F]flortaucipir SUVR in larger-sized ROIs.
References
- 1.Dani M, Brooks DJ, Edison P. Tau imaging in neurodegenerative diseases. Eur J Nucl Med Mol Imaging. 2016;43(6):1139-1150. doi: 10.1007/s00259-015-3231-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson KA, Schultz A, Betensky RA, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79(1):110-119. doi: 10.1002/ana.24546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139(pt 5):1551-1567. doi: 10.1093/brain/aww027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schöll M, Lockhart SN, Schonhaut DR, et al. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89(5):971-982. doi: 10.1016/j.neuron.2016.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schöll M, Ossenkoppele R, Strandberg O, et al. ; Swedish BioFINDER study . Distinct 18F-AV-1451 tau PET retention patterns in early- and late-onset Alzheimer’s disease. Brain. 2017;140(9):2286-2294. doi: 10.1093/brain/awx171 [DOI] [PubMed] [Google Scholar]
- 6.Smith R, Wibom M, Olsson T, et al. Posterior accumulation of tau and concordant hypometabolism in an early-onset Alzheimer’s disease patient with presenilin-1 mutation. J Alzheimers Dis. 2016;51(2):339-343. doi: 10.3233/JAD-151004 [DOI] [PubMed] [Google Scholar]
- 7.Ossenkoppele R, Rabinovici GD, Smith R, et al. Discriminative accuracy of [18F]flortaucipir positron emission tomography for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2018;320(11):1151-1162. doi: 10.1001/jama.2018.12917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowe VJ, Curran G, Fang P, et al. An autoradiographic evaluation of AV-1451 tau PET in dementia. Acta Neuropathol Commun. 2016;4(1):58. doi: 10.1186/s40478-016-0315-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marquié M, Normandin MD, Vanderburg CR, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015;78(5):787-800. doi: 10.1002/ana.24517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sander K, Lashley T, Gami P, et al. Characterization of tau positron emission tomography tracer [18F]AV-1451 binding to postmortem tissue in Alzheimer’s disease, primary tauopathies, and other dementias. Alzheimers Dement. 2016;12(11):1116-1124. doi: 10.1016/j.jalz.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 11.Smith R, Puschmann A, Schöll M, et al. 18F-AV-1451 tau PET imaging correlates strongly with tau neuropathology in MAPT mutation carriers. Brain. 2016;139(pt 9):2372-2379. doi: 10.1093/brain/aww163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell TW, Nissanov J, Han LY, et al. Novel method to quantify neuropil threads in brains from elders with or without cognitive impairment. J Histochem Cytochem. 2000;48(12):1627-1638. doi: 10.1177/002215540004801206 [DOI] [PubMed] [Google Scholar]
- 13.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 3rd ed, revised Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944. doi: 10.1212/WNL.34.7.939 [DOI] [PubMed] [Google Scholar]
- 15.Baker SL, Maass A, Jagust WJ. Considerations and code for partial volume correcting [18F]-AV-1451 tau PET data. Data Brief. 2017;15:648-657. doi: 10.1016/j.dib.2017.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thurfjell L, Lilja J, Lundqvist R, et al. Automated quantification of 18F-flutemetamol PET activity for categorizing scans as negative or positive for brain amyloid: concordance with visual image reads. J Nucl Med. 2014;55(10):1623-1628. doi: 10.2967/jnumed.114.142109 [DOI] [PubMed] [Google Scholar]
- 17.Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 2001;8(5):443-459. doi: 10.1136/jamia.2001.0080443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42(3, pt 1):631-639. doi: 10.1212/WNL.42.3.631 [DOI] [PubMed] [Google Scholar]
- 19.Braak H, Del Tredici K. Spreading of tau pathology in sporadic Alzheimer’s disease along cortico-cortical top-down connections. Cereb Cortex. 2018;28(9):3372-3384. doi: 10.1093/cercor/bhy152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gómez-Isla T, Hollister R, West H, et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol. 1997;41(1):17-24. doi: 10.1002/ana.410410106 [DOI] [PubMed] [Google Scholar]
- 21.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239-259. doi: 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- 22.Jack CR Jr, Wiste HJ, Schwarz CG, et al. Longitudinal tau PET in ageing and Alzheimer’s disease. Brain. 2018;141(5):1517-1528. doi: 10.1093/brain/awy059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Driscoll I, Troncoso JC, Rudow G, et al. Correspondence between in vivo (11)C-PiB-PET amyloid imaging and postmortem, region-matched assessment of plaques. Acta Neuropathol. 2012;124(6):823-831. doi: 10.1007/s00401-012-1025-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131(pt 6):1630-1645. doi: 10.1093/brain/awn016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1. Correlation of neuropathological rating with [18F]flortaucipir retention.
eFigure 2. Correlation of AT8 immunohistochemistry with [18F]flortaucipir SUVR in larger-sized ROIs.