Abstract
Importance
Meta-analyses of treatments for posttraumatic stress disorder (PTSD) suggest that trauma-focused psychotherapies produce greater benefits than antidepressant medications alone.
Objective
To determine the relative efficacy of prolonged exposure therapy plus placebo, prolonged exposure therapy plus sertraline hydrochloride, and sertraline plus enhanced medication management in the treatment of PTSD.
Design, Setting, and Participants
The Prolonged Exposure and Sertraline Trial was a randomized, multisite, 24-week clinical trial conducted at the Veterans Affairs Ann Arbor Healthcare System, Veterans Affairs San Diego Healthcare System, Ralph H. Johnson Veterans Affairs Medical Center, and Massachusetts General Hospital Home Base Veterans Program between January 26, 2012, and May 9, 2016. Participants and clinicians were blinded to pill condition, and outcome evaluators were blinded to assignment. Participants completed assessments at weeks 0 (intake), 6, 12, 24, and 52 (follow-up). Participants (N = 223) were service members or veterans of the Iraq and/or Afghanistan wars with combat-related PTSD and significant impairment (Clinician-Administered PTSD Scale score, ≥50) of at least 3 months’ duration. Analyses were on an intent-to-treat basis.
Intervention
Participants completed up to thirteen 90-minute sessions of prolonged exposure therapy by week 24. Sertraline dosage was titrated during a 10-week period and continued until week 24; medication management was manualized.
Main Outcomes and Measures
The primary outcome was symptom severity of PTSD in the past month as assessed by the Clinician-Administered PTSD Scale score at week 24.
Results
Of 223 randomized participants, 149 completed the study at 24 weeks, and 207 (180 men and 27 women; mean [SD] age, 34.5 [8.3 years]) were included in the intent-to-treat analysis. Modified intent-to-treat analysis using a mixed model of repeated measures showed that PTSD symptoms decreased significantly during the 24 weeks (sertraline plus enhanced medication management, 33.8 points; prolonged exposure therapy plus sertraline, 32.7 points; and prolonged exposure therapy plus placebo, 29.4 points; β,–9.39; 95% CI, −11.62 to −7.16; P < .001); however, slopes did not differ by treatment group (prolonged exposure therapy plus placebo group, –9.39; sertraline plus enhanced medication management group, –10.37; and prolonged exposure therapy plus sertraline group, –9.99; P = .81).
Conclusions and Relevance
No difference in change in PTSD symptoms or symptom severity at 24 weeks was found between sertraline plus enhanced medication management, prolonged exposure therapy plus placebo, and prolonged exposure therapy plus sertraline.
Trial Registration
ClinicalTrials.gov Identifier: NCT01524133
This randomized clinical trial compares the relative efficacy of prolonged exposure therapy plus placebo, prolonged exposure therapy plus sertraline hydrochloride, and sertraline plus enhanced medication management in the treatment of posttraumatic stress disorder among combat veterans.
Key Points
Question
How do prolonged exposure therapy, sertraline hydrochloride, and their combination compare with regard to reducing the severity of posttraumatic stress disorder symptoms during 24 weeks of treatment?
Findings
This randomized clinical trial showed that, in a modified intent-to-treat analysis (n = 207) using a mixed model of repeated measures, the severity of posttraumatic stress disorder symptoms decreased significantly during the 24 weeks of treatment; however, slopes did not differ by treatment arms and at 24 weeks.
Meaning
No difference in change in posttraumatic stress disorder symptoms or symptom severity at 24 weeks was found across the 3 groups of sertraline plus enhanced medication management, prolonged exposure plus placebo, and prolonged exposure plus sertraline.
Introduction
Clinical practice guidelines for posttraumatic stress disorder (PTSD) have presented both trauma-focused psychotherapies and selective serotonin reuptake inhibitors (SSRIs) as effective, strongly recommended treatments.1,2,3 The American Psychological Association4 and the Veterans Affairs (VA) and Department of Defense recommended trauma-focused psychotherapy vs medication for the treatment of PTSD1 based on meta-analyses comparing effect sizes across studies that rarely involved direct head-to-head comparisons of psychotherapy vs medication.5,6 Without direct comparisons, effect sizes across studies may not accurately reflect efficacy, owing to differences in study designs and comparators. Furthermore, although combined medication and psychotherapy is the most common treatment practice for veterans with PTSD,7 current guidelines are unable to make specific recommendations.8 The few extant comparisons of trauma-focused psychotherapy vs SSRIs or combined treatment have significant limitations in design or generalizability or have focused on refractory conditions or augmentation strategies.9,10,11,12,13,14
The present study was designed to address these critical gaps in guidance for clinicians, especially those who serve military service members and veterans. The study provides a comparison of 2 effective treatments for PTSD—prolonged exposure therapy and sertraline hydrochloride—and whether their combination enhances either treatment alone. Prolonged exposure therapy was selected owing to the abundance of research supporting its efficacy.1,15 Of the 2 SSRIs approved by the US Food and Drug Administration for the treatment of PTSD,1 sertraline is generally tolerated better than paroxetine hydrochloride and has more robust data on long-term efficacy.5,16 To control for placebo effects and nonspecific effects of therapy (eg, therapist alliance or consistency of administration), prolonged exposure therapy was combined with pill placebo or sertraline (double-blinded), and sertraline was administered using a manualized enhanced medication management protocol.17 In this context, sertraline and prolonged exposure therapy plus sertraline were administered under matched conditions, with psychotherapists and pharmacotherapists administering treatment modalities according to manualized protocols, under expert supervision. We examined the relative efficacy of prolonged exposure therapy plus placebo, prolonged exposure therapy plus sertraline, and sertraline plus enhanced medication management among 223 veterans with combat-related PTSD on our primary outcome of PTSD severity as assessed by blinded clinicians18 and on our secondary outcomes of clinically meaningful change, remission, response, and self-reported PTSD.19
Based on previous studies,20 we hypothesized that larger reductions in symptom severity would be achieved with prolonged exposure therapy plus sertraline than with prolonged exposure therapy plus placebo and that larger reductions in symptom severity would be achieved with prolonged exposure therapy plus placebo than with sertraline plus enhanced medication management. Finally, based on concerns that sertraline might interfere with learning and reducing symptom severity using prolonged exposure therapy, we hypothesized that treatment dropout in the group treated with prolonged exposure therapy plus sertraline would be greater than in either the group treated with sertraline plus enhanced medication management or the group treated with prolonged exposure therapy plus placebo.
Methods
Design
The Prolonged Exposure and Sertraline Trial (PROGrESS) is a randomized clinical trial approved by the institutional review boards at the Veterans Affairs Ann Arbor Healthcare System, the Veterans Affairs San Diego Healthcare System, the Ralph H. Johnson Veterans Affairs Medical Center, and the Massachusetts General Hospital Home Base Veterans Program and the Department of Defense Human Research Protection Office. The study is registered at ClinicalTrials.gov, and the trial protocol is available in Supplement 1. A data safety and monitoring board reviewed the conduct of the study. Participants provided written informed consent before enrollment. Participants and clinicians were blinded to pill condition through week 24, and independent evaluators were blinded to treatment assignments for the duration of the study.
Participants
Participants were recruited from the following 4 sites: the Veterans Affairs Ann Arbor Healthcare System, the Veterans Affairs San Diego Healthcare System, the Ralph H. Johnson Veterans Affairs Medical Center, and the Massachusetts General Hospital Home Base Veterans Program. Inclusion criteria were service members or veterans of the Iraq or Afghanistan wars with combat-related PTSD and significant impairment (Clinicians-Administered PTSD Scale [CAPS]5 score, ≥50) of at least 3 months’ duration. Exclusion criteria were the following: (1) current, imminent risk of suicide; (2) active psychosis; (3) alcohol or substance dependence (in the past 8 weeks); (4) inability to attend weekly appointments for the treatment period; (5) prior intolerance to or failure of adequate trial of prolonged exposure therapy or sertraline; (6) medical illness likely to result in imminent hospitalization or contraindication to study treatments; (7) serious cognitive impairment (eg, confusion or inability to track discussion); and (8) concurrent use of antidepressants or antipsychotics, benzodiazepines, prazosin hydrochloride, and sleep agents (eg, zolpidem tartrate), which were allowed if the dosage was stable for 2 weeks by baseline. Veterans with mild traumatic brain injury were not excluded.
Procedures
Full details of the study methods, selection of participants, randomization, blinding, and outcome assessments are published elsewhere.17 Key procedures are reviewed here. Veterans and service members recruited between January 26, 2012, and May 9, 2016, were assessed with a review of their medical records, CAPS,5 and the Mini International Neuropsychiatric Interview.21 Once eligibility was determined, randomization (with masked allocation) occurred using a secure centralized interactive web-based application (Treatment Assignment Tool; University of Michigan). Randomization was stratified by site with treatment assignments randomly permuted in varying block sizes within the site.
Maintenance of the blinding was prioritized. All pills were encapsulated to protect the blinding. All evaluators were blinded to both medication and therapy assignments. Only 19 unblinding incidents occurred, with an alternate evaluator assigned for those cases. Independent evaluators completed training and achieved 90% or more agreement on CAPS prior to conducting assessments. Interrater reliability was conducted throughout the study period on 20% of randomly selected taped CAPS and Mini International Neuropsychiatric Interview assessments. Correlations on the CAPS ranged from 0.98 to 0.99, and the percentage agreement for Mini International Neuropsychiatric Interview diagnostic outcomes was 85% to 100%, with a κ coefficient of 0.86 for major depressive episode and 0.85 for generalized anxiety disorder. All raters attended fidelity calls to ensure consistency of rating across sites and over time. Calls occurred bimonthly for CAPS and annually for the Mini International Neuropsychiatric Interview. After completion of week 24 outcome measures, patients and clinicians were unblinded, and participants were offered open prolonged exposure therapy and/or sertraline or treatment outside of the study. Participants received $50 per assessment for weeks 0 (intake), 6, 12, 24, and 52.
Measures
Self-report and clinician-administered clinical measures occurred at weeks 0 (intake), 6, 12, 24, 36, and 52. Blinding was broken at week 24.
The primary outcome was severity of PTSD symptoms in the past month measured by the CAPS,5 a clinician interview assessing symptom severity and diagnostic status. Current severity of PTSD symptoms was assessed in relation to targeting the most distressing war zone trauma. The DSM IV-TR CAPS version22 was used, as the DSM-523 was not available at study initiation.
The secondary outcome was self-reported symptoms of PTSD (PTSD Checklist [PCL] Specific Stressor Version),19 clinically meaningful change, response, and remission. Clinically meaningful change was defined as a reduction of 20 points or more in the CAPS score or a CAPS score of 35 or less, response was defined as a reduction of 50% or more in CAPS score, and remission was defined as a CAPS score of 35 or less; all definitions are based on week 24 or last observed CAPS score up to week 24.
Treatment
Active treatment began at week 0 and was maintained through week 24. Sertraline therapy was titrated through week 10 and continued until week 24. Early response was defined as 2 consecutive PCL scores below 28. Enhanced medication management elements ended at week 12 or with early response. Previous investigations24,25 and recent evidence26 support these criteria, documenting 18% of individuals with military-related PTSD as early responders to prolonged exposure therapy.
Prolonged Exposure Therapy
Participants were scheduled for 13 standard, 90-minute prolonged exposure therapy sessions by week 12 and were allowed to complete all sessions by week 24. Prolonged exposure therapy sessions included recorded sessions and in vivo exposure homework.27 All study therapists were trained with a Veterans Affairs prolonged exposure therapy 4-day workshop and demonstrated fidelity on at least 2 supervised cases. Prolonged exposure therapy fidelity was ensured via structured weekly supervision telephone calls and independent audio recording of a random 20% of sessions (381 sessions). The therapy staff were 15 certified therapists across 4 study sites (3 from the Veterans Affairs Ann Arbor Healthcare System, 2 from the Veterans Affairs San Diego Healthcare System, 5 from the Ralph H. Johnson Veterans Affairs Medical Center, and 5 from the Massachusetts General Hospital Home Base Veterans Program). The mean (SD) number of prolonged exposure therapy cases per therapist was 8.7 (7.7) (median number, 6; range, 1-30). The analyzed fidelity measure consisted of 22 items per session, assessing prolonged exposure therapy components and therapist behaviors, and components or prescriptions not related to prolonged exposure therapy. All sites achieved a mean fidelity per session of at least 94%.
Pharmacotherapy
Medication doses were flexibly adjusted between 50 and 200 mg/d, with the last dosage increase at week 10 to ensure stable dosing by week 12. Medication was continued until week 24. Medication management (sertraline or placebo) was fully manualized to standardize pharmacotherapy delivery as brief (approximately 15 minutes) medication management, when administered alongside prolonged exposure therapy, or as enhanced medication management. Enhanced medication management was approximately 30 minutes for those randomized to receive sertraline alone to balance time, psychoeducation, and clinician support compared with prolonged exposure therapy conditions.17 Thus, enhanced medication management added 15 minutes of psychoeducation and/or active listening to the 15-minute routine medical management. Both medication management and enhanced medication management included clear instructions to not talk about the trauma details, included elements of exposure, or gave guidance on addressing certain PTSD-specific symptoms, such as avoidance. Prior to participation, pharmacotherapists were trained and certified on the manual and study procedures, and they participated in cross-site monthly supervision. Enhanced medication management and medication management sessions were recorded, and a randomly selected 20% were rated for fidelity and avoidance of proscribed elements of prolonged exposure therapy. Overall adherence across conditions was 96.7%.
Statistical Analysis
The primary analytic cohort is a modified intent-to-treat cohort, excluding veterans who consented but who were not dispensed any medication or placebo. The study design had 82% power to detect a 0.48 standardized effect size (corresponding to a mean [SD] difference of 11.4 [24.0] points in CAPS score) between prolonged exposure therapy plus placebo and sertraline plus enhanced medication management, and between prolonged exposure therapy plus placebo and prolonged exposure therapy plus sertraline at 24 weeks (primary end point) based on 2-sided .025-level tests using a longitudinal data model.17 The α was chosen at .025 to account for 2 comparisons of interest.
To compare week 24 outcomes and pace of recovery, we used a mixed model of repeated measures with week 0, 6, 12, and 24 assessments as dependent variables, and with indicators for sertraline plus enhanced medication management and for prolonged exposure therapy plus sertraline, ln (time), interactions of ln (time) by indicators for sertraline plus enhanced medication management and for prolonged exposure therapy plus sertraline and study sites (stratification factor) as predictors. In the CAPS model, log-transformed time was used to model nonlinear slopes of time, and the interaction term of ln (time) by group was used to test for treatment effects on the rate of symptom changes over time. The model included random intercepts and slopes with autoregressive covariance structure, and, based on the model, predicted mean CAPS scores at week 24 were compared between 2 pairs of treatment groups. We examined the extent and pattern of missing data and used logistic regression model to evaluate baseline factors predictive of missing week 24 CAPS score and included them as covariates in sensitivity analysis. For the PCL, polynomial terms of time were included to model curvilinear trends. We examined adherence to treatment assignment (retention), with adherence to medication defined as taking medication or placebo at week 24, and adherence to prolonged exposure therapy defined as completing 13 therapy sessions within 24 weeks. Early responders were considered adherent to treatment. Treatment adherence was defined for combination therapy (eg, prolonged exposure therapy plus placebo) as completion of both therapies. Binary outcomes included remission, response, and clinically meaningful change, and they were compared across treatment groups using logistic regression models, adjusting for site, baseline CAPS score, and sex.
Results
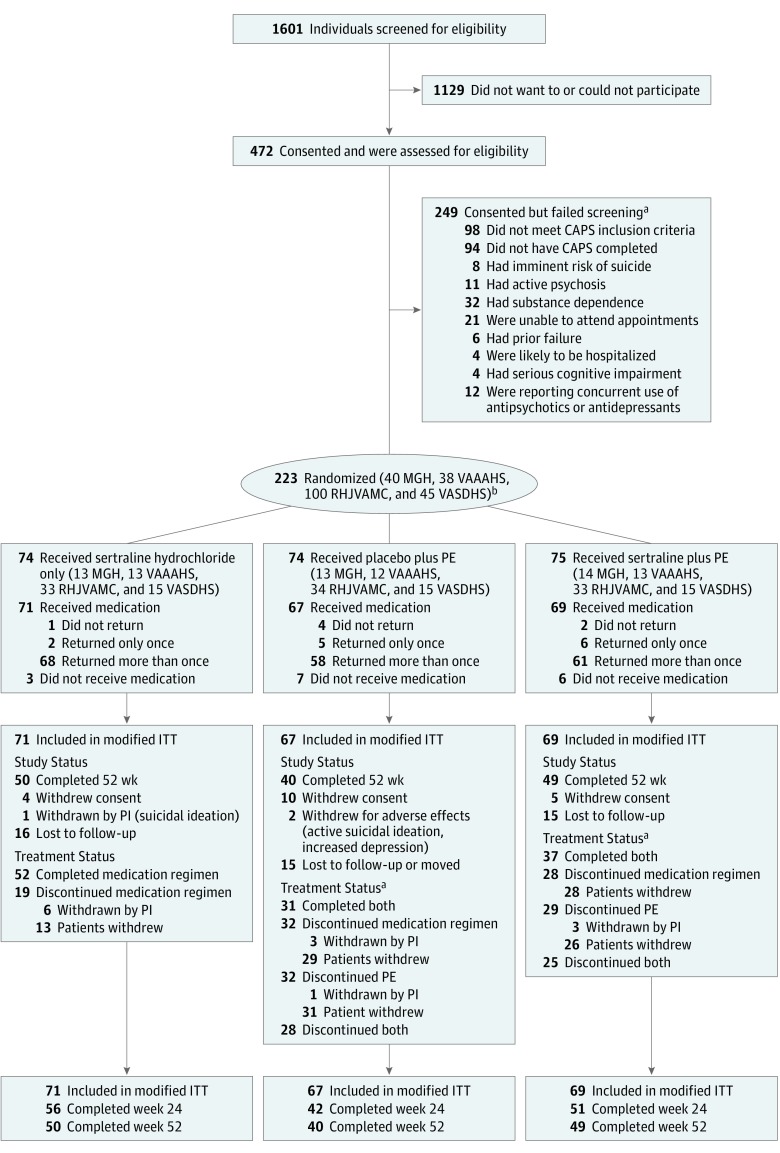
Figure 1 shows the CONSORT diagram; 472 participants underwent eligibility assessments after providing informed consent, 223 were randomized, and 207 participants (33, 34, 95, and 45 at each of the 4 sites) were dispensed medication (primary intent-to-treat cohort). After flexible dosage titration to tolerability and response, the mean (SD) week 12 sertraline hydrochloride dosage was 170.7 (46.9) mg/d for the sertraline plus enhanced medication management group, 171.6 (45.0) mg/d for the sertraline plus prolonged exposure therapy group, and 197.4 (11.3) mg/d for the prolonged exposure therapy plus placebo group (P < .001). The week 12 dosage for prolonged exposure therapy plus placebo differed from the 2 sertraline groups combined (P < .001). As previously noted, concurrent treatment with antidepressants or antipsychotics, benzodiazepines, prazosin, or sleep agents (eg, zolpidem) was allowed if the dosage was stable for 2 weeks. At baseline, the difference in concomitant psychiatric medications was significant across groups: allowed psychiatric medications at stable dosages were present in 9 of 71 patients (12.7%) in the sertraline plus enhanced medication management group, 20 of 67 patients (29.9%) in the prolonged exposure therapy plus placebo group, and 16 of 69 patients (23.2%) in the sertraline plus prolonged exposure therapy group (P = .04).
Figure 1. CONSORT Diagram of Participants in the Prolonged Exposure and Sertraline Trial.
CAPS indicates Clinician Administered PTSD Scale; ITT, intent to treat; LTF, lost to follow-up; MGH, Massachusetts General Hospital Home Base Veterans Program; PE, prolonged exposure therapy; PI, principal investigator; PTSD, posttraumatic stress disorder; RHJVAMC, Ralph H. Johnson Veterans Affairs Medical Center; VAAAHS, Veterans Affairs Ann Arbor Healthcare System; and VASDHS, Veterans Affairs San Diego Healthcare System.
aSome individuals had more than 1 exclusion.
bGroups not mutually exclusive.
Modified Intent-to-Treat Cohort
Patient characteristics were comparable across groups, except for sex, marital status, and baseline function (Table 1). The prolonged exposure therapy plus sertraline group had fewer men and fewer married participants. Completion of week 24 CAPS did not differ significantly across treatment groups (56 of 71 [78.9%] in the sertraline plus enhanced medication management group, 42 of 67 [62.7%] in the prolonged exposure therapy plus placebo group, and 51 of 69 [73.9%] in the sertraline plus prolonged exposure therapy group; P = .10).
Table 1. Baseline Demographic Characteristics of Enrolled Intent-to-Treat Cohort.
| Characteristic | Participants With PTSD, No. (%) | |||
|---|---|---|---|---|
| Sertraline Hydrochloride (n = 71) | PE Plus Placebo (n = 67) | PE Plus Sertraline (n = 69) | Total (N = 207) | |
| Age, mean (SD), y | 33.7 (8.2) | 34.7 (8.3) | 35.1 (8.5) | 34.5 (8.3) |
| Male sex | 66 (93.0) | 59 (88.1) | 55 (79.7) | 180 (87.0) |
| Race | ||||
| White | 43 (60.6) | 36 (53.7) | 40 (58.0) | 119 (57.5) |
| Black | 20 (28.2) | 20 (29.9) | 22 (31.9) | 62 (30.0) |
| Other | 8 (11.3) | 11 (16.4) | 7 (10.1) | 26 (12.6) |
| Hispanic or Latino ethnicity | 14 (19.7) | 7 (10.4) | 10 (14.5) | 31 (15.0) |
| Marital statusa | ||||
| Married | 42 (59.2) | 36 (53.7) | 30 (43.5) | 108 (52.1) |
| Never married | 19 (26.8) | 11 (16.4) | 15 (21.7) | 45 (21.7) |
| Divorced | 8 (11.3) | 14 (20.9) | 17 (24.6) | 39 (18.8) |
| Separated | 1 (1.4) | 6 (9.0) | 7 (10.1) | 14 (6.8) |
| Educational level | ||||
| High school (or equivalent) | 31 (43.7) | 23 (34.3) | 22 (31.9) | 76 (36.7) |
| Some college (13-15 y) | 32 (45.1) | 27 (40.3) | 34 (49.3) | 93 (44.9) |
| Bachelor’s degree or above (≥16 y) | 8 (11.3) | 17 (25.4) | 13 (18.8) | 38 (18.4) |
| Work status | ||||
| Full time | 36 (50.7) | 34 (50.7) | 36 (52.2) | 106 (51.2) |
| Part time | 6 (8.5) | 9 (13.4) | 8 (11.6) | 23 (11.1) |
| Not working | 29 (40.8) | 24 (35.8) | 25 (36.2) | 78 (37.7) |
| Served in Iraq | 56 (78.9) | 53 (79.1) | 58 (84.1) | 167 (80.7) |
| Served in Afghanistanb | 32 (45.1) | 36 (53.7) | 30 (43.5) | 98 (47.3) |
| CAPS score, mean (SD)c | 75.5 (15.0) | 80.9 (13.2) | 76.0 (14.2) | 77.4 (14.3) |
| CAPS subscale B score, mean (SD) | 19.6 (6.0) | 20.6 (7.2) | 18.8 (6.7) | 19.6 (6.6) |
| CAPS subscale C score, mean (SD) | 29.0 (8.2) | 32.1 (7.1) | 29.9 (7.4) | 30.3 (7.7) |
| CAPS subscale D score, mean (SD) | 27.0 (5.0) | 28.2 (4.6) | 27.4 (4.8) | 27.5 (4.8) |
| Major depressive disorder | 43 (60.5) | 52 (77.6) | 48 (69.6) | 143 (69.1) |
| Panic disorder | 9 (12.7) | 7 (10.4) | 6 (8.7) | 22 (10.7) |
| Agoraphobia | 16 (22.5) | 14 (20.9) | 11 (15.9) | 41 (19.8) |
| Alcohol abusea | 7 (9.9) | 8 (12.9) | 6 (8.7) | 21 (10.1) |
| Substance abused | 2 (2.8) | 1 (1.5) | 2 (2.9) | 5 (2.4) |
Abbreviations: CAPS, Clinician-Administered PTSD Scale; PE, prolonged exposure therapy; PTSD posttraumatic stress disorder.
Does not include alcohol dependence because that was an exclusion criterion. Two patients from sertraline hydrochloride group have missing data. Percentages were calculated from a denominator of 207.
One participant had an unknown marital status, and 1 participant had an unknown Afghan war status. Percentages were calculated from a denominator of 207.
Total CAPS score from 17 items for the past month.
Does not include substance dependence because that was an exclusion criterion.
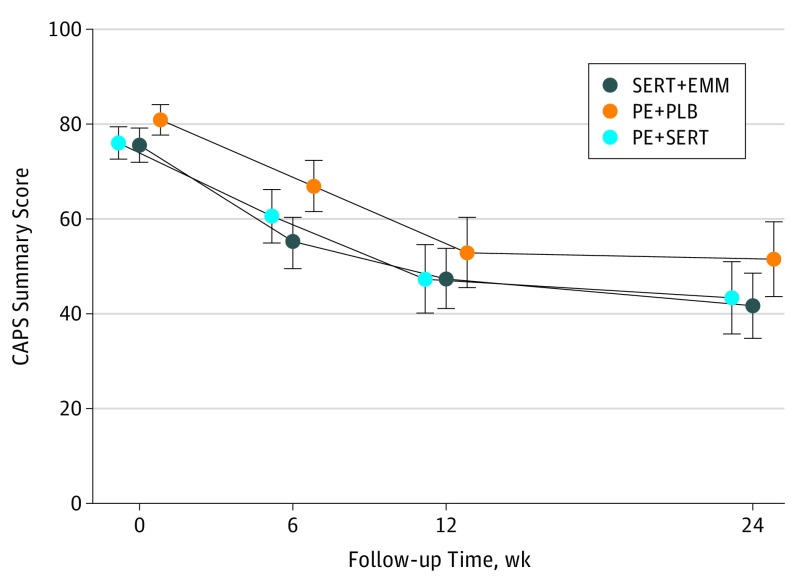
Unadjusted descriptive statistics of primary and secondary outcomes are shown in Table 2, and unadjusted mean cross-sectional CAPS scores are shown in Figure 2. Changes in unadjusted CAPS scores showed significant symptom reductions at week 24 (33.8 points for sertraline plus enhanced medication management [P < .001], 32.7 points for prolonged exposure therapy plus sertraline [P < .001], and 29.4 points for prolonged exposure therapy plus placebo [P < .001]). The primary model of longitudinally assessed CAPS scores showed no significant difference at week 24 between prolonged exposure therapy plus placebo and sertraline plus enhanced medication management (mean [SD] difference in score, 9.11 [4.65]; P = .05) or between prolonged exposure therapy plus placebo and prolonged exposure therapy plus sertraline (mean [SD] difference in score, 6.69 [4.77]; P = .16) (Table 3); the predicted mean scores were 41.9 for the sertraline plus enhanced medication management group, 51.0 for the prolonged exposure therapy plus placebo group, and 44.4 for the prolonged exposure therapy plus sertraline group. The symptoms of PTSD decreased significantly (β, –9.39; 95% CI, −11.62 to −7.16; P < .001) over 24 weeks in the prolonged exposure therapy plus placebo group, and the rate of the decrease in the CAPS scores did not differ significantly for the sertraline plus enhanced medication management group (β, –0.98; P = .52) or for the prolonged exposure therapy plus sertraline group (β, –0.60; P = .70) (Table 3; Figure 2).
Table 2. Unadjusted Summary Statistics of Primary Outcome and Secondary Outcomes During 24 Weeks.
| Outcome | Sertraline Hydrochloride Plus EMM (n = 71) | PE Plus Placebo (n = 67) | PE Plus Sertraline (n = 69) |
|---|---|---|---|
| Total CAPS score, mean (SD) | |||
| Week 0 (n = 207) | 75.5 (15.0) | 80.9 (13.2) | 76.0 (14.2) |
| Week 6 (n = 172) | 54.9 (21.9) | 66.9 (19.2) | 60.6 (20.9) |
| Week 12 (n = 159) | 47.4 (24.4) | 52.9 (24.9) | 47.3 (26.4) |
| Week 24 (n = 149) | 41.7 (25.7) | 51.5 (25.3) | 43.3 (27.2) |
| Total PCL score, mean (SD) | |||
| Week 0 (n = 207) | 56.2 (10.0) | 59.6 (9.6) | 56.6 (11.6) |
| Week 6 (n = 168) | 48.1 (14.4) | 51.5 (13.6) | 46.9 (16.2) |
| Week 12 (n = 154) | 42.8 (15.5) | 43.0 (14.7) | 40.5 (17.7) |
| Week 24 (n = 146) | 41.5 (16.6) | 42.3 (13.9) | 40.5 (19.2) |
| Remissiona | |||
| No. | 28 | 14 | 26 |
| % (95% CI) | 39.4 (28.0 to −51.7) | 20.9 (11.9 to 32.6) | 37.7 (26.3 to 50.2) |
| Responsea | |||
| No. | 29 | 18 | 26 |
| % (95% CI) | 40.8 (29.3 to 53.2) | 26.9 (16.8 to 39.2) | 37.7 (26.3 to 50.2) |
| Clinically meaningful changea | |||
| No. | 44 | 35 | 39 |
| % (95% CI) | 62.0 (49.7 to 73.2) | 52.2 (39.7 to 64.6) | 56.5 (44.0 to 68.4) |
Abbreviations: CAPS, Clinician-Administered PTSD Scale; EMM, enhanced medication management; PCL, PTSD checklist; PE, prolonged exposure therapy; PTSD, posttraumatic stress disorder.
Remission is defined as a CAPS score of 35 or less, response is defined as 50% or higher reduction in CAPS score from baseline, and clinically meaningful change is defined as a reduction of 20 points or more in the CAPS score from baseline or a CAPS score of 35 or less. All definitions are based on week 24 CAPS scores or the last observed CAPS scores if week 24 scores are missing, and participants were considered nonremitted, not responsive, and without clinically meaningful change if all follow-up CAPS scores were missing.
Figure 2. Cross-sectional Mean Scores of Clinician-Administered PTSD Scale (CAPS) Showing Change in Posttraumatic Stress Disorder Symptoms During Treatment.
PE indicates prolonged exposure therapy; PLB, placebo; PTSD, posttraumatic stress disorder; and SERT, sertraline hydrochloride. Error bars represent 95% CIs.
Table 3. Mixed-Effects Model of Primary Outcome (CAPS 17-Item Total Score) Using Follow-up Data at Weeks 6, 12, and 24 and Marginal Mean Scores at Week 24 Estimated Based on the Modela.
| Model | Coefficient (SE) | z Score | P Value | 95% CI |
|---|---|---|---|---|
| Constant | 80.76 (2.96) | 27.26 | <.001 | 74.95 to 86.57 |
| Study arm (with PE plus placebo as reference) | ||||
| Sertraline hydrochloride plus EMM | −5.95 (2.60) | −2.29 | .02 | −11.04 to −0.87 |
| PE plus sertraline | −4.74 (2.62) | −1.81 | .07 | −9.87 to 0.38 |
| Study site (with site 1 as reference) | ||||
| Site 2 | 0.84 (3.48) | 0.24 | .81 | −5.98 to 7.66 |
| Site 3 | 1.19 (2.88) | 0.41 | .68 | −4.46 to 6.83 |
| Site 4 | −0.81 (3.26) | −0.25 | .80 | −7.21 to 5.58 |
| ln (time + 1) (with PE plus placebo as reference)b | −9.39 (1.14) | −8.25 | <.001 | −11.62 to −7.16 |
| ln (time + 1) by sertraline plus EMM | −0.98 (1.52) | −0.64 | .52 | −3.96 to 2.00 |
| ln (time + 1) by PE plus sertraline | −0.60 (1.56) | −0.39 | .70 | −3.66 to 2.45 |
| Marginal CAPS mean score at week 24 | ||||
| PE plus placebo | 51.04 (3.49) | 14.64 | <.001 | 44.20 to 57.87 |
| Sertraline plus EMM | 41.93 (3.07) | 13.66 | <.001 | 35.91 to 47.94 |
| PE plus sertraline | 44.35 (3.26) | 13.62 | <.001 | 44.20 to 57.87 |
| Comparison between groups at week 24 (primary contrasts)c | ||||
| PE plus placebo vs sertraline plus EMM | 9.11 (4.65) | 1.96 | .05 | 0.01 to 18.22 |
| PE plus placebo vs PE plus sertraline | 6.69 (4.77) | 1.40 | .16 | −2.66 to 16.04 |
Abbreviations: CAPS, Clinician-Administered PTSD Scale; EMM, enhanced medication management; PE, prolonged exposure therapy; PTSD, posttraumatic stress disorder.
The model is based on CAPS scores at weeks 0, 6, 12 and 24 and had random intercepts and slopes with autoregressive covariance structure. The CAPS score was also evaluated using longer-term data by including weeks 36 and 52 and no differences in slope were found across groups (P = .83).
Time is in weeks and log-transformed to depict the pattern of decreasing symptoms at a decreasing rate seen in Figure 2. Coefficients of ln (time + 1) estimate the treatment effect as changes in symptom scores, and they do not differ between sertraline hydrochloride plus EMM vs PE plus placebo (P = .52) and between PE plus sertraline vs PE plus placebo (P = .70).
The standardized effect sizes based the between-group difference in CAPS scores are 0.38 (9.11/23.7) for PE plus placebo vs sertraline plus EMM and 0.28 (6.69/23.7) for PE plus placebo vs PE plus sertraline, where 23.7 is the common SD of the changes in CAPS score from baseline to week 24.
Secondary outcomes of self-reported symptoms of PTSD (PCL) estimated from a mixed model of repeated measures did not differ significantly across groups (eFigure in Supplement 2). The predicted mean difference in PCL scores at week 24 was 0.01 between the prolonged exposure therapy plus placebo group and the sertraline plus enhanced medication management group (P = .99) and 2.6 between the prolonged exposure therapy plus placebo group and the prolonged exposure therapy plus sertraline group 2.6 (P = .28).
Sensitivity Analysis
Missing data for the week 24 CAPS scores occurred for 15 of 71 participants (21.1%) in the sertraline plus enhanced medication management group, 25 of 67 participants (37.3%) in the prolonged exposure therapy plus placebo group, and 18 of 69 participants (26.1%) in the prolonged exposure therapy plus sertraline group. Missing data were associated with race/ethnicity and marital status, and the primary model of CAPS, adjusting for marital status and race/ethnicity, did not show a difference in the week 24 outcomes by treatment groups. The dropout rate from the blinded study medication was 26.8% (19 of 71) for the sertraline plus enhanced medication management group, 47.8% (32 of 67) for the prolonged exposure therapy plus placebo group, and 40.6% (28 of 69) for the prolonged exposure therapy plus sertraline group, with a median time of discontinuation of therapy of 12 weeks for the sertraline plus enhanced medication management group, 5 weeks for the prolonged exposure therapy plus placebo group, and 5 weeks for the prolonged exposure therapy plus sertraline group. The dropout rate was 47.8% (32 of 67) in the prolonged exposure therapy plus placebo group and 42.0% (29 of 69) in the prolonged exposure therapy plus sertraline group, with a median time of discontinuation of prolonged exposure therapy of 5 weeks in both groups. Adherence (retention) to the entire treatment condition (ie, both the prolonged exposure therapy and the pill for the prolonged exposure therapy plus placebo group and the prolonged exposure therapy plus sertraline group) differed across groups whether unadjusted or adjusted, with the highest rate of adherence in the sertraline plus enhanced medication management group (52 of 71 [73.2%]), and the lower rates of adherence in the prolonged exposure therapy plus placebo group (31 of 67 [46.3%]) and the prolonged exposure therapy plus sertraline group (37 of 69 [53.6%]) (unadjusted P = .005 and adjusted P = .006). Similar to the primary modified intent-to-treat analysis, sensitivity analysis examining the adherent subset found no differences in CAPS scores by treatment group.
Clinically Meaningful Change, Response, and Remission Outcomes
None of the dichotomized response (χ2 = 2.07; P = .36), clinical response (χ2 = 1.37; P = .50), and remission (χ2 = 3.43; P = .18) outcomes differed significantly by treatment group (Table 2) after adjusting for site, baseline CAPS score, and sex.
Discussion
This head-to-head randomized clinical trial comparing sertraline plus enhanced medication management, prolonged exposure therapy plus placebo, and prolonged exposure therapy plus sertraline was initiated to answer fundamental questions about the efficacy of these treatments alone or in combination in a population of veterans. All treatments led to significant reductions in the severity of PTSD symptoms. However, contrary to our hypotheses and findings in meta-analyses, no significant differences were observed across the 3 study groups in severity of PTSD symptoms for either clinician-assessed measures or self-report measures. These results are unlikely to be the result of type II error because the study was well powered for these comparisons. The high rates of clinically meaningful change observed among veterans in this trial (eg, ranging from 52% to 62%) are noteworthy, given the proportion of participants with chronic treatment-resistant PTSD. There were no significant differences in response rates or remission rates across treatment groups.
Although we hypothesized greater effects for combination treatment than for either treatment alone and greater effects for prolonged exposure therapy plus placebo than for sertraline plus enhanced medication management, the results that we observed were not entirely unexpected. A previous randomized clinical trial of eye movement desensitization and reprocessing vs fluoxetine showed no differences 12 weeks after treatment,12 and a study comparing a hybrid trauma-focused exposure-based acceptance and commitment therapy and medical management (sertraline supplemented with a sleep aid), or their combination, showed no significant differences after treatment.9 Finally, prolonged exposure therapy resulted in statistically higher rates of remission of PTSD compared with paroxetine, but the combination of prolonged exposure therapy and paroxetine did not differentiate from either alone.11
Importantly, this study was designed to deliver sertraline and prolonged exposure therapy plus sertraline under matched conditions that included rigorous training and ongoing supervision of psychotherapists and pharmacotherapists. To balance clinical attention and expectations, the group receiving sertraline without prolonged exposure therapy received 30 minutes of enhanced medication management, with sertraline expected to support medication adherence.1 These enhancements may have contributed to the somewhat larger effect size obtained for the sertraline plus enhanced medication management condition compared with previous medication-only trials. The present study did not include a prolonged exposure therapy without a pill condition, which resulted in increased patient burden during prolonged exposure therapy in clinical practice. Thus, the quality of the prolonged exposure therapy provided was high, but the overall effect may have been affected by the placebo. Moreover, we used 24 weeks as our primary outcome, with high levels of adherence to medication and a graded 10-week titration schedule to minimize adverse effects. This longer duration of medication management may have allowed participants to achieve greater benefit from sertraline compared with shorter trials, as previous studies have shown.28
Contrary to our hypotheses, while sertraline plus enhanced medication management performed better than expected, in the purist effectiveness comparison of prolonged exposure therapy plus sertraline vs prolonged exposure therapy plus placebo, there was no evidence for added benefit for active medication. It is possible that participants in both the prolonged exposure therapy plus placebo group and the prolonged exposure therapy plus sertraline group attributed changes to the pill, reducing motivation for exposure components. The combined prolonged exposure therapy treatments had a greater burden for participants owing to the requirement to attend 2 different appointments and more time required per week in addition to homework, which may have contributed to the higher attrition among the participants who received prolonged exposure therapy compared with the participants who received sertraline alone. The present study design allowed for early response, and the prolonged exposure therapy plus sertraline group did show significantly more early responders (13 of 69 [18.8%]) than did the other 2 groups (6 of 67 participants [9.0%] in the prolonged exposure therapy plus placebo group and 4 of 71 participants [5.6%] in the sertraline plus enhanced medication management group were early responders). However, the overall slopes of change and the results of the intent-to-treat analysis did not differ. There were significant differences in rates of adherence, with adherence being lower in both the prolonged exposure therapy plus sertraline group and the prolonged exposure therapy plus placebo group.
Limitations
Although our results are informative, limitations are apparent. Based on study design, only combat veterans were included, suggesting that an extension to other trauma populations and demographic groups that are not represented is necessary. In addition, only participants who were not currently taking an SSRI and were willing to receive prolonged exposure therapy and/or sertraline could be randomized. This restriction made recruitment challenging because many veterans with PTSD were already receiving an SSRI,7 and many veterans are unwilling to take psychotropic medication. Despite this fact, the retention rate (ranging from 46.3% [31 of 67] to 73.2% [52 of 71]) was similar to that seen in other studies of PTSD in veterans. Nonetheless, additional research needs to focus on enhancing treatment retention, including delivering prolonged exposure therapy over compressed time frames.29 Third, the enhanced medication management protocol is not standard medication management but does show excellent results. This protocol may provide a possible guide to enhance routine medication treatment and achieve the magnitude of effect found in this study.
Conclusions
In this first direct comparison of 2 of the most commonly administered treatments of PTSD (sertraline and prolonged exposure therapy) and their combination (sertraline plus prolonged exposure therapy) for veterans, we found no significant differences between the 3 treatment groups. These results require additional replication and may suggest changes to future clinical guidelines, particularly when SSRIs are administered under similar conditions to this study.
Trial protocol
eFigure. Means of PCL-S Showing Change in PTSD Over Treatment by Study Arm
Data Sharing Statement
References
- 1.US Department of Veterans Affairs Management of posttraumatic stress disorder and acute stress reaction 2017. https://www.healthquality.va.gov/guidelines/mh/ptsd/. Updated August 29, 2018. Accessed October 26, 2018.
- 2.Institute of Medicine Treatment of PTSD: an assessment of the evidence, report brief. Washington, DC: Institute of Medicine; 2007. [Google Scholar]
- 3.Forbes D, Creamer M, Bisson JI, et al. A guide to guidelines for the treatment of PTSD and related conditions. J Trauma Stress. 2010;23(5):537-552. doi: 10.1002/jts.20565 [DOI] [PubMed] [Google Scholar]
- 4.American Psychological Association Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD). https://www.apa.org/ptsd-guideline/. Accessed October 26, 2018.
- 5.Lee DJ, Schnitzlein CW, Wolf JP, Vythilingam M, Rasmusson AM, Hoge CW. Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: systemic review and meta-analyses to determine first-line treatments. Depress Anxiety. 2016;33(9):792-806. doi: 10.1002/da.22511 [DOI] [PubMed] [Google Scholar]
- 6.Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, Friedman MJ. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatry. 2013;74(6):e541-e550. doi: 10.4088/JCP.12r08225 [DOI] [PubMed] [Google Scholar]
- 7.Bohnert KM, Sripada RK, Mach J, McCarthy JF. Same-day integrated mental health care and PTSD diagnosis and treatment among VHA primary care patients with positive PTSD screens. Psychiatr Serv. 2016;67(1):94-100. doi: 10.1176/appi.ps.201500035 [DOI] [PubMed] [Google Scholar]
- 8.Haller M, Myers US, McKnight A, Angkaw AC, Norman SB. Predicting engagement in psychotherapy, pharmacotherapy, or both psychotherapy and pharmacotherapy among returning veterans seeking PTSD treatment. Psychol Serv. 2016;13(4):341-348. doi: 10.1037/ser0000093 [DOI] [PubMed] [Google Scholar]
- 9.Buhmann CB, Nordentoft M, Ekstroem M, Carlsson J, Mortensen EL. The effect of flexible cognitive-behavioural therapy and medical treatment, including antidepressants on post-traumatic stress disorder and depression in traumatised refugees: pragmatic randomised controlled clinical trial. Br J Psychiatry. 2016;208(3):252-259. doi: 10.1192/bjp.bp.114.150961 [DOI] [PubMed] [Google Scholar]
- 10.Otto MW, Hinton D, Korbly NB, et al. Treatment of pharmacotherapy-refractory posttraumatic stress disorder among Cambodian refugees: a pilot study of combination treatment with cognitive-behavior therapy vs sertraline alone. Behav Res Ther. 2003;41(11):1271-1276. doi: 10.1016/S0005-7967(03)00032-9 [DOI] [PubMed] [Google Scholar]
- 11.Popiel A, Zawadzki B, Pragłowska E, Teichman Y. Prolonged exposure, paroxetine and the combination in the treatment of PTSD following a motor vehicle accident: a randomized clinical trial—The ‘TRAKT’ study. J Behav Ther Exp Psychiatry. 2015;48:17-26. doi: 10.1016/j.jbtep.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 12.van der Kolk BA, Spinazzola J, Blaustein ME, et al. A randomized clinical trial of eye movement desensitization and reprocessing (EMDR), fluoxetine, and pill placebo in the treatment of posttraumatic stress disorder: treatment effects and long-term maintenance. J Clin Psychiatry. 2007;68(1):37-46. doi: 10.4088/JCP.v68n0105 [DOI] [PubMed] [Google Scholar]
- 13.Simon NM, Connor KM, Lang AJ, et al. Paroxetine CR augmentation for posttraumatic stress disorder refractory to prolonged exposure therapy. J Clin Psychiatry. 2008;69(3):400-405. doi: 10.4088/JCP.v69n0309 [DOI] [PubMed] [Google Scholar]
- 14.Rothbaum BO, Cahill SP, Foa EB, et al. Augmentation of sertraline with prolonged exposure in the treatment of posttraumatic stress disorder. J Trauma Stress. 2006;19(5):625-638. doi: 10.1002/jts.20170 [DOI] [PubMed] [Google Scholar]
- 15.De Jongh A, Resick PA, Zoellner LA, et al. Critical analysis of the current treatment guidelines for complex PTSD in adults. Depress Anxiety. 2016;33(5):359-369. doi: 10.1002/da.22469 [DOI] [PubMed] [Google Scholar]
- 16.Khoo AL, Zhou HJ, Teng M, et al. Network meta-analysis and cost-effectiveness analysis of new generation antidepressants. CNS Drugs. 2015;29(8):695-712. doi: 10.1007/s40263-015-0267-6 [DOI] [PubMed] [Google Scholar]
- 17.Rauch SAM, Simon NM, Kim HM, et al. Integrating biological treatment mechanisms into randomized clinical trials: design of PROGrESS (PROlonGed ExpoSure and Sertraline Trial). Contemp Clin Trials. 2018;64:128-138. doi: 10.1016/j.cct.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 18.Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8(1):75-90. doi: 10.1002/jts.2490080106 [DOI] [PubMed] [Google Scholar]
- 19.Adkins JW, Weathers FW, McDevitt-Murphy M, Daniels JB. Psychometric properties of seven self-report measures of posttraumatic stress disorder in college students with mixed civilian trauma exposure. J Anxiety Disord. 2008;22(8):1393-1402. doi: 10.1016/j.janxdis.2008.02.002 [DOI] [PubMed] [Google Scholar]
- 20.Schneier FR, Neria Y, Pavlicova M, et al. Combined prolonged exposure therapy and paroxetine for PTSD related to the World Trade Center attack: a randomized controlled trial. Am J Psychiatry. 2012;169(1):80-88. doi: 10.1176/appi.ajp.2011.11020321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(20)(suppl 20):22-33. [PubMed] [Google Scholar]
- 22.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text rev Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- 23.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 5th ed Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 24.Foa EB, Hembree EA, Cahill SP, et al. Randomized trial of prolonged exposure for posttraumatic stress disorder with and without cognitive restructuring: outcome at academic and community clinics. J Consult Clin Psychol. 2005;73(5):953-964. doi: 10.1037/0022-006X.73.5.953 [DOI] [PubMed] [Google Scholar]
- 25.de Kleine RA, Hendriks GJ, Kusters WJ, Broekman TG, van Minnen A. A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biol Psychiatry. 2012;71(11):962-968. doi: 10.1016/j.biopsych.2012.02.033 [DOI] [PubMed] [Google Scholar]
- 26.Clapp JD, Kemp JJ, Cox KS, Tuerk PW. Patterns of change in response to prolonged exposure: implications for treatment outcome. Depress Anxiety. 2016;33(9):807-815. doi: 10.1002/da.22534 [DOI] [PubMed] [Google Scholar]
- 27.Foa EB, Hembree EA, Rothbaum BO In: Barlow DH, ed. Prolonged Exposure Therapy for PTSD: Therapist Guide. New York, NY: Oxford University Press; 2007. doi: 10.1093/med:psych/9780195308501.001.0001 [DOI] [Google Scholar]
- 28.Londborg PD, Hegel MT, Goldstein S, et al. Sertraline treatment of posttraumatic stress disorder: results of 24 weeks of open-label continuation treatment. J Clin Psychiatry. 2001;62(5):325-331. doi: 10.4088/JCP.v62n0503 [DOI] [PubMed] [Google Scholar]
- 29.Harvey MM, Rauch SAM, Zalta AK, et al. Intensive treatment models to address posttraumatic stress among post-9/11 warriors: the Warrior Care Network. Focus. 2017;15(4):378-383. doi: 10.1176/appi.focus.20170022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eFigure. Means of PCL-S Showing Change in PTSD Over Treatment by Study Arm
Data Sharing Statement