Key Points
Question
What is the evidence from randomized clinical trials on effectiveness and appropriate administration of antibiotics in rhinoplasty procedures?
Findings
In a systematic review and meta-analysis, a pooled study sample of 589 participants were extracted from 5 randomized clinical trials. Postoperative antibiotic therapy did not significantly alter the rate of infections after rhinoplasty procedures.
Meanings
Based on these findings, preventive antibiotic therapy in rhinoplasty does not appear to be indicated.
Abstract
Importance
Although antibiotic prophylaxis following rhinoplasty is widespread, the evidence on antibiotic prophylaxis effectiveness and the superiority of particular administration regimens is controversial. To date, a meta-analysis on the topic has not been performed.
Objective
To systematically review the association between use of preventive antibiotics and postoperative complications in patients undergoing rhinoplasty and quantify the review through meta-analysis.
Data Sources
MEDLINE, Embase, CINAHL, Central (Cochrane Controlled Register of Trials), Scopus, and Web of Science were searched with prospectively designed search phrases on February 16, 2018. All databases were searched from database inception. Key search terms included rhinoplasty, nasal valve repair, and antibacterial agent.
Study Selection
Randomized clinical trials (RCTs) with adults (≥18 years) undergoing rhinoplasty and including systemic antibiotic medications administered in the absence of other reasons for use of an antibiotic (eg, localized or systemic infection), without restrictions on language or the time of publication, were included in the study. Interventions of interest were classified into 3 types: (1) single-dose systemic antibiotic administered within 24 hours before the first incision, (2) multidose systemic antibiotic treatment started within 24 hours before the first incision and continuing after the operation, and (3) systemic antibiotic therapy (single dose or multidose) started within 24 hours after the first incision. The following comparisons were made: for the interventions of type 1, no antibiotic; for the interventions of types 2 or 3, no antibiotic or an intervention of type 1.
Data Extraction and Synthesis
Data extraction was compliant with PRISMA guidelines and Cochrane Handbook for Systematic Reviews of Interventions. Two independent reviewers assessed the relevance of the remaining records at abstract and full-text stages. Meta-analysis pooled with random-effects model.
Main Outcomes and Measures
Difference in infectious complication rate between groups.
Results
A total of 262 records were identified; of these, only 5 RCTs fulfilled predetermined population, intervention, comparison, and outcome criteria. The pooled study sample consisted of 589 participants. No significant differences in outcome of preventive antibiotic therapy given either preoperatively or postoperatively were found, with a pooled risk ratio of 0.92 (95% CI, 0.35-2.43; P = .86).
Conclusions and Relevance
This study appears to be the first Cochrane-protocol systematic review and meta-analysis investigating preventive antibiotics in rhinoplasty. This study’s results suggest that pooled evidence from the 5 RCTs does not support the use of preventive antibiotic therapy in rhinoplasty.
Level of Evidence
1.
This systematic review and meta-analysis compares the pooled results of randomized clinical trials that examined the effect of antibiotic preventive therapy on postoperative complications in patients undergoing rhinoplasty.
Introduction
Surgical site infections are a significant clinical problem associated with elevated postoperative morbidity and demanding substantial use of health care resources.1 To avoid postoperative infection, antibiotic prophylaxis following rhinoplasty is used in over 90% of the procedures.2 While this practice is widespread, the evidence on antibiotic prophylaxis effectiveness and the superiority of particular administration regimens is controversial. It has previously been reported that 28% of rhinoplasty surgeons do not use antibiotics, 17% use them only perioperatively, 24% prescribe antibiotics up to 3 days after the surgical procedure, 31% administer them up to 7 days, and 3% use antibiotic irrigation.3 Use of grafts in rhinoplasty has been associated with an even more prolonged use of postoperative antibiotics.3
A narrative review by Georgiou et al4 has advised against antibiotic prophylaxis after rhinoplasty. An extensive retrospective review by Cabouli et al5 suggested a low rate of infection after rhinoplasty (0.6%) as a reason against routine postoperative antibiotic prophylaxis. A low incidence of postoperative infections (0.48%) has also been reported by Yoder and Weimert6 among a mixed septoplasty/septorhinoplasty cohort. Slavin et al7 have found no evidence of bacteremia after rhinoplasty.
Published in 2017, a clinical practice guideline suggested that harm caused by routine postoperative antibiotics might outweigh the risks related to possible postoperative infection.2 The clinical practice guideline cited 2 randomized clinical trials (RCTs) that reported no difference in infection incidence between groups.8,9 However, the clinical practice guideline included the results of a single trial on revision rhinoplasty that reported higher infection rates in the placebo group.10 Thus, the clinical practice guideline has recommended against routine prescription of antibiotic therapy for more than 24 hours after the operation.2 However, no definitive recommendations were offered concerning optimal preoperative, perioperative, or postoperative antibiotic prophylaxis in rhinoplasty.2
To our knowledge, there have not been previous meta-analyses on the topic. The objective of this systematic review and meta-analysis was to investigate whether there is evidence that supports the routine use of postoperative antibiotic prophylaxis in rhinoplasty.
Methods
This review protocol was based on the Cochrane Handbook for Systematic Reviews of Interventions.11 The population, intervention, comparison, and outcome criteria were defined as described below.
Population
Adults (age ≥18 years) undergoing rhinoplasty were the sample population. Rhinoplasty was understood as a surgical procedure that alters the shape or appearance of the nose while preserving or enhancing the nasal airway. The reasons for the procedure might be functional, cosmetic, or both. Adjunctive procedures on nasal septum, nasal turbinates, or paranasal sinuses alone, without an effect on nasal shape or appearance, were not considered rhinoplasty.
Types of Studies
Randomized clinical trials published in peer-reviewed academic journals with abstracts available and without restrictions on language or time of publication were included for analysis. Pilot reports, theses, conference proceedings, letters, editorials, and retrospective studies were excluded. Controlled clinical trials were included if the number of RCTs was less than 3.
Intervention
Systemic antibiotics administered in the absence of reasons for antibiotic use (eg, localized or systemic infection) other than preventing surgical complications were the interventions considered. Interventions of interest were classified as 3 types: (1) single-dose systemic antibiotic therapy administered within 24 hours before the first incision, (2) multidose systemic antibiotic treatment started within 24 hours before the first incision and continuing after the operation, and (3) a systemic antibiotic treatment (single dose or multidose) started within 24 hours after the first incision.
Comparison
For interventions of type 1, use vs no use of antibiotic was compared. For intervention types 2 and 3, use of no antibiotic or a type 1 intervention was compared. These interventions of interest were designed prior to search of the database.
Outcome
The outcome of the studies was difference in any infectious complication rate between groups. The risk rates of infections were reported as risk ratios (RRs).
Database Search Strategy
Without restriction on the time or language of publication, the databases of MEDLINE, Embase, CINAHL, Central (Cochrane Controlled Register of Trials), Scopus, and Web of Science were searched on February 16, 2018. All databases were searched from database inception. Search terms included rhinoplasty, nasal valve repair, and antimicrobial agents. Other terms are found in the eAppendix in the Supplement.
Duplicates, conference proceedings, theses, and other publications that were not RCTs were excluded. Two independent reviewers (B.N. and C.K.K.) assessed the relevance of the remaining records, first based on titles and abstracts, and then based on full texts. Possible disagreements between the reviewers were resolved by consensus or by a decision made by a third reviewer (K.L.).
Data Extraction
The data needed for meta-analysis were extracted from the included trials using a standardized form based on recommendations by the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0, part 7.6.11
Bias Assessments
The methodologic quality of the RCTs was assessed according to the Cochrane Collaboration’s domain-based evaluation framework.12 Main domains were assessed in the following sequence: (1) selection bias (randomized sequence generation and allocation concealment), (2) performance bias (blinding of participants and study personnel), (3) detection bias (blinding of outcome assessment), (4) attrition bias (eg, incomplete outcome data due to dropouts), (5) reporting bias (selective reporting), and (6) other sources of bias. The scores for each bias domain and the final score for risk of systematic bias were graded as low, high, or unclear.13
Statistical Analysis
The interrater reliability of independent author review of screened records was assessed using the κ statistic. The risk rates of infections in intervention and control groups are reported as RRs. The RRs were calculated along with their 95% CIs and 2-tailed P values considering findings significant at P < .05. The test for heterogeneity was conducted using the I2 statistic describing the percentage of variation across studies originating more from heterogeneity than from chance. Potential publication bias was not analyzed because of the small number of identified trials. Expecting wide range of variability in studies’ settings, a random-effects metasynthesis was used regardless of particular percentages of heterogeneity. The I2 rank was classified as follows: 0% to 24%, no heterogeneity; 25% to 49%, low heterogeneity; 50% to 74%, moderate heterogeneity; and 75% or more, high heterogeneity.
The analysis was conducted using Comprehensive Meta Analysis software, version 3.3 (Biostat; http://www.meta-analysis.com).
Results
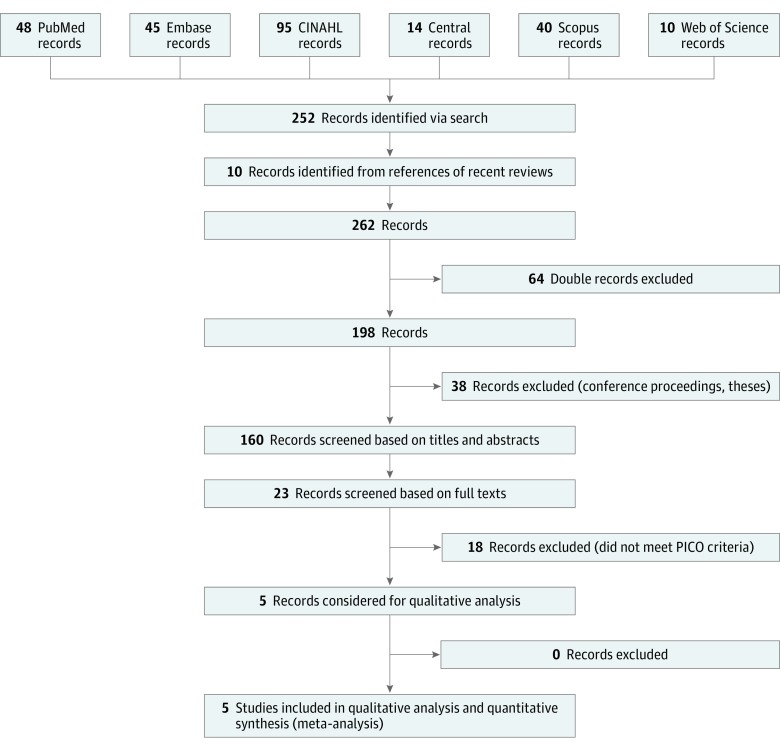
The initial search yielded 252 records; 10 records were added from references of recent reviews. After exclusion of 64 duplicates, 198 records remained. Thirty-eight records were then excluded as not being RCTs (eg, conference proceedings, theses, case reports). The remaining 160 records were screened based on titles and abstracts with moderate κ of agreement 0.41 (SE, 0.11; 95% CI, 0.21-0.62). Twenty-three records were assessed based on their full texts. Finally, 5 studies were considered to be relevant for the qualitative and quantitative analysis (Figure 1).8,9,10,14,15 The included RCTs were published between 1971 and 2006. The pooled sample consisted of 589 patients: 298 cases and 291 controls.
Figure 1. PRISMA Flow Diagram.
Databases accessed using relevant search clauses yielded 262 studies after excluding duplicates and other studies that did not meet criteria. Using the population, intervention, comparison, and outcome framework, 23 records were screened based on full texts and 5 randomized clinical trials were deemed eligible for inclusion in the meta-analyses. PICO indicates population, intervention, comparison of control, and outcome.
Table 1 describes prophylactic antibiotic regimens used in the included trials. The most common antibiotics were penicillin or amoxicillin, with or without β-lactamase inhibition. Antibiotic treatments started on induction or perioperatively were administered intravenously, whereas therapy with antibiotics started postoperatively was administered orally. Dosages and durations varied with the duration of postoperative administration, ranging from 5 to 12 days. Two RCTs compared groups with antibiotic prophylaxis of different lengths of treatment,8,9 and 3 RCTs compared antibiotics with placebo or no treatment (Table 2).10,14,15 The overall rate of infections within the pooled sample was 5.54% in cases and 6.53% in controls.
Table 1. Basic Characteristics of the Selected Trials.
| Source | Sample Size, No.a | Women, % | Age, Mean (SD), y | Cases | Controls |
|---|---|---|---|---|---|
| Postoperative vs Perioperative | |||||
| Andrews et al,8 2006 United Kingdom | 164 | NR | NR | Postoperative amoxicillin wih clavulanate, 375 mg orally, for 3 or 7 d or erythromycin, 500 mg orally, for 4 or 7 d | Amoxicillin with clavulanate, 1200 mg intravenously or erythromycin, 1000 mg orally for 3 doses (induction, 6 h, and 12 h after surgery) |
| Postoperative vs Preoperative | |||||
| Rajan et al,9 2005 Australia | 200 | 44 | 33 (8.4) in case cohort; 32 (9.2) in control cohort | Amoxicillin with clavulanate, 2200 mg intravenously at induction and amoxicillin with clavulanate, 1000 mg orally for 2 or 7 d postoperatively | Amoxicillin with clavulanate, 2200 mg intravenously at induction |
| Postoperative vs Placebo or None | |||||
| Eschelman et al,14 1971 United Statesa | 36 | NR | NR | Penicillin, 600 000 IU intramuscularly or 250 mg orally at induction and then 4 or 5-10 d postoperatively | Placebo |
| Eschelman et al,14 1971 United Statesa | 40 | NR | NR | Ampicillin, 500 mg intramuscularly or orally at induction and then 4 or 5-10 d postoperatively | Placebo |
| Schäfer and Pirsig,10 1988 Germany | 100 | 29 | 31 (12) | Propicillin, 1000 IU orally 6 h postoperatively, then 3 times/d for 12 d | Placebo |
| Weimert et al,15 1980 United States | 68 | NR | NR | Ampicillin, 500 mg 12 h before and up to 5 d after surgery (oral dosage implied) | None |
Abbreviation: NR, not reported.
Eschelman et al14 conducted 2 trials within their study: the first included 17 patients who received penicillin and 19 patients who received placebo, and the second included 21 patients who received ampicillin and the initial 19 patients who received placebo. Therefore, after removing the 19 patients who received placebo patients and were accounted for twice, the total pooled study sample is 589 patients (298 cases and 291 controls).
Table 2. Postoperative Infection Rates Reported by the Selected Trials.
| Source | Cases | Controls | Recorded Infectious Complications | ||||
|---|---|---|---|---|---|---|---|
| Infection, No. (%) | Sample Size, No. | Infection, % | Sample Size, No. | ||||
| Yes | No | Yes | No | ||||
| Postoperative vs Perioperative | |||||||
| Andrews et al,8 2006 | 9 (11.0) | 73 (89.0) | 82 | 6 (7.3) | 76 (93.7) | 82 | Vestibular cellulitis, nasal/septal cellulitis, donor-site infection |
| Postoperative vs Preoperative | |||||||
| Rajan et al,9 2005 | 3 (3.0) | 97 (97.0) | 100 | 0 | 100 (100) | 100 | Vestibulitis |
| Postoperative vs Placebo or None | |||||||
| Eschelman et al,14 1971 (all rhinoplasties) | 1 (2.6) | 37 (97.4) | 38 | 1 (5.3) | 18 (94.7) | 19 | Maxillary sinusitis |
| Schäfer and Pirsig,10 1988 | 4 (8.3) | 44 (91.7) | 48 | 14 (26.9) | 38 (73.1) | 52 | Upper respiratory tract infection, purulence, septal abscess, candidal dermatitis |
| Weimert et al,151980 | 1 (3.3) | 29 (96.7) | 30 | 1 (2.6) | 37 (97.4) | 38 | Vestibulitis |
The eTable in the Supplement describes the risks of systematic bias among the RCTs. Of 5 studies, 4 were considered to have low risks. The overall risk of bias in the study by Weimert and Yoder15 was considered unclear.
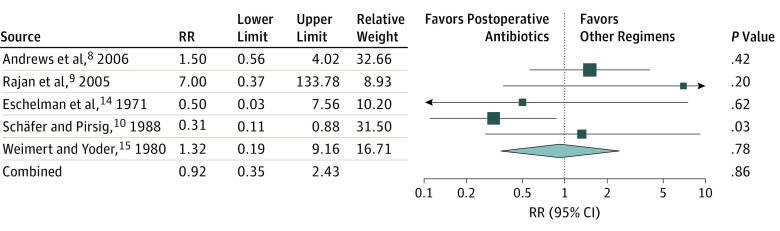
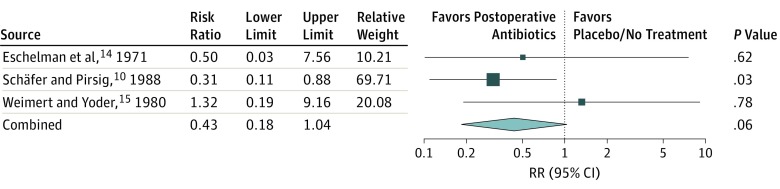
The pooled RR of infection when comparing postoperative antibiotics with any or all other antibiotic regimens in the RCTs, including placebo or no treatment, preoperative, or perioperative antibiotics is presented in Figure 2. The pooled RR was 0.92 (95% CI, 0.35-2.43; P = .86) with low heterogeneity (I2 = 44%). The pooled RR of infection when comparing postoperative antibiotics with placebo or no treatment (excluding preoperative or perioperative antibiotics) is presented in Figure 3. The pooled RR was 0.43 (95% CI, 0.18-1.04; P = .06) with no heterogeneity observed (I2 = 0%).
Figure 2. The Pooled Risk Ratio (RR) of Infection When Comparing Postoperative Antibiotics With Placebo or No Treatment and Preoperative or Perioperative Antibiotics.
The pooled RR was 0.92 (95% CI, 0.35-2.43; P = .86) with low random effects heterogeneity (I2), 44%.
Figure 3. The Pooled Risk Ratio (RR) of Infection When Comparing Postoperative Antibiotics With Placebo or No Treatment (Excluding Preoperative or Perioperative Antibiotics).
The pooled RR was 0.43 (95% CI, 0.18-1.04; P = .06) with random effects heterogeneity (I2), 0%.
Discussion
In this systematic review and meta-analysis of 5 RCTs with overall low risks of systematic bias, routine antibiotic prophylaxis after rhinoplasty did not demonstrate an association with infection rates. The differences in RRs of infection were not statistically significant when comparing postoperative with preoperative and perioperative antibiotics or with placebo.
The selected RCTs were notable regarding publication timing and the antibiogram landscape from which they arose. The most recent RCT included in this review was more than 10 years old, and the RCTs span 4 decades; this difference may affect the generalizability of the results as modern surgery, antibiotic assortment and doses, and national guidelines have significantly changed across the times studied. We noted in this review that, in general, the RCTs published more recently used antibiotics with wider extended-spectrum coverage than the older RCTs—this difference is perhaps the result of increasing concerns or evidence for antibiotic resistance and the greater need for effective antibiotic stewardship.
Parallel to concerns for antibiotic stewardship include a general global pandemic of multidrug-resistant infections, including methicillin-resistant Staphylococcus aureus (MRSA) infections.16 While cases series of MRSA infections following rhinoplasty have been documented,17 this issue has not been systematically studied and remains a research gap.1 Current literature, limited to case reports and series, does not advocate routine MRSA preoperative screening.17 Such reviews defer to individual clinician judgment regarding antibiotic coverage for patients with colonized MRSA who are undergoing rhinoplasty.1,17
Prior studies report a range of postrhinoplasty infection from 0% to 26%,4,5,6,8,9,14,15,17,18 with the largest retrospective reviews reporting infection rates as low as 0.6%.5 This percentage is in contrast to our review’s overall rate of infection among RCTs as 6.0%. This discrepancy, as well as prior literature’s wide published range of dates, may be secondary to various factors. Studies prospectively defining infectious complications prior to outcome measurement were few,8,14 with only 1 RCT a priori explicitly classifying anticipated infectious complications.8 Commonly, descriptions of postrhinoplasty infection mention peri-incisional purulence, frank abscess of any nasal compartment/subunit, nasal vestibulitis, and nasal cellulitis (Table 2). However, specific measurement of other types of infectious complications vary widely among studies. For example, paranasal sinusitis is specifically identified as a postoperative infectious complication in few studies.6,14
In the selected RCTs, the breadth in specificity and variety of a defined infectious complication is notable (Table 2). The higher rate of infection among the reviewed RCTs compared with prior literature also may be attributable to study design. All prior studies reporting infection rates from 0% to 0.6% were either retrospective medical record reviews or uncontrolled cohort studies, which are likely more susceptible to inherent differential biases,4,5,7 in contrast to the selected RCTs and their overall low assessed bias (eTable in the Supplement).
The role of antibiotics in packing and/or splints post rhinoplasty is understudied. Among our selected RCTs, studies that mentioned packing had included it for all of their rhinoplasty patients.8,9,10 Similarly, the study that mentioned internal splints placed them for all patients9; otherwise, packing/splinting among the other RCT cohorts was ambiguous. To our knowledge, there have been no prospective, controlled cohort studies investigating the role of postoperative vs perioperative or placebo antibiotics in rhinoplasty patients with nasal packing or internal splints.
Another consideration is the role of antibiotics in revision rhinoplasty and grafting. Andrews et al8 examined perioperative amoxicillin with clavulanate vs postoperative amoxicillin with clavulanate in “complex nasal surgery” only, which they defined as septorhinoplasty that involved nasal grafting and/or revision surgery, and found no difference in the incidence of infection. Schäfer and Pirsig10 specifically examined postoperative propicillin vs placebo in revision rhinoplasty only, which in most cases in their cohort included septal reconstruction, osteotomies, wedge resection, and free “transplants” or grafting, and did find benefit to postoperative prophylaxis. The other RCT cohorts excluded or did not specify or quantify which patients underwent grafting or revision surgery. It is thus suggested that prophylactic therapy with antibiotics should be considered in revision cases or cases with grafting, but that perioperative administration may provide similar prophylactic benefit as postoperative administration.8,10 Further RCTs specifically investigating prophylaxis in grafting and revision rhinoplasty are needed.
Limitations
There are limitations to this meta-analysis. Pooling the results reported by different trials is always approximation. Even though the heterogeneity in this meta-analysis was small, the included studies varied in terms of their samples, settings, and antibiotic treatment regimens. Some of the included trials lacked precise descriptions of surgical techniques used (eg, the extent of grafting, a graft type, or revision rates). Many potentially important confounding factors were not reported (eg, comorbidities, sociodemographics, allergies).
Conclusions
This systematic review and meta-analysis found no evidence to support the routine use of postoperative antibiotic prophylaxis in rhinoplasty. Like most surgical procedures, however, rhinoplasty is a heterogeneous operation in terms of scope, incisions, length of surgery, and use of grafts or foreign materials. These factors were not explored rigorously in this or other studies. It shall remain important to be vigilant regarding infection risk, and the above recommendations are not a replacement for clinical judgment on a case-by-case basis.
eAppendix. Search Terms Used
eTable 1. Risk of Systematic Bias of the Included Studies
References
- 1.Owens CD, Stoessel K. Surgical site infections: epidemiology, microbiology and prevention. J Hosp Infect. 2008;70(suppl 2):3-10. doi: 10.1016/S0195-6701(08)60017-1 [DOI] [PubMed] [Google Scholar]
- 2.Ishii LE, Tollefson TT, Basura GJ, et al. Clinical Practice Guideline: improving nasal form and function after rhinoplasty. Otolaryngol Head Neck Surg. 2017;156(2 Suppl):S1-S30. [DOI] [PubMed] [Google Scholar]
- 3.Perrotti JA, Castor SA, Perez PC, Zins JE. Antibiotic use in aesthetic surgery: a national survey and literature review. Plast Reconstr Surg. 2002;109(5):1685-1693. doi: 10.1097/00006534-200204150-00034 [DOI] [PubMed] [Google Scholar]
- 4.Georgiou I, Farber N, Mendes D, Winkler E. The role of antibiotics in rhinoplasty and septoplasty: a literature review. Rhinology. 2008;46(4):267-270. [PubMed] [Google Scholar]
- 5.Cabouli JL, Guerrissi JO, Mileto A, Cerisola JA. Local infection following aesthetic rhinoplasty. Ann Plast Surg. 1986;17(4):306-309. doi: 10.1097/00000637-198610000-00007 [DOI] [PubMed] [Google Scholar]
- 6.Yoder MG, Weimert TA. Antibiotics and topical surgical preparation solution in septal surgery. Otolaryngol Head Neck Surg. 1992;106(3):243-244. doi: 10.1177/019459989210600307 [DOI] [PubMed] [Google Scholar]
- 7.Slavin SA, Rees TD, Guy CL, Goldwyn RM. An investigation of bacteremia during rhinoplasty. Plast Reconstr Surg. 1983;71(2):196-198. doi: 10.1097/00006534-198302000-00008 [DOI] [PubMed] [Google Scholar]
- 8.Andrews PJ, East CA, Jayaraj SM, Badia L, Panagamuwa C, Harding L. Prophylactic vs postoperative antibiotic use in complex septorhinoplasty surgery: a prospective, randomized, single-blind trial comparing efficacy. Arch Facial Plast Surg. 2006;8(2):84-87. doi: 10.1001/archfaci.8.2.84 [DOI] [PubMed] [Google Scholar]
- 9.Rajan GP, Fergie N, Fischer U, Romer M, Radivojevic V, Hee GK. Antibiotic prophylaxis in septorhinoplasty? a prospective, randomized study. Plast Reconstr Surg. 2005;116(7):1995-1998. doi: 10.1097/01.prs.0000191181.73298.b3 [DOI] [PubMed] [Google Scholar]
- 10.Schäfer J, Pirsig W. Preventive antibiotic administration in complicated rhinosurgical interventions—a double-blind study [in German]. Laryngol Rhinol Otol (Stuttg). 1988;67(4):150-155. [PubMed] [Google Scholar]
- 11.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Updated March 2011. London, United Kingdom: The Cochrane Collaboration; 2011. [Google Scholar]
- 12.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S. The judgement: The Cochrane Collaboration's tool for assessing risk of bias In: Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Updated March 2011. London, United Kingdom: The Cochrane Collaboration, 2011:8.5.3. [Google Scholar]
- 14.Eschelman LT, Schleuning AJ II, Brummett RE. Prophylactic antibiotics in otolaryngologic surgery: a double-blind study. Trans Am Acad Ophthalmol Otolaryngol. 1971;75(2):387-394. [PubMed] [Google Scholar]
- 15.Weimert TA, Yoder MG. Antibiotics and nasal surgery. Laryngoscope. 1980;90(4):667-672. doi: 10.1288/00005537-198004000-00014 [DOI] [PubMed] [Google Scholar]
- 16.Holden MT, Hsu LY, Kurt K, et al. A genomic portrait of the emergence, evolution, and global spread of a methicillin-resistant Staphylococcus aureus pandemic. Genome Res. 2013;23(4):653-664. doi: 10.1101/gr.147710.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lohr GD, Hollabaugh B, Waters P, Tiwana PS. Methicillin-resistant Staphylococcus aureus and antibiotic use in septorhinoplasty: case report and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123(6):e177-e181. doi: 10.1016/j.oooo.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 18.Cochran CS, Ducic Y, DeFatta RJ. Current concepts in the postoperative care of the rhinoplasty patient. South Med J. 2008;101(9):935-939. doi: 10.1097/SMJ.0b013e3181807a79 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Search Terms Used
eTable 1. Risk of Systematic Bias of the Included Studies