This experiment assesses whether the application of polyethylene glycol in addition to neurorrhaphy can improve functional outcomes and synkinesis in a rat model of facial nerve injury.
Key Points
Question
Does the application of polyethylene glycol in addition to facial nerve neurorrhaphy improve functional and anatomical outcomes after facial nerve injury?
Findings
In this animal experiment, the right facial nerve of 36 rats was cut and immediately repaired using neurorrhaphy with or without the addition of polyethylene glycol. Compared with suture alone, the addition of polyethylene glycol showed no significant benefit in functional outcomes, motoneuron survival, or specificity of regrowth to target at any time points.
Meaning
The addition of polyethylene glycol to suturing may not be warranted in the surgical repair of facial nerve injury.
Abstract
Importance
Functional and anatomical outcomes after surgical repair of facial nerve injury may be improved with the addition of polyethylene glycol (PEG) to direct suture neurorrhaphy. The application of PEG has shown promise in treating spinal nerve injuries, but its efficacy has not been evaluated in treatment of cranial nerve injuries.
Objective
To determine whether PEG in addition to neurorrhaphy can improve functional outcomes and synkinesis after facial nerve injury.
Design, Setting, and Subjects
In this animal experiment, 36 rats underwent right facial nerve transection and neurorrhaphy with addition of PEG. Weekly behavioral scoring was done for 10 rats for 6 weeks and 14 rats for 16 weeks after the operations. In the 16-week study, the buccal branches were labeled and tissue analysis was performed. In the 6-week study, the mandibular and buccal branches were labeled and tissue analysis was performed. Histologic analysis was performed for 10 rats in a 1-week study to assess the association of PEG with axonal continuity and Wallerian degeneration. Six rats served as the uninjured control group. Data were collected from February 8, 2016, through July 10, 2017.
Intervention
Polyethylene glycol applied to the facial nerve after neurorrhaphy.
Main Outcomes and Measures
Functional recovery was assessed weekly for the 16- and 6-week studies, as well as motoneuron survival, amount of regrowth, specificity of regrowth, and aberrant branching. Short-term effects of PEG were assessed in the 1-week study.
Results
Among the 40 male rats included in the study, PEG addition to neurorrhaphy showed no functional benefit in eye blink reflex (mean [SEM], 3.57 [0.88] weeks; 95% CI, −2.8 to 1.9 weeks; P = .70) or whisking function (mean [SEM], 4.00 [0.72] weeks; 95% CI, −3.6 to 2.4 weeks; P = .69) compared with suturing alone at 16 weeks. Motoneuron survival was not changed by PEG in the 16-week (mean, 132.1 motoneurons per tissue section; 95% CI, −21.0 to 8.4; P = .13) or 6-week (mean, 131.1 motoneurons per tissue section; 95% CI, −11.0 to 10.0; P = .06) studies. Compared with controls, neither surgical group showed differences in buccal branch regrowth at 16 (36.9 motoneurons per tissue section; 95% CI, −14.5 to 22.0; P = .28) or 6 (36.7 motoneurons per tissue section; 95% CI, −7.8 to 18.5; P = .48) weeks or in the mandibular branch at 6 weeks (25.2 motoneurons per tissue section; 95% CI, −14.5 to 15.5; P = .99). Addition of PEG had no advantage in regrowth specificity compared with suturing alone at 16 weeks (15.3% buccal branch motoneurons with misguided projections; 95% CI, −7.2% to 11.0%; P = .84). After 6 weeks, the number of motoneurons with misguided projections to the mandibular branch showed no advantage of PEG treatment compared with suturing alone (12.1% buccal branch motoneurons with misguided projections; 95% CI, −8.2% to 9.2%; P = .98). In the 1-week study, improved axonal continuity and muscular innervation were not observed in PEG-treated rats.
Conclusions and Relevance
Although PEG has shown efficacy in treating other nervous system injuries, PEG in addition to neurorraphy was not beneficial in a rat model of facial nerve injury. The addition of PEG to suturing may not be warranted in the surgical repair of facial nerve injury.
Level of Evidence
NA.
Introduction
Various treatments for facial nerve injury exist; however, none provide full functional recovery. Limitations to functional recovery include the time required for degeneration and regrowth, number of outgrowths crossing the injury site, and nonspecific regeneration resulting in a disrupted myotopic organization in the central nervous system. Each contributes to impaired recovery and facial synkinesis after surgical repair of facial nerve injury.1 The use of polyethylene glycol (PEG) in addition to neurorrhaphy has shown promise in mitigating these limitations.2,3,4 However, the efficacy of PEG has only been evaluated in a mixed sensory and motor nerve but not in the treatment of a purely motor nerve, such as the rat facial nerve.
The PEG treatment protocol used by the laboratory of Bittner et al3,4 includes application of a calcium-free solution followed by the antioxidant methylene blue. After microsuture neurorrhaphy, the PEG solution is applied to the suture site, resulting in nonspecific fusing of the apposed neuronal membranes.3 Last, the site is bathed in a calcium-containing solution to initiate sealing of any neuronal membranes not sealed by PEG. Sequential application of these solutions acts to expel vesicles and prevent their accumulation on the axotomized ends of the nerve, inhibits calcium influx that would initiate axonal injury signaling, and likely removes the hydration barrier surrounding the axolemma, lowering the activation energy of membrane fusion.3,5 These actions have been shown to alter wallerian degeneration distal to the repair site, prevent oxidative stress, and improve recovery times.3,4,6 Polyethylene glycol has also been implicated in altering specificity of regrowth. Robinson and Madison7 observed PEG-impaired regrowth specificity after sciatic nerve injury, but other groups8 have challenged these findings.
Whether such PEG-mediated benefits will be reproducible and of therapeutic significance in treating facial nerve injury remains unknown. We hypothesize that adding PEG to neurorrhaphy will improve functional recovery, amount of axonal regrowth, and specificity of regrowth. To test this hypothesis, behavioral scoring and anatomical analysis were performed at 3 times in a rat model.
Methods
Overview of Methods
We used a total of 40 male Wistar rats (200-400 g; Envigo), including 14 in the 16-week study, 10 in the 6-week study, 10 in the 1-week study, and 6 in an uninjured control group. Animals at each point were divided into those undergoing facial nerve axotomy (FNA) and suture neurorrhaphy with or without application of PEG. The Institutional Animal Care and Use Committee of Indiana University School of Medicine approved all experimental protocols.
Data were collected from February 8, 2016, through July 10, 2017. For all surgical procedures, animals were anesthetized using 2.5% to 3.0% isoflurane in 99% oxygen at a rate of 1 L/min. All surgical sites were closed using two to three 9-mm wound clips, which were removed at 1 postoperative week. Immediately after surgery, 1 mL of buprenorphine hydrochloride was administered for pain management.
Surgical Groups
FNA and Neurorrhaphy
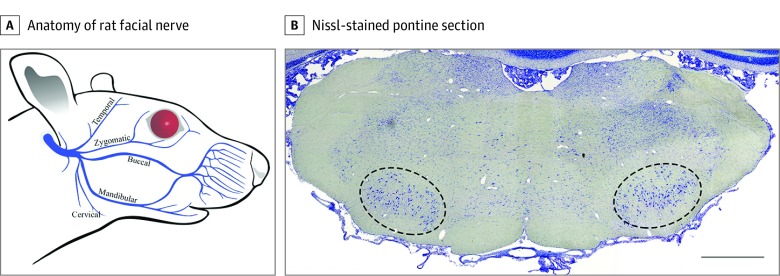
A 1.5-cm posterior-auricular incision was made on the right side of the head to expose the main trunk of the facial nerve as it exits the stylomastoid foramen. Once 5 to 6 mm of the main trunk was fully exposed, the nerve was fully transected 2 to 3 mm distal from its exit of the stylomastoid foramen (Figure 1A).9 Only the injured (right) facial motor nucleus (FMN) was quantified (Figure 1B). Neurorrhaphy was performed using two 10-0 nylon sutures (AROSurgical) with care to only pass the needle through the epineurium and prevent fraying and swelling of the nerve ending and subsequent protrusions from the suture site.
Figure 1. Anatomy of the Rat Facial Nerve and Facial Nucleus.
A, The main trunk of the facial nerve forms 5 main branches after exiting the stylomastoid foramen (adapted from Hohman et al,9 JAMA Facial Plast Surg. 2014;16(1):20-24. doi:10.1001/jamafacial.2013.1431). B, Nissl-stained pontine section shows the rat facial motor nucleus outlined bilaterally. Scale bar = 1000 μm.
Addition of PEG to Neurorrhaphy
For all repairs including PEG, before transection, the area was rinsed with a calcium-free solution (Plasma-Lyte; Baxter International, Inc). After transection, the area was rinsed a second time with the calcium-free solution, and 2 drops of methylene blue were applied to both ends of the transected nerve. The area was rinsed a third time with the calcium-free solution to improve visibility. Neurorrhaphy was then performed as described in the previous section. We applied a 500mM PEG solution (3.35 kDa; Sigma Aldrich) in sterile water to the suture site for 60 to 90 seconds. After PEG application, the area was rinsed 5 to 6 times with calcium-containing lactated Ringer solution.10,11
Functional Outcome Measures
Facial nerve axotomy was confirmed by observation of ipsilateral facial paralysis characterized by a loss of eye blink reflex, complete loss of vibrissae movement, and abnormal posterior vibrissae orientation.12 Animals in the 16- and 6-week studies were observed weekly to monitor recovery of eye blink reflex and vibrissae movement. Eye blink reflex was tested by delivery of a brief direct stream of air to the eye using a rubber bulb syringe. Recovery of eye blink reflex was defined by the ability to close the eyelid further than halfway owing to voluntary muscular contraction. To monitor recovery of vibrissae movement, animals’ heads were stabilized using a rodent restraint cone (AIMS Inc) with the end cut, allowing the head to emerge and the animal to whisk freely. Recovery of vibrissae movement was defined by the ability of vibrissae to move in synchrony during consecutive whisks.
Branch Labeling
Buccal branch labeling was performed on the 14 animals in the 16-week study. A 1-cm incision was made 5 mm dorsal to the maxilla to expose the buccal branch. Once exposed, the buccal branch was transected, and the proximal end was bathed in 2 μL of 4% fluorogold (Fluorochrome, LLC). Immediately after fluorogold application, a small piece of absorbable gelatin (Gelfoam; Pfizer) was applied to the proximal end of the nerve to sequester fluorogold to the area.
Buccal and mandibular branch labeling was performed on the 10 animals in the 6-week study. Fluorogold labeling was performed as described above. To expose the mandibular branch, a 1-cm anterior-auricular incision was made in the rostral-caudal direction. Once exposed, the mandibular branch was transected and DiI crystals (Thermo Scientific) were applied to the proximal nerve end. A small piece of absorbable gelatin was applied to the proximal end of the mandibular branch to keep the DiI in contact with the nerve.
Tissue Analysis
In all experiments, 1 week after branch labeling, animals were anesthetized using a mixture of ketamine hydrochloride and xylazine hydrochloride and perfused with phosphate-buffered saline solution followed by 4% paraformaldehyde. The brain and brainstem were removed, postfixed in 4% paraformaldehyde overnight, and transferred to a 30% sucrose solution until sectioned.
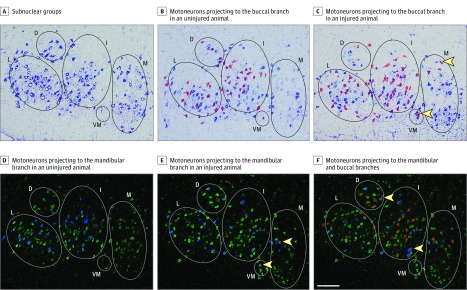
The pons from each animal was prepared for cryosectioning. Five slide sets were collected (Superfrost slides; Thermo Scientific) with sections cut at 20 μm. One slide per set was Nissl stained to allow for visualization of the entire FMN and prevent double counting. Tissue with only buccal branch labeling (16- and 1-week studies) was stained with cresyl violet, and tissue with buccal and mandibular branch labeling (6-week study) was stained with fluorescent Nissl (NeuroTrace, ThermoFisher Scientific). Fluorescent Nissl staining was used owing to cresyl violet stain ablation of DiI tracing. A map of the rat FMN was used to assess myotopic organization (Figure 2A).13 The middle 7 to 8 stained FMN sections were used for quantification. Sections were quantified if all subnuclei were visible and the map fit without resizing. Image processing and counting were performed blinded using photo editing software (Photoshop CS6; Adobe). In the 16- and 6-week studies, total number of motoneurons, number of motoneurons with buccal branch projections, and number of motoneurons with misguided projections to the buccal branch were quantified. In the 6-week study, the numbers of motoneurons with mandibular branch projections, motoneurons with misguided projections to the mandibular branch, and double-labeled motoneurons were counted.
Figure 2. Organization of the Facial Motor Nucleus.
A, Nissl-stained rat facial motor nucleus is subdivided into the following 5 main subnuclear groups: lateral (L), dorsal (D), intermediate (I), ventromedial (VM), and medial (M). B, Overlay of motoneurons projecting to the buccal branch (red) in an uninjured animal. C, Buccal branch projecting motoneurons (red) from an injured animal; yellow arrows indicate motoneurons with misguided projections. D, Overlay of motoneurons projecting to the mandibular branch (blue) in an uninjured animal. E, Mandibular branch projecting motoneurons (blue) from an injured animal; yellow arrows indicate motoneurons with misguided projections. F, Overlay of motoneurons projecting to the buccal (red) and mandibular (blue) branches; yellow arrows indicate motoneurons projecting to both branches. Scale bar = 200 μm.
Bilateral orbicularis oculi tissue from animals in the 1-week study was collected to examine neuromuscular junction innervation. Orbicularis oculi tissue was cryosectioned at 20 μm and collected on slides. Immunocytochemistry was performed on sections from the injured and uninjured sides using primary antibody (antineurofilament, 1:200 dilution; Aves Laboratories) overnight at 4°C, followed by a 1-hour incubation with a donkey antichicken secondary antibody (Alexa Fluor 488, 1:200 dilution; Jackson ImmunoResearch) and a conjugated α-bungarotoxin primary antibody (Alex-555, 1:800 dilution; Molecular Probes). All imaging was performed on an inverted fluorescent microscope (Bx50; Olympus).
Data Analysis
One-way analysis of variance using the Tukey post hoc test was performed on measures with 3 groups. Unpaired t tests were performed on measures of double-labeled motor neurons and behavior data.
Results
Functional Outcomes
Among the 40 male rats, ipsilateral facial paralysis consisting of a loss of eye blink reflex and complete loss of vibrissae movement was seen in all animals immediately after FNA. Passive eyelid movement due to retraction of the eye was observed (owing to orbicularis oculi contraction) but distinguished from active eyelid closure by video analysis. After reaching recovery, eye blink reflex on the injured side was slower and often resulted in incomplete eyelid opening after blinking compared with the uninjured side. Visible vibrissae movement typically began 2 to 3 weeks after injury and repair and consisted of independent uncoordinated vibrissae osculation. After reaching recovery, whisking function remained slow and consisted of smaller whisking amplitudes compared with the uninjured side.
In our 16-week study, the addition of PEG to neurorrhaphy resulted in a mean (SEM) time to eye blink recovery of 3.57 (0.88) weeks (95% CI, −2.8 to 1.9 weeks; P = .70), which provided no benefit beyond neurorrhaphy alone (mean [SEM] time to recovery, 4.00 [0.72] weeks; 95% CI −3.6 to 2.4; P = .69). Mean (SEM) time to recovery of whisking function in the PEG group was 5.00 (0.82) weeks, compared with 5.57 (1.20) weeks in the neurorrhaphy-alone group. In the 6-week study, not all animals reached recovery by the end of the study; thus, the mean number of weeks for recovery was not calculated. Of interest, the percentage of rats reaching recovery for eye blink reflex (2 of 5 [40%] vs 1 of 5 [20%]) and whisking (4 of 5 [80%] vs 3 of 5 [60%]) by week 6 favored the suture-only group (Table).
Table. Recovery of Function in the 6-Week Study.
| Function | Postoperative Time, wk | No. (%) Animals Recovering | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Eye blink recovery | |||||||
| Suture (n = 5) | 0 | 0 | 0 | 1 | 0 | 1 | 2 (40) |
| Suture plus PEG (n = 5) | 0 | 0 | 0 | 1 | 0 | 0 | 1 (20) |
| Whisking recovery | |||||||
| Suture (n = 5) | 0 | 0 | 0 | 0 | 2 | 2 | 4 (80) |
| Suture plus PEG (n = 5) | 0 | 0 | 0 | 0 | 1 | 2 | 3 (60) |
Abbreviation: PEG, polyethylene glycol.
Motoneuron Survival and Amount of Axonal Regrowth
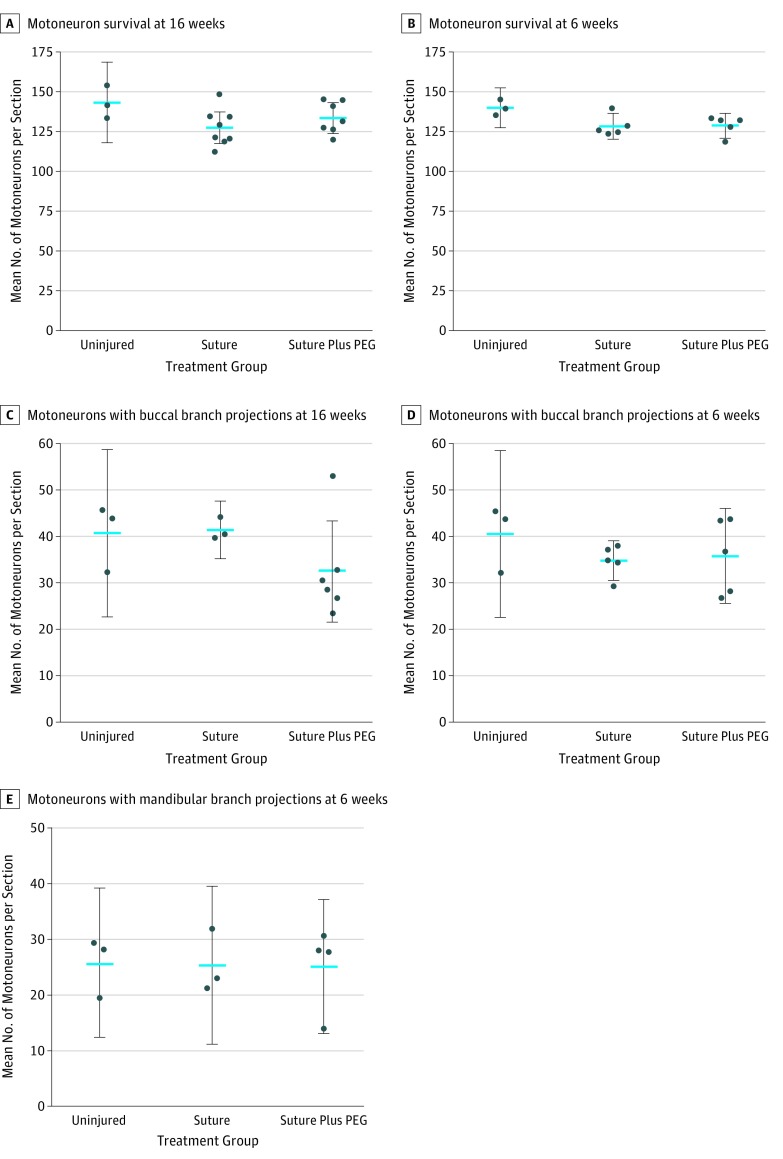
Motoneuron survival after peripheral nerve injury in Wistar rats is high, with most studies showing 75% or greater survival.14,15,16 Motoneuron survival of 90% was seen in the 16-week (mean, 132.1 motoneurons per tissue section; 95% CI, −21.0 to 8.4; P = .13) and 6-week (mean, 131.1 motoneurons per tissue section; 95% CI, −11.0 to 10.0; P = .06) studies, indicating that PEG had no added benefits or adverse effects on motoneuron survival (Figure 3A and B).
Figure 3. Motoneuron Survival and Amount of Regrowth.
Blue lines indicate mean values; error bars, 95% CIs. PEG indicates polyethylene glycol.
To examine regrowth, the number of motoneurons routing projections to the buccal branch was quantified in the 16-week study by counting the number of fluorogold-labeled motoneurons (Figure 3C). Although not significant, a decrease in the number of fluorogold-labeled motoneurons was seen in the PEG-treated animals (mean, 132.1 motoneurons per tissue section; 95% CI, −8.3 to 26.0; P = .29). Based on this decrease and plateaued functional outcomes 5 weeks after repair, our 6-week study examined axonal regrowth in the buccal and mandibular branches. Six weeks after repair, no significant differences were seen between groups (mean, 36.7 motoneurons per tissue section; 95% CI, −12 to 10; P = .48). Both surgical groups showed a decreasing number of motoneurons with buccal branch projections compared with uninjured controls, whereas the number of motoneurons with mandibular branch projections were unaltered (mean, 25.2 motoneurons per section; 95% CI, −14.0 to 15.0; P = .99) (Figure 3D and E).
Specificity of Regrowth
After injury, the myotopic organization of the FMN is disrupted.17 Disruption results from regenerating axons being misguided to incorrect targets. This miswiring contributes to a loss of coordinated muscle recruitment and facial synkinesis.18 In uninjured animals, motoneurons from the lateral, dorsal, and intermediate subnuclei projected to the buccal and mandibular branches (Figure 2B and D). After repair, motoneurons in the ventromedial and medial subnuclei also projected to the buccal and mandibular branches, contributing to disrupted myotopic organization (Figure 2C and E).
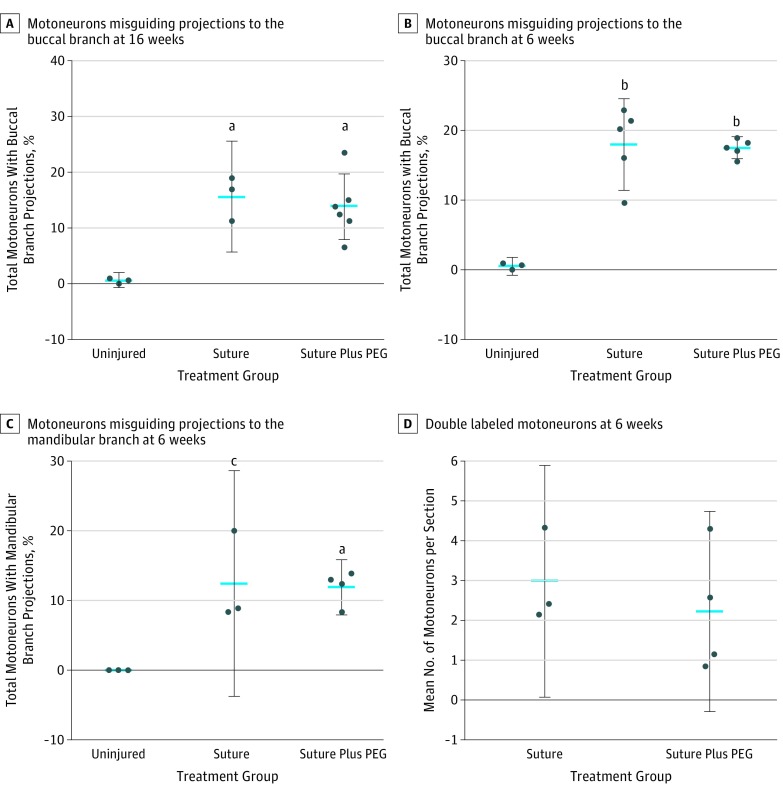
The 16- and 6-week studies showed a significant increase in the number of motoneurons with misguided projections to the buccal branch. The addition of PEG showed no benefit over suturing alone at 16 weeks (mean, 15.3% motoneurons with misguided projections; 95% CI, −7.2% to 11.0%; P = .84) and 6 weeks (mean, 17.3% motoneurons with misguided projections; 95% CI, −5.5% to 6.5%; P = .97) (Figure 4A and B). Similarly, in the 6-week study, we found a significant increase in the number of motoneurons with misguided projections to the mandibular branch in both surgical groups (mean, 8.5% motoneurons with misguided projections; 95% CI, −21.5% to −3.2%; P = .01). The addition of PEG showed no benefit beyond suturing alone (mean, 12.1% motoneurons with misguided projections; 95% CI, −8.2% to 9.2%; P = .98) (Figure 4C).
Figure 4. Misguided Regrowth and Aberrant Branching.
Blue lines indicate mean values; error bars, 95% CIs. PEG indicates polyethylene glycol.
aP < .01.
bP < .001.
cP = .01.
In the rat facial motor system, it is well established that motoneurons guide their axonal projections to only 1 branch of the facial nerve.19,20,21 We found double-labeled motoneurons, indicating motoneurons guiding outgrowths to multiple facial nerve branches (Figure 2F). These aberrant branching motoneurons are another contributor to facial synkinesis. No significant difference in aberrant branching was seen between surgical groups (mean, 2.53 motoneurons with projections to the buccal and mandibular branches; 95% CI, −3.54 to 2.06; P = .53) (Figure 4D).
Wallerian Degeneration
One of the first changes seen in the wallerian degeneration of motor axons after peripheral nerve injury is denervation of the neuromuscular junction 12 to 20 hours after injury.22,23 Neuromuscular junction reinnervation begins 3 weeks after injury, with numerous neuromuscular junctions being reinnervated by 4 weeks, but this time is highly dependent on the distance of regrowth required.24,25 To assess the association of PEG with wallerian degeneration at the level of the neuromuscular junction, innervation of orbicularis oculi tissue was evaluated from the injured and uninjured side of each animal in the 1-week study. We found fully innervated neuromuscular junctions on the uninjured side of all animals but were unable to find any innervated neuromuscular junctions on the injured side of animals in either surgical group from the 1-week study (eFigure 1 in the Supplement).
Discussion
Numerous studies have shown the efficacy of PEG in the treatment of spinal nerve and cord injury, but to our knowledge, use of PEG has not been evaluated in the repair of cranial nerve injuries.2,3,4 Based on the ability of PEG to improve functional and anatomical outcomes after other nervous system injuries, we hypothesized that PEG would improve recovery from facial nerve injury. However, the addition of PEG provided no additional benefit beyond suturing alone.
This series of studies began with the 16-week study showing no acceleration of functional recovery when PEG was added to neurorrhaphy. These functional outcome data in conjunction with the anatomical data from the 16-week study provided the basis for the 6-week study. Of interest, in the 6-week study, both functional outcome measures were impaired by the addition of PEG, resulting in fewer animals reaching recovery by week 6.
Facial synkinesis is a result of various factors after repair, including the amount of axonal regrowth, specificity of regrowth, and aberrant branching.1 Mattsson et al15 evaluated the amount of regrowth through neuronal labeling via intramuscular injection and, 6 weeks after neurorrhaphy, they found that the number of labeled facial motoneurons began to increase beyond uninjured levels and gradually increased until week 16. To assess the amount of regrowth in specific branches, branch labeling was used at 16 and 6 weeks after repair. Although not significant, the number of motoneurons projecting to the buccal branch decreased in both surgical groups 6 weeks after repair. In the 16-week study, this decrease reached control levels in the suture-only group but not in the PEG-treated group. This trend coincides with unpublished data from our laboratory (C.L.W., K.J.J., and B.L.B.; completed September 2017) that demonstrated a decrease in the number of motoneurons with buccal branch projections at 16 postoperative weeks when PEG was combined with systemic testosterone administration, which has been shown to accelerate axon regrowth.26 In the mandibular branch, the number of labeled motoneurons was similar to uninjured levels in both surgical groups by week 6. These results suggest that PEG limits the amount of long-term regrowth in some facial nerve branches. This limitation results from fewer axonal outgrowths being recruited to a branch or fewer outgrowths being maintained over time. The number of outgrowths is heavily affected by the amount of trophic influence to which the nerve branch is subjected.19 With the mandibular branch of the rat projecting to the whisker pad, upper lip, and lower lip, it may have greater trophic influence on outgrowths, resulting in the recruitment and/or maintenance of a greater number of outgrowths compared with branches such as the buccal, which projects to only 1 area.
Specificity of regrowth was evaluated 16 and 6 weeks after repair by assessing myotopic organization of the FMN and aberrantly branching motoneurons. In both surgical groups at both points, a significant disruption in the myotopic organization of the FMN occurred. In addition, similar numbers of aberrantly branching motoneurons were found in both surgical groups, denoting motoneurons routing outgrowths to the buccal and mandibular branches. These outcomes indicate that PEG offers no benefit in relation to specificity of regrowth after injury.
Previous studies have found that PEG helps to retard wallerian degeneration after injury.3 The first anatomical sign of wallerian degeneration by motoneurons is neuromuscular junction denervation; therefore, we evaluated the neuromuscular junction for signs of wallerian degeneration in our 1-week study.22,23 We found no evidence of innervated neuromuscular junctions on the injured side of either group, but fully innervated neuromuscular junctions were found on the uninjured side of all animals. Although PEG may alter degeneration of axons near the injury site, we found no evidence of PEG leading to full retardation of wallerian degeneration based on our assessment of neuromuscular junction innervation. However, this finding does not exclude the possibility of wallerian degeneration near the repair site. It is plausible that enough wallerian degeneration occurs in the nerve to reduce target musculature innervation, because the present study did not assess the extent of axonal regrowth distal to the injury site. Based on the present study, it is therefore difficult to determine whether PEG fusion and thus reduction in distal wallerian degeneration occur in our facial nerve injury model compared with what is described in sciatic nerve repair.
Limitations
Functional and anatomical outcomes of the present study demonstrate that PEG provides no benefit beyond neurorrhaphy alone in the surgical repair of facial nerve injury, but many questions remain unanswered. Although PEG fusion may limit the number of outgrowths, polyinnervation of the facial musculature may contribute to impaired functional outcomes. Guntinas-Lichius et al27 found polyneural innervation despite a reduction in the number of outgrowths. In the present study, polyneural innervation was not evaluated and may further elucidate the association of PEG with anatomical and functional outcomes. Also pertinent is the potential for autonomic reinnervation of skeletal muscle, which not only has the potential to affect recovery of whisking function, but may have implications in polyneural innervation seen when the number of outgrowths after repair is decreased.28 We did not histologically evaluate the suture site or distal nerve after repair, and to our knowledge, no studies using PEG have done so. Thus, we cannot rule out the possibility that the nonspecific fusing of the neuronal membranes via PEG may produce a physical barrier altering regrowth. Future studies are needed to examine this possibility and further clarify the effect of PEG on neuronal regeneration. Last, future studies should also evaluate regrowth in other branches of the facial nerve. This evaluation will help to determine whether PEG affects regrowth in branches with various distances to target innervation.
One limitation of the facial nerve injury model compared with a sciatic model is the size of the nerve. In the sciatic nerve, PEG fusion is confirmed by compound action potential measurement. The rat facial nerve model does not allow for compound action potential measurements owing to anatomical restrictions.
Conclusions
Polyethylene glycol fusion has shown efficacy in the surgical repair of spinal nerve injuries, but this finding was not replicated in facial nerve injury repair. This discrepancy is likely a result of differences between injury models. The sciatic nerve is a mixed sensory and motor nerve at the level of injury, and sciatic axons have a greater distance to regenerate before reaching their targets. Based on the findings of the present study, we do not recommend the use of PEG for the treatment of facial nerve injury because it provided no benefit beyond suturing alone.
eFigure. Immunostained Orbuclaris Oculi Tissue Sections
References
- 1.Crumley RL. Mechanisms of synkinesis. Laryngoscope. 1979;89(11):1847-1854. [DOI] [PubMed] [Google Scholar]
- 2.Riley DC, Bittner GD, Mikesh M, et al. Polyethylene glycol–fused allografts produce rapid behavioral recovery after ablation of sciatic nerve segments. J Neurosci Res. 2015;93(4):572-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bittner GD, Mikesh M, Ghergherehchi CL. Polyethylene glycol–fusion retards wallerian degeneration and rapidly restores behaviors lost after nerve severance. Neural Regen Res. 2016;11(2):217-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bittner GD, Sengelaub DR, Trevino RC, et al. The curious ability of polyethylene glycol fusion technologies to restore lost behaviors after nerve severance. J Neurosci Res. 2016;94(3):207-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bamba R, Riley DC, Kelm ND, Does MD, Dortch RD, Thayer WP. A novel technique using hydrophilic polymers to promote axonal fusion. Neural Regen Res. 2016;11(4):525-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo J, Borgens R, Shi R. Polyethylene glycol improves function and reduces oxidative stress in synaptosomal preparations following spinal cord injury. J Neurotrauma. 2004;21(8):994-1007. [DOI] [PubMed] [Google Scholar]
- 7.Robinson GA, Madison RD. Polyethylene glycol fusion repair prevents reinnervation accuracy in rat peripheral nerve. J Neurosci Res. 2016;94(7):636-644. [DOI] [PubMed] [Google Scholar]
- 8.Bittner GD, Sengelaub DR, Trevino RC, Ghergherehchi CL, Mikesh M. Robinson and Madison have published no data on whether polyethylene glycol fusion repair prevents reinnervation accuracy in rat peripheral nerve. J Neurosci Res. 2017;95(3):863-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hohman MH, Kleiss IJ, Knox CJ, Weinberg JS, Heaton JT, Hadlock TA. Functional recovery after facial nerve cable grafting in a rodent model. JAMA Facial Plast Surg. 2014;16(1):20-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghergherehchi CL, Bittner GD, Hastings RL, et al. Effects of extracellular calcium and surgical techniques on restoration of axonal continuity by polyethylene glycol fusion following complete cut or crush severance of rat sciatic nerves. J Neurosci Res. 2016;94(3):231-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bittner GD, Rokkappanavar KK, Peduzzi JD. Application and implications of polyethylene glycol–fusion as a novel technology to repair injured spinal cords. Neural Regen Res. 2015;10(9):1406-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kujawa KA, Kinderman NB, Jones KJ. Testosterone-induced acceleration of recovery from facial paralysis following crush axotomy of the facial nerve in male hamsters. Exp Neurol. 1989;105(1):80-85. [DOI] [PubMed] [Google Scholar]
- 13.Semba K, Egger MD. The facial “motor” nerve of the rat: control of vibrissal movement and examination of motor and sensory components. J Comp Neurol. 1986;247(2):144-158. [DOI] [PubMed] [Google Scholar]
- 14.Johnson IP, Duberley RM. Motoneuron survival and expression of neuropeptides and neurotrophic factor receptors following axotomy in adult and ageing rats. Neuroscience. 1998;84(1):141-150. [DOI] [PubMed] [Google Scholar]
- 15.Mattsson P, Janson AM, Aldskogius H, Svensson M. Nimodipine promotes regeneration and functional recovery after intracranial facial nerve crush. J Comp Neurol. 2001;437(1):106-117. [DOI] [PubMed] [Google Scholar]
- 16.Dai CF, Kanoh N, Li KY, Wang Z. Study on facial motoneuronal death after proximal or distal facial nerve transection. Am J Otol. 2000;21(1):115-118. [DOI] [PubMed] [Google Scholar]
- 17.Choi D, Raisman G. Somatotopic organization of the facial nucleus is disrupted after lesioning and regeneration of the facial nerve: the histological representation of synkinesis. Neurosurgery. 2002;50(2):355-362. [DOI] [PubMed] [Google Scholar]
- 18.Madison RD, Archibald SJ, Lacin R, Krarup C. Factors contributing to preferential motor reinnervation in the primate peripheral nervous system. J Neurosci. 1999;19(24):11007-11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Streppel M, Azzolin N, Dohm S, et al. Focal application of neutralizing antibodies to soluble neurotrophic factors reduces collateral axonal branching after peripheral nerve lesion. Eur J Neurosci. 2002;15(8):1327-1342. [DOI] [PubMed] [Google Scholar]
- 20.Dohm S, Streppel M, Guntinas-Lichius O, et al. Local application of extracellular matrix proteins fails to reduce the number of axonal branches after varying reconstructive surgery on rat facial nerve. Restor Neurol Neurosci. 2000;16(2):117-126. [PubMed] [Google Scholar]
- 21.Skouras E, Angelov DN. Experimental studies on post-transectional facial nerve regrowth and functional recovery of paralyzed muscles of the face in rats and mice. Int J Exp Clin Anat. 2010;4:1-27. [Google Scholar]
- 22.Miledi R, Slater CR. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970;207(2):507-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manolov S. Initial changes in the neuromuscular synapses of denervated rat diaphragm. Brain Res. 1974;65(2):303-316. [DOI] [PubMed] [Google Scholar]
- 24.Gonzenbach HR, Waser PG. Electron microscopic studies of degeneration and regeneration of rat neuromuscular junctions. Brain Res. 1973;63:167-174. [DOI] [PubMed] [Google Scholar]
- 25.Saito A, Zacks SI. Fine structure observations of denervation and reinnervation of neuromuscular junctions in mouse foot muscle. J Bone Joint Surg Am. 1969;51(6):1163-1178. [PubMed] [Google Scholar]
- 26.Tanzer L, Jones KJ. Neurotherapeutic action of testosterone on hamster facial nerve regeneration: temporal window of effects. Horm Behav. 2004;45(5):339-344. [DOI] [PubMed] [Google Scholar]
- 27.Guntinas-Lichius O, Irintchev A, Streppel M, et al. Factors limiting motor recovery after facial nerve transection in the rat: combined structural and functional analyses. Eur J Neurosci. 2005;21(2):391-402. [DOI] [PubMed] [Google Scholar]
- 28.Heaton JT, Sheu SH, Hohman MH, et al. Rat whisker movement after facial nerve lesion: evidence for autonomic contraction of skeletal muscle. Neuroscience. 2014;265:9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Immunostained Orbuclaris Oculi Tissue Sections