Key Points
Question
Is electronic cigarette vaping associated with random pattern flap viability in rats as is traditional cigarette smoking?
Findings
In this cohort study involving 45 rats, the rate of flap necrosis was not significantly different between rats exposed to cigarette smoke and those exposed to electronic cigarette vapor. Both groups had considerably more necrosis than rats that were unexposed.
Meaning
Exposure to vapor and smoke may be equally detrimental to random pattern flap viability, suggesting that vaping should not be seen as a better alternative to smoking in the context of wound healing.
Abstract
Importance
Smoking is a known risk to wound healing, but whether electronic cigarettes present the same risk remains unknown.
Objective
To evaluate the rate of flap necrosis in the e-cigarette vapor–exposed group and the unexposed control and to detect a difference in the rate of flap necrosis between the traditional cigarette smoke–exposed group and the unexposed control.
Design, Setting, and Participants
From March 10, 2018, to May 4, 2018, a cohort study was conducted on 45 male Sprague-Dawley rats at Boston University School of Medicine. Each rat weighed approximately 100 g at the beginning of the study and was randomized to 1 of 3 groups: negative control (n = 15), experimental (exposed to e-cigarette vapor; n = 15), and positive control (exposed to traditional cigarette smoke; n = 15). Rats in the experimental and positive control groups were exposed to electronic cigarette vapor and traditional cigarette smoke in a smoking chamber for 30 minutes twice a day for 30 consecutive days. Levels of serum cotinine were monitored and maintained between 150 ng/mL and 200 ng/mL. After 30 days, random pattern dorsal skin flaps were raised.
Main Outcomes and Measures
Percentage of flap necrosis for each group.
Results
All 45 rats survived the surgical procedure and postoperative recovery, and all rats thrived and gained weight over the course of the study. The highest rate of flap necrosis was found in the positive control cohort, with a mean (SD) of 68.7% (8.6%), followed by the experimental cohort, with a mean (SD) of 65.9% (11.8%); the negative control cohort had the least amount of flap necrosis, with a mean (SD) of 50.8% (9.4%). The percentage of flap necrosis in the negative control rats (95% CI, 46.0-55.6; P < .001) was substantially lower than that for both the positive control rats (95% CI, 64.3-73.0; P < .001) and the experimental rats (95% CI, 59.9-71.8; P < .001). No statistically significant difference in flap necrosis was noted between the rats in the experimental cohort and the rats in the positive control cohort (95% CI, 59.9-71.8 vs 95% CI, 64.3-73.0; P = .46).
Conclusions and Relevance
Smoking and vaping appear to be equally detrimental to wound healing and to be associated with a statistically significant increase in flap necrosis compared with the unexposed group. The results suggest that vaping should not be seen as a better alternative to cigarette smoking in the context of wound healing.
Level of Evidence
NA.
This study investigates whether exposure to electronic cigarette vapor is less likely than exposure to cigarette smoke to lower the viability of random pattern flaps in laboratory rats.
Introduction
Smoking is a known risk to wound healing.1,2,3,4,5,6 Whenever possible, surgeons recommend smoking cessation for several months prior to a surgical procedure and often modify surgical technique for people who are active smokers.7
Vaping involves inhaling the vapor of an often flavored, nicotine-containing liquid from a battery-powered vaping pen or electronic cigarette (e-cigarette).3 Vaping creates a heavy mist, but it has been presumed to be safer than cigarette smoking and even considered a potential path to smoking cessation because it does not use tobacco in the process. However, few studies have examined whether e-cigarette vaping is safer than traditional cigarette smoking, especially in the perioperative period.
Vaping is gaining popularity among youth, first-time, and current smokers; however, evidence of its safety, particularly its association with wound healing,8,9 is inadequate. Current literature suggests that e-cigarette fluids, although they may have a decreased total number of chemicals compared with cigarettes, contain higher levels of established carcinogens, such as formaldehyde.8 The US Food and Drug Administration, which analyzed e-cigarettes, found that the fluids contain many other chemicals known to cause cancer, such as diethylene glycol.8 Other studies show that vaping pens can release heavy metals, chemicals, and glass particles, which are found in the welding material and tubing for the device.10,11 Manufacturers of e-cigarettes have not published a complete list of compounds that make up the various e-cigarette fluids.
The unfavorable association of traditional cigarette smoking with wound healing and flap viability has been well documented.2,4,5,6,12,13,14 However, the literature on how e-cigarettes affect wound healing and flap viability is lacking. In response to the call by the Food and Drug Administration for more research on e-cigarettes,15 we examined the association of e-cigarette vaping with wound healing in rats and compared this cohort with rats exposed to traditional cigarettes and with a negative control group. Specifically, our study used random flap necrosis as a model for wound healing, as did previous studies that examined wound healing in rats.2,6,14 Random pattern flaps are areas of skin that receive blood supply from cutaneous vessels, unlike axial flaps that receive blood from named arteries. Healing of random pattern flaps has been found to be impaired by the use of cigarettes in both human and murine models.2,5,6,14 This impaired healing has been associated with the vasoconstrictive actions of nicotine.12,14 However, no studies have been conducted on whether e-cigarettes similarly reduce the viability of these flaps. In this context, the primary goals of our study were (1) to detect a difference in the rate of flap necrosis (expressed as a continuous variable in the form of a percentage) between the experimental group (e-cigarette vapor–exposed) and the negative control (unexposed) and (2) to detect a difference in the rate of flap necrosis between the positive control group (traditional cigarette smoke–exposed) and the negative control.
Methods
This study was conducted from March 10, 2018, to May 4, 2018, at Boston University School of Medicine (Massachusetts). The Boston University Institutional Animal Care and Use Committee approved the protocol for this study.
Forty-five male Sprague-Dawley rats, weighing approximately 100 g at the beginning of the study, were randomized into 3 groups: negative control (n = 15), experimental (exposed to e-cigarette vapor; n = 15), and positive control (exposed to traditional cigarette smoke; n = 15). Sprague-Dawley rats were chosen because this species has a known pharmacokinetic model for calculating equivalent levels of nicotine and cotinine concentrations in humans.1,16 Exclusively male rats were used because the presence of estrogen is known to enhance flap viability.14
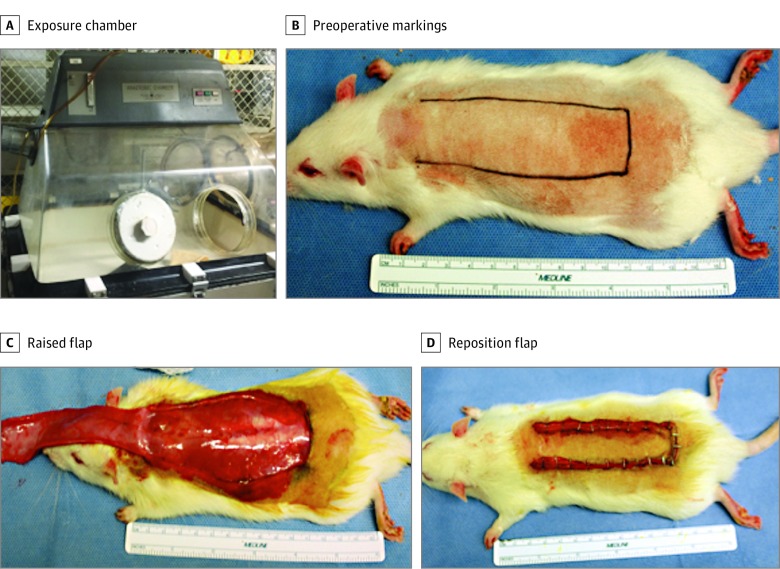
Our exposure protocol was based on previously employed methods described by Karayel et al13 and Manchio et al.14 Rats in the experimental group were exposed to e-cigarette vapor and rats in the positive control group were exposed to traditional cigarette smoke by being placed into a smoking chamber for 30 minutes twice a day for 30 consecutive days. The smoking chamber was a modified anaerobic box chamber (Figure 1) designed by the Nutrition and Cancer Biology Laboratory of the US Department of Agriculture Human Nutrition Research Center on Aging and obtained from Tufts University School of Medicine (Boston, Massachusetts).14
Figure 1. Study Design.
A, Traditional cigarette and e-cigarette exposure chamber modified from an anaerobic chamber. B, Preoperative markings for dorsal random pattern flap. C, The flap was raised, showing the undersurface. D, The flap was placed into its original position and secured with staples.
Cigarettes (Marlboro Gold; Philip Morris International) were used for the positive control group. Electronic cigarettes (Blu [Imperial Brands PLC; this brand was selected because it is the most common brand of e-cigarettes, holding approximately 45% of the market share3) with fluid containing 24 mg/mL (or 2.4%) nicotine were used for the experimental group (to convert to micromoles per liter, multiply by 6.164).
To determine the effective exposure of the animals to the smoke or vapor, we checked the serum cotinine levels with an ELISA test kit (Calbiotech; Calbiotech, Inc). Serum cotinine levels were monitored for 3 consecutive days at the start of the exposure and then twice a week using a cotinine ELISA kit to ensure comparable levels and exposures between the smoking and vaping groups. Continued exposure to cigarettes and vapor resulted in serum cotinine levels between 150 ng/mL and 200 ng/mL for both positive control and experimental groups, with the day-30 mean (SD) cotinine level before the surgical procedure being 168.3 (8.0) ng/mL for the positive control group and 178.4 (10.2) ng/mL for the experimental group (Table 1). Serum cotinine levels of 150 to 200 ng/mL were maintained because these levels have been shown to be equivalent to humans smoking approximately 1 pack per day1,16 (To convert to nanomoles per liter, multiply by 5.68).
Table 1. Physical and Physiological Characteristics.
| Cohort | Mean (SD) | |||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 Weight, g | Day 30 Weight, g | Serum Cotinine Levels, ng/mLa | ||||||
| Day 7 | Day 8 | Day 14 | Day 15 | Day 29 | Day 30 | |||
| Negative control (n = 15) | 99.9 (2.23) | 310.9 (17.4) | 1.8 (0.05) | 1.8 (0.11) | 1.6 (1.1) | 1.0 (0.44) | NA | NA |
| Positive control (n = 15) | 99.7 (2.25) | 322.7 (21.6) | 85.4 (5.5) | 106.2 (5.7) | 162.5 (9.3) | 169.9 (3.8) | 168.2 (7.0) | 168.3 (8.0) |
| Experimental (n = 15) | 100.1 (3.09) | 323.6 (25.5) | 130.6 (6.4) | 134.9 (6.5) | 159.4 (6.6) | 160.1 (5.1) | 163.3 (10.7) | 178.4 (10.2) |
Abbreviation: NA, not applicable.
SI conversion factor: To convert ng/mL to nmol/L, multiply by 5.68.
Serum cotinine levels were determined on the basis of saphenous vein blood collection and subsequent cotinine ELISA testing at various time points for 5 randomly selected rats. The goal was to stabilize cotinine levels in the experimental (exposed to e-cigarette vapor) and positive control (exposed to traditional cigarette smoke) groups between 150 ng/mL and 200 ng/mL (approximately 1 pack of cigarettes per day).
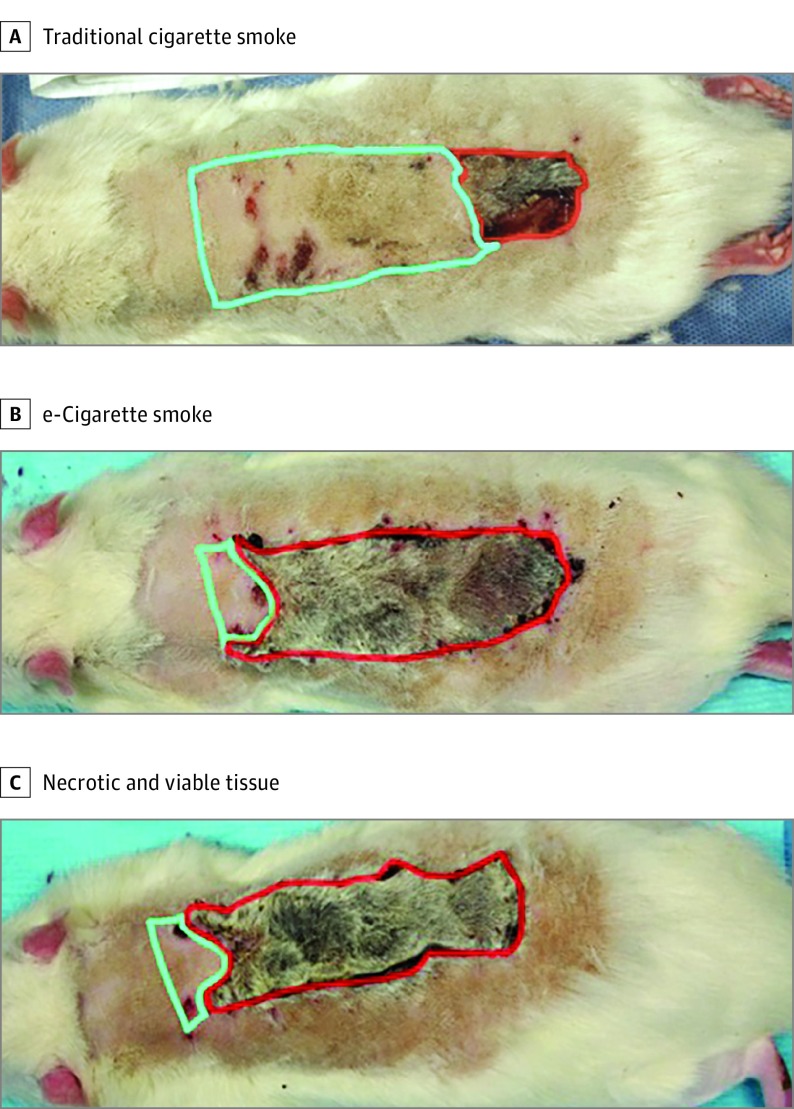
At the end of 30 days, we raised a random pattern flap on the dorsum of all animal subjects. The rats were anesthetized with isoflurane and placed prone on the operating table. The dorsal hair was shaved, and the surgical field was cleaned with povidone-iodine (Betadine; Purdue Pharma LP). A standard rostral MacFarlane 3-cm by 9-cm flap was created, including all skin layers to the underlying fascia.13,14 The flap was reapproximated to the original position using skin staples (Figure 2). The rats were kept in separate cages for 2 weeks after the procedure. Flaps were monitored on a daily basis for viability and necrosis. A flap’s viability or necrosis was determined by its appearance, turgor, and capillary refill, as described in a previous study (Figure 2).6,17
Figure 2. Representative Flaps.
A, Rat exposed to traditional cigarette smoke. B, Rat exposed to e-cigarette vapor. C, Areas of necrotic tissue outlined in red with viable tissue outlined in green. Measurements of ratio of necrotic tissue to total flap area were used to determine the percentage of flap necrosis (using OsiriX medical imaging software).
After 2 weeks, all rats were anesthetized with isoflurane and placed prone on the operating table. A digital single-lens reflex camera (Canon EOS Rebel T3i; Canon) was used to obtain images. OsiriX, version 9.5 (Pixmeo SARL) medical imaging software was used to determine the percentage of flap necrosis by precisely delineating between viable and nonviable tissues. (See eFigure in the Supplement for the study flowchart.)
Statistical Analysis
Multiple comparisons of percentage dorsal flap necrosis were done with a 1-way analysis of variance. Subsequent post hoc analysis for pairwise differences between groups used an unpaired 2-tailed t test with a Bonferroni correction to account for multiple comparisons (α<.02). All other significance levels were carried out with a 2-sided P < .05. Graphs were created and statistical analyses were performed on Microsoft Excel, version 16.16.1 (Microsoft), with the mean and SE of the percentage of dorsal flap necrosis provided.
Results
Overview
All 45 rats survived the surgical procedure and postoperative recovery, and all rats thrived and gained weight over the course of the study. Flap size remained constant across the 3 groups, with similar number of staples used in similar locations to hold the flap in place. Early attempts at autophagy of the dorsal flap were observed in all 3 groups during the recovery period and were prevented with head cones. Physical characteristics of the rat cohorts are included in Table 1.
Dorsal Skin Flap Necrosis
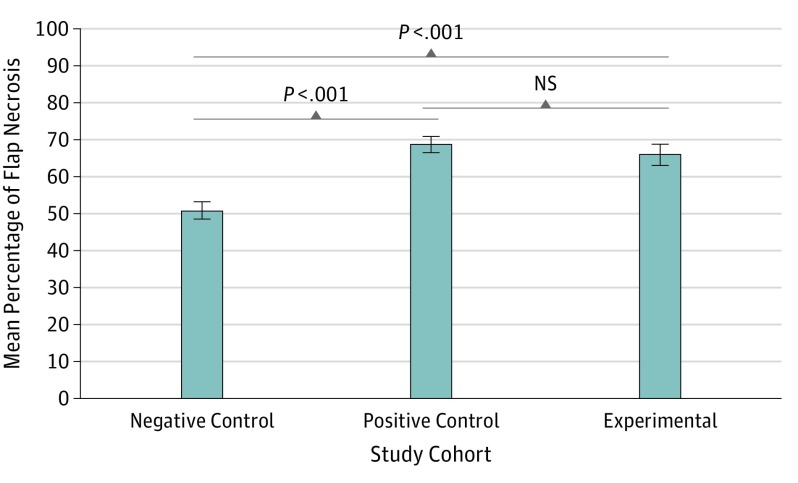
As intended by their design, all flaps demonstrated some degree of necrosis after 2 weeks. The highest level of flap necrosis was found in the positive control cohort (exposed to cigarette smoke), with a mean (SD) of 68.7% (8.6%), followed by the experimental cohort (exposed to e-cigarette vapor) with a mean (SD) of 65.9% (11.8%); the negative control cohort (unexposed) had the least amount of flap necrosis, with a mean (SD) of 50.8% (9.4%) (Table 2). The percentage of flap necrosis in the negative control rats (95% CI, 46.0-55.6; P < .001) was substantially lower than that for both the positive control rats (95% CI, 64.3-73.0; P < .001) and the experimental rats (95% CI, 59.9-71.8; P < .001) (Table 2 and Figure 3). No statistically significant difference in flap necrosis was observed between the rats in the experimental cohort and the rats in the positive control cohort (95% CI, 59.9-71.8 vs 95% CI, 64.3-73.0; P = .46) (Figure 3).
Table 2. Summary of Mean Necrotic Flap Area.
| Cohort | % Flap Necrosis, Mean (SD) [95% CI] | P Valuea |
|---|---|---|
| Negative control (n = 15) | 50.8 (9.4) [46.0-55.6] | <.001 |
| Positive control (n = 15) | 68.7 (8.6) [64.3-73.0] | <.001 |
| Experimental (n = 15) | 65.9 (11.8) [59.9-71.8] | <.001 |
The P value represents the result of a 1-way analysis of variance for multiple comparisons between groups.
Figure 3. Percentage of Random Flap Necrosis.
No statistically significant difference in flap necrosis was observed between the experimental and positive control cohorts (95% CI, 59.9-71.8 vs 95% CI, 64.3-73.0; P = .46). Error bars represent the SE of the mean. P values represent the results from a post hoc, unpaired, 2-tailed t test. Values were obtained from dividing the area of necrotic tissue by the total flap area (using OsiriX medical imaging software). NS indicates no significance.
Discussion
Recognizing the paucity of data regarding the association of e-cigarettes with human physiology, researchers have examined induced gene expression on cells exposed to traditional cigarette smoke and e-cigarette vapor. Changes in gene expression associated with oxidative stress and apoptosis, among others, were found to be higher in the group exposed to traditional cigarette smoke compared with the group exposed to e-cigarette vapor.18 Initially, this finding may indicate that e-cigarettes are less harmful overall than traditional cigarettes, but the implications of e-cigarettes for wound healing and patient outcomes remain unknown. Most of these studies point to the vasoconstrictive and immunogenic properties of nicotine as a factor in tissue ischemia and poor reconstructive surgical outcomes.13,14 Therefore, e-cigarettes, many of which use fluids with equal or higher concentrations of nicotine than contained in traditional cigarettes, could also affect wound healing in a deleterious manner.
To our knowledge, this study is the first to examine the association between e-cigarettes and wound healing. We found a substantially higher rate of flap necrosis in rats exposed to e-cigarette vapor and cigarette smoke when compared with unexposed rats. The exposed groups had the same degree of flap necrosis at similar nicotine levels; no substantial difference in wound healing was found when comparing the 2 groups, and no substantial difference in serum cotinine levels was observed. Because of the high variability in vaping brands, little data exist that compare the cotinine levels in typical vaping usage with standard cigarette usage, but Pulvers et al19 found that the cotinine levels of cigarette smokers who switched to vaping remained the same after switching. This finding has clinical relevance for controlling cotinine, as smokers who use e-cigarettes as a healthier alternative would likely receive the same nicotine dose compared with traditional cigarettes. Our findings support the theory that, regardless of the delivery system, nicotine reduces wound healing. We speculate that nicotine, because it affects cutaneous and microvascular blood distribution, is associated with flap necrosis.
This study shows a statistically significant association between flap necrosis and exposure to vapor and smoke but no association with nonexposure. The baseline mean (SD) rate of necrosis in the negative control group was relatively high at 50.8% (9.4%). Previous studies in this area that used models similar to ours have reported necrosis levels between 15% and 30%. This higher rate of flap necrosis among the negative controls in our study could be attributed to the flaps being rostrally based instead of caudally based, as seen in other investigations.14 The behavioral patterns of the rats combined with the relatively small sample size (n = 15) could have also contributed to the higher percentage of flap necrosis. Future studies in rats with larger sample sizes and that correlate the percentage of flap necrosis with gradients of e-cigarette exposure should be conducted to further validate the results from this study. In addition, clinical studies of reconstructive surgical outcomes for patients who use e-cigarettes should be done to expand on the issues brought up in this study.
Limitations
The limitation of this study relates to the generalizability of results from the rat species to humans. Although the Sprague-Dawley rat is a verified pharmacokinetic model for cigarette smoking in humans, it has not been verified for e-cigarette vaping. However, the marker of nicotine exposure, serum cotinine, was able to be reliably measured and tracked. This study used commercial cigarettes and e-cigarettes instead of a standardized research alternative. However, this alternative does not exist for e-cigarettes; although theoretically nicotine levels could vary from pack to pack of cigarettes, the serum cotinine levels were stable.
Conclusions
This study demonstrates that e-cigarette vaping appears not to be safer than cigarette smoking in the context of wound healing and that both types of exposure appear to adversely affect healing from a surgical procedure. Surgeons are advised to appropriately counsel their patients and to regard those who use e-cigarettes as having equivalent perioperative healing risk as those who smoke cigarettes.
eFigure. Study Flowchart
References
- 1.Matta SG, Balfour DJ, Benowitz NL, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl). 2007;190(3):269-319. doi: 10.1007/s00213-006-0441-0 [DOI] [PubMed] [Google Scholar]
- 2.Nolan J, Jenkins RA, Kurihara K, Schultz RC. The acute effects of cigarette smoke exposure on experimental skin flaps. Plast Reconstr Surg. 1985;75(4):544-551. doi: 10.1097/00006534-198504000-00018 [DOI] [PubMed] [Google Scholar]
- 3.Scheffler S, Dieken H, Krischenowski O, Förster C, Branscheid D, Aufderheide M. Evaluation of e-cigarette liquid vapor and mainstream cigarette smoke after direct exposure of primary human bronchial epithelial cells. Int J Environ Res Public Health. 2015;12(4):3915-3925. doi: 10.3390/ijerph120403915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taub PJ, Matarasso A. E-cigarettes and potential implications for plastic surgery. Plast Reconstr Surg. 2016;138(6):1059e-1066e. doi: 10.1097/PRS.0000000000002742 [DOI] [PubMed] [Google Scholar]
- 5.Craig S, Rees TD. The effects of smoking on experimental skin flaps in hamsters. Plast Reconstr Surg. 1985;75(6):842-846. doi: 10.1097/00006534-198506000-00015 [DOI] [PubMed] [Google Scholar]
- 6.van Adrichem LN, Hoegen R, Hovius SE, et al. The effect of cigarette smoking on the survival of free vascularized and pedicled epigastric flaps in the rat. Plast Reconstr Surg. 1996;97(1):86-96. doi: 10.1097/00006534-199601000-00015 [DOI] [PubMed] [Google Scholar]
- 7.Theocharidis V, Katsaros I, Sgouromallis E, et al. Current evidence on the role of smoking in plastic surgery elective procedures: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2018;71(5):624-636. doi: 10.1016/j.bjps.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 8.American Lung Association E-cigarettes and lung health. http://www.lung.org/stop-smoking/smoking-facts/e-cigarettes-and-lung-health.html. Accessed January 12, 2017.
- 9.Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students–United States, 2011-2015. MMWR Morb Mortal Wkly Rep. 2016;65(14):361-367. doi: 10.15585/mmwr.mm6514a1 [DOI] [PubMed] [Google Scholar]
- 10.Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5(2):67-86. doi: 10.1177/2042098614524430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin JW, Jo SH, Kim KH, et al. Are glass fiber particles released during the use of electronic cigarettes? development of a semi-quantitative approach to detect glass particle emission due to vaping. Environ Res. 2018;165:267-273. doi: 10.1016/j.envres.2018.04.032 [DOI] [PubMed] [Google Scholar]
- 12.Gazzalle A, Teixeira LF, Pellizzari AC, et al. Effect of side-stream smoking on random-pattern skin flap survival in rats. Ann Plast Surg. 2014;72(4):463-466. doi: 10.1097/SAP.0b013e318262395c [DOI] [PubMed] [Google Scholar]
- 13.Karayel H, Kaya B, Caydere M, Terzioğlu A, Aslan G. Prevention of unfavourable effects of cigarette smoke on flap viability using botulinum toxin in random pattern flaps: an experimental study. Plast Surg (Oakv). 2015;23(3):177-182. doi: 10.1177/229255031502300309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manchio JV, Litchfield CR, Sati S, Bryan DJ, Weinzweig J, Vernadakis AJ. Duration of smoking cessation and its impact on skin flap survival. Plast Reconstr Surg. 2009;124(4):1105-1117. doi: 10.1097/PRS.0b013e3181b5a360 [DOI] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration Vaporizers, e-cigarettes, and other Electronic Nicotine Delivery Systems (ENDS). https://www.fda.gov/tobaccoproducts/labeling/productsingredientscomponents/ucm456610.htm. Accessed January 5, 2017.
- 16.Yamazaki H, Horiuchi K, Takano R, et al. Human blood concentrations of cotinine, a biomonitoring marker for tobacco smoke, extrapolated from nicotine metabolism in rats and humans and physiologically based pharmacokinetic modeling. Int J Environ Res Public Health. 2010;7(9):3406-3421. doi: 10.3390/ijerph7093406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milch HS, Schubert SY, Hammond S, Spiegel JH. Enhancement of ischemic wound healing by inducement of local angiogenesis. Laryngoscope. 2010;120(9):1744-1748. doi: 10.1002/lary.21068 [DOI] [PubMed] [Google Scholar]
- 18.Moses E, Wang T, Corbett S, et al. Molecular impact of electronic cigarette aerosol exposure in human bronchial epithelium. Toxicol Sci. 2017;155(1):248-257. doi: 10.1093/toxsci/kfw198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pulvers K, Emami AS, Nollen NL, et al. Tobacco consumption and toxicant exposure of cigarette smokers using electronic cigarettes. Nicotine Tob Res. 2018;20(2):206-214. doi: 10.1093/ntr/ntw333 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Study Flowchart