Key Points
Question
What is the effect of systemic hydrocortisone, initiated between 7 and 14 days after birth, on death or bronchopulmonary dysplasia among very preterm infants receiving mechanical ventilation?
Findings
In this randomized clinical trial that included 372 infants, there was no significant difference in the composite outcome of death or bronchopulmonary dysplasia at 36 weeks’ postmenstrual age between the hydrocortisone and placebo groups (71% vs 74%, respectively).
Meaning
These findings do not support the practice of initiating hydrocortisone between 7 and 14 days after birth to reduce the risk of the composite outcome of death or bronchopulmonary dysplasia in mechanically ventilated very preterm infants.
Abstract
Importance
Dexamethasone initiated after the first week of life reduces the rate of death or bronchopulmonary dysplasia (BPD) but may cause long-term adverse effects in very preterm infants. Hydrocortisone is increasingly used as an alternative, but evidence supporting its efficacy and safety is lacking.
Objective
To assess the effect of hydrocortisone initiated between 7 and 14 days after birth on death or BPD in very preterm infants.
Design, Setting, and Participants
Double-blind, placebo-controlled randomized trial conducted in 19 neonatal intensive care units in the Netherlands and Belgium from November 15, 2011, to December 23, 2016, among preterm infants with a gestational age of less than 30 weeks and/or birth weight of less than 1250 g who were ventilator dependent between 7 and 14 days of life, with follow-up to hospital discharge ending December 12, 2017.
Interventions
Infants were randomly assigned to receive a 22-day course of systemic hydrocortisone (cumulative dose, 72.5 mg/kg) (n = 182) or placebo (n = 190).
Main Outcomes and Measures
The primary outcome was a composite of death or BPD assessed at 36 weeks’ postmenstrual age. Twenty-nine secondary outcomes were analyzed up to hospital discharge, including death and BPD at 36 weeks’ postmenstrual age.
Results
Among 372 patients randomized (mean gestational age, 26 weeks; 55% male), 371 completed the trial; parents withdrew consent for 1 child treated with hydrocortisone. Death or BPD occurred in 128 of 181 infants (70.7%) randomized to hydrocortisone and in 140 of 190 infants (73.7%) randomized to placebo (adjusted risk difference, −3.6% [95% CI, −12.7% to 5.4%]; adjusted odds ratio, 0.87 [95% CI, 0.54-1.38]; P = .54). Of 29 secondary outcomes, 8 showed significant differences, including death at 36 weeks’ postmenstrual age (15.5% with hydrocortisone vs 23.7% with placebo; risk difference, −8.2% [95% CI, −16.2% to −0.1%]; odds ratio, 0.59 [95% CI, 0.35-0.995]; P = .048). Twenty-one outcomes showed nonsignificant differences, including BPD (55.2% with hydrocortisone vs 50.0% with placebo; risk difference, 5.2% [95% CI, −4.9% to 15.2%]; odds ratio, 1.24 [95% CI, 0.82-1.86]; P = .31). Hyperglycemia requiring insulin therapy was the only adverse effect reported more often in the hydrocortisone group (18.2%) than in the placebo group (7.9%).
Conclusions and Relevance
Among mechanically ventilated very preterm infants, administration of hydrocortisone between 7 and 14 days after birth, compared with placebo, did not improve the composite outcome of death or BPD at 36 weeks’ postmenstrual age. These findings do not support the use of hydrocortisone for this indication.
Trial Registration
Netherlands National Trial Register Identifier: NTR2768
In this randomized trial, administration of hydrocortisone 7 to 14 days after birth had no effect on mortality or bronchopulmonary dysplasia (BPD) among mechanically ventilated very preterm infants compared with placebo.
Introduction
Bronchopulmonary dysplasia (BPD) is a common serious complication of preterm birth, affecting almost half of infants born at gestational ages of less than 28 weeks.1,2 Infants with BPD are more likely to die early,3 and those who survive have an increased risk of long-term pulmonary and neurodevelopmental morbidity.4,5
Pulmonary inflammation is an important risk factor in the development of BPD, providing the rationale for treating at-risk infants with corticosteroids.6 Until the early 2000s, dexamethasone was used widely because randomized clinical trials showed that dexamethasone reduced the risk of death or BPD.7,8 However, this benefit may be outweighed by an increased risk of neurodevelopmental impairment.7 As a result, clinicians started using dexamethasone less frequently, in lower doses, and at later postnatal ages.9,10 Furthermore, international guidelines recommended investigating whether hydrocortisone would be an effective and safe alternative to dexamethasone.11,12
To date, hydrocortisone has been studied only as a prophylactic treatment (ie, started at ≤7 days of life), and reports conflict on the effect on death or BPD.7 Although current practice surveys show that corticosteroid treatment is almost exclusively used after the first week of life,9,10 and hydrocortisone treatment has already been implemented in many neonatal units across the world, a clinical trial investigating the efficacy and safety of hydrocortisone treatment initiated between 7 and 14 days after birth is lacking.9,13
This multicenter randomized clinical trial tested the hypothesis that systemic hydrocortisone treatment initiated between 7 and 14 days after birth in very preterm infants receiving mechanical ventilation decreased the incidence of death or BPD at 36 weeks’ postmenstrual age.14
Methods
Study Design
This double-blind, placebo-controlled, superiority randomized trial was performed at 19 neonatal intensive care units in the Netherlands and Belgium. The human research ethics committees at each participating center approved the trial. All study sites were monitored by an independent clinical research associate. The study protocol appears in Supplement 1 and has been previously published.14
Study Population
Infants were included after written informed consent was obtained from both parents. Infants born at a gestational age of less than 30 weeks and/or with a birth weight of less than 1250 g who were ventilator dependent between 7 and 14 days’ postnatal age and at high risk of developing BPD were eligible. High risk of developing BPD was defined as having a respiratory index (product of mean airway pressure and the fraction of inspired oxygen) equal to or greater than 3.5 for more than 12 h/d for at least 48 hours. During the initial months of the trial, participating centers noted that many infants receiving ventilation and considered at high risk of BPD had a respiratory index of less than 3.5 and were treated with corticosteroids outside the trial. Based on this feedback, the respiratory index threshold was reduced to 3.0 and finally to 2.5 (in May 2012 and December 2012, respectively) via approved protocol amendments. Infants were ineligible if they had chromosomal defects or major congenital malformations or had received corticosteroids for improving lung function in the first week of life. Because ethnic background affects the risk of death or BPD,15 ethnicity as reported by the attending physician based on fixed categories was collected.
Randomization
Eligible infants were randomly allocated in a 1:1 ratio to either hydrocortisone or placebo, stratified by study center and gestational age (<27 weeks or ≥27 weeks) and using randomly permuted block sizes between 2 and 8. Multiple-birth infants were randomized independently unless the parents or caregivers explicitly requested that siblings were to be allocated to the same treatment group. The randomization sequence was generated electronically with Alea randomization software (Alea Clinical/FormsVision). The hydrocortisone and placebo vials appeared identical, and the investigators, caregivers, and parents were blinded to group assignment.16
Intervention
Infants allocated to the intervention group were given hydrocortisone sodium succinate, 5 mg/kg per day in 4 doses per day for 7 days, followed by 3.75 mg/kg per day in 3 doses per day for 5 days, subsequently lowering the frequency by 1 dose every 5 days. This resulted in 22 days of treatment with a cumulative dose of 72.5 mg/kg. Infants allocated to the control group received a placebo (mannitol) using a dosing schedule with volumes and duration similar to those of hydrocortisone. The use of open-label hydrocortisone during the trial was strongly discouraged but could be considered in infants with severe and progressive pulmonary deterioration (respiratory index >10 for more than 6 consecutive hours) or who received at least 10 days of trial medication but did not show an improvement in pulmonary condition. Trial medication was stopped when open-label hydrocortisone was initiated.
Study Outcomes
The primary outcome was a composite of death or BPD at 36 weeks’ postmenstrual age. Patients were categorized as having BPD if they required positive pressure support, required supplemental oxygen with a fraction of inspired oxygen exceeding 0.30 (severe BPD), or had a fraction of inspired oxygen of 0.22 to 0.29 and a failed oxygen reduction test (moderate BPD).17,18 In 5 infants, the indicated oxygen reduction test was not performed. For these infants, a committee of 3 independent clinical experts masked to treatment allocation assessed the severity of BPD diagnosis.
In addition to the components of the primary outcome, the following prespecified secondary outcomes were assessed: mortality at 28 days and at hospital discharge, BPD at 28 days, failure to extubate at days 3, 7, 14, and 21, total duration of mechanical ventilation and supplemental oxygen, use of open-label hydrocortisone, hospital length of stay, necrotizing enterocolitis, gastrointestinal bleeding, spontaneous intestinal perforation, intraventricular hemorrhage, periventricular leukomalacia, retinopathy of prematurity, hypertension, hyperglycemia, nosocomial infection (ie, sepsis, pneumonia, meningitis), patent ductus arteriosus, and growth as assessed by body weight and head circumference at 36 weeks’ postmenstrual age. In addition, long-term health and developmental outcomes will be assessed at 2 years’ corrected age but are not reported in this article. More details on definitions can be found in the published statistical analysis plan, available in Supplement 2.16
Suspected serious adverse drug reactions and serious adverse events were reported to the data and safety monitoring board and the trial’s principal investigator.
Statistical Analysis
Previous studies comparing dexamethasone treatment initiated between 7 and 14 days after birth with placebo in very preterm infants undergoing mechanical ventilation have shown a 25% absolute risk difference in the primary outcome of death or BPD in favor of dexamethasone.19 Ideally, the treatment effect of hydrocortisone should be similar to dexamethasone to consider it a good alternative. If safety is improved, a slightly reduced treatment effect of hydrocortisone might also change clinical practice. For this reason, the target sample size of 175 patients in each treatment group was based on an estimated probability of death or BPD of 0.60 in the placebo group, a 15% absolute risk reduction with hydrocortisone treatment, 80% power, and a 2-tailed type I error of .05. Anticipating 10% dropout, we aimed to include 200 infants in each group.
Analyses were performed according to the intention-to-treat principle including all randomized infants regardless of protocol deviations. Crude absolute risk differences and crude odds ratios were calculated for the primary outcome and its components. For the primary outcome, a generalized linear model with a binomial distribution and identity link was used to estimate the absolute risk difference adjusted for the stratification factors. A logistic regression model correcting for the stratification factors of gestational age and study center was used to estimate the adjusted odds ratio for the primary outcome. To check the robustness of the main results, we performed preplanned sensitivity analyses including per-protocol (including only infants treated according to the study protocol) and as-treated analyses (including infants based on actual treatment received), use of an alternative BPD definition (infants receiving positive pressure or high flow with room air, classified as mild BPD), a generalized estimating equations model to account for clustering of outcomes within multiple births, and examination of potential confounding and effect modification using a multivariable model including treatment and gestational age, chorioamnionitis,20 respiratory index at randomization,21 sex,22 and multiple birth23 as preselected baseline risk factors for BPD and subgroup analyses. Preplanned subgroup analyses based on gestational age (<27 weeks vs ≥27 weeks), chorioamnionitis (yes vs no), respiratory index at randomization (less than or equal to vs greater than the median), sex (male vs female), multiple birth (single vs multiple), and study center steroid preference (hydrocortisone vs dexamethasone) were performed and statistically tested with interaction effects of the specific subgroup and treatment in logistic regression models. A post hoc mixed-effects logistic regression model with site as random effect was performed as an additional sensitivity analysis to check the robustness of the preplanned main analysis of the composite outcome.
To allow more insight into the observed differential death rates at 36 weeks postmenstrual age, post hoc sensitivity and subgroup analyses were performed.24,25 First, we studied the treatment effect in the per-protocol population. Second, we studied the treatment effect adjusted for postulated risk factors for death: gestational age,2 small for gestational age (<10th percentile of the Fenton growth charts),26,27 severity of lung disease as measured by respiratory index,21 sex,22 and multiple birth.23 Third, exploratory subgroup analyses were performed using these risk factors. Fourth, time to death was estimated for both treatment groups using a Kaplan-Meier survival curve and compared with a log-rank test. The proportionality assumption was established by graphical examination and use of a time-dependent covariate in a Cox model.
The effect of hydrocortisone compared with placebo on the occurrence of short-term secondary outcomes was analyzed using linear, logistic, or competing risk (with death occurring before the event of interest considered a competing risk) regression, as appropriate. Time to event was calculated as the time between randomization and the event of interest, death, or discharge home (censoring event), whichever occurred first.
In case of missing data, every attempt was undertaken to retrieve the data from both levels III and II (referral) hospitals because many infants were transferred back to referral hospitals once clinically stable. Because the primary outcome and most secondary outcomes were assessed before hospital discharge, we anticipated no or minimal missing values.
For all treatment effect estimators, 95% confidence intervals are presented; all analyses were performed using 2-sided tests and P<.05 was regarded as statistically significant. No adjustments for multiple comparisons were made, so secondary outcome analyses should therefore be interpreted as exploratory. For statistical analysis and computing, we used SPSS version 24 (IBM Corp) and R version 3.4.3 (R Foundation for Statistical Computing).
An independent data and safety monitoring board conducted interim analyses for safety outcomes every 3 months during the first 2 years of the study. After this period, the frequency of safety monitoring was reduced to thrice and then twice annually. The data and safety monitoring board advised on whether the trial should be stopped for safety concerns or futility according to the predefined charter.16 Hence, there was no α spending associated with the interim analyses.
Results
Study Population
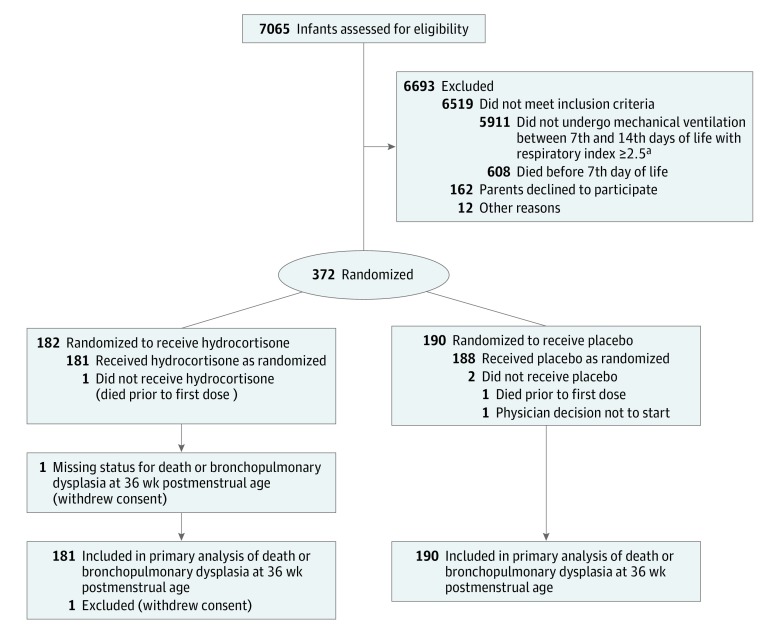
Between November 15, 2011, and December 23, 2016, 7065 infants were screened and 372 infants were randomized, 182 to the hydrocortisone group and 190 to the placebo group (Figure 1). Parents of 1 infant in the hydrocortisone group withdrew consent and this infant was excluded from the intention-to-treat analyses. Because the dropout rate was lower than expected, the minimum required sample size (n = 350) for the primary outcome analysis was attained in December 2016 and further inclusion was stopped. Follow-up to hospital discharge ended on December 12, 2017. Baseline characteristics of mothers and infants were broadly similar in both groups, with some differences in birth weight, proportion of infants small for gestational age, and multiple births (Table 1). Of the 70 multiple births in the hydrocortisone group, 33 siblings participated in the trial, of whom 29 were allocated to the same treatment group per parental request. In the placebo group, 22 siblings from 54 multiple births participated in the trial, of whom 12 were allocated to the same treatment group.
Figure 1. Recruitment, Randomization, and Participant Flow in a Trial of Hydrocortisone vs Placebo Among Very Preterm Infants Receiving Mechanical Ventilation.
aRespiratory index was defined as mean airway pressure × fraction of inspired oxygen.
Table 1. Maternal and Infant Characteristics.
| Characteristics | Hydrocortisone (n = 182) | Placebo (n = 190) |
|---|---|---|
| Maternal characteristicsa | ||
| Age, median (IQR), y | 30 (27-34) | 30 (27-34) |
| Ethnic origin, No. (%)b | ||
| Caucasian | 138 (75.8) | 140 (73.7) |
| Mediterranean | 12 (6.6) | 10 (5.2) |
| African | 13 (7.1) | 22 (11.6) |
| Asian | 7 (3.9) | 11 (5.8) |
| Latin American | 5 (2.8) | 1 (0.5) |
| Unknown | 7 (3.8) | 6 (3.2) |
| Clinical chorioamnionitis, No. (%)c | 51 (28.0) | 53 (27.9) |
| Cesarean delivery, No. (%) | 98 (53.8) | 111 (58.4) |
| Antenatal corticosteroids (any), No. (%) | 159 (87.4) | 172 (90.5) |
| Pregnancy-induced hypertension, No. (%) | 33 (18.1) | 45 (23.6) |
| Infant characteristics | ||
| Gestational age, median (IQR), wk | 25.4 (24.9-26.4) | 25.6 (24.7-26.4) |
| Birth weight, median (IQR), g | 775 (644-865) | 710 (629-810) |
| Male sex, No. (%) | 96 (52.8) | 109 (57.4) |
| Small for gestational age, No. (%)d | 26 (14.3) | 38 (20.0) |
| Multiple birth, No. (%) | 70 (38.5) | 54 (28.4) |
| Apgar 5-min score, median (IQR) | 7 (6-8) [n=180] | 7 (6-8) |
| Duration of invasive respiratory support before randomization, median (IQR), d | 9 (7-11) | 9 (7-11) |
| Age at randomization, median (IQR), d | 10 (8-13) | 11 (9-13) |
| Fraction of inspired oxygen at randomization, median (IQR) | 0.35 (0.30-0.45) | 0.34 (0.29-0.40) |
| Respiratory index at randomization, median (IQR)e | 4.3 (3.3-5.3) | 3.9 (3.1-5.0) |
Abbreviation: IQR, interquartile range.
Maternal characteristics have been summarized at the infant level (ie, women were counted multiple times if they had multiple infants).
Reported by attending physicians and selected from fixed categories.
Clinical chorioamnionitis was defined as maternal fever without other cause (eg, urinary tract infection or pneumonia), prenatal antibiotic use without source of infection other than chorioamnionitis, or preterm premature rupture of membranes.
Defined as less than the 10th percentile on the Fenton growth chart.
Respiratory index was defined as mean airway pressure × fraction of inspired oxygen.
In the per-protocol analysis for the primary outcome, 173 infants were analyzed in the hydrocortisone group and 162 in the placebo group. In the as-treated analysis, a total of 288 participants were analyzed as hydrocortisone treated vs 80 infants in the placebo group (eFigure in Supplement 3).
Primary Outcome
All participating infants were assessed for the primary outcome. In total, 73 infants (20%) died and 195 survivors (53%) were diagnosed as having BPD. The observed rate of death or BPD was 70.7% (128/181 infants) in the hydrocortisone group and 73.7% (140/190 infants) in the placebo group. After adjustment for stratification factors, the risk difference between hydrocortisone and placebo treatment for the primary outcome was −3.6% (95% CI, −12.7% to 5.4%) and the adjusted odds ratio was 0.87 (95% CI, 0.54-1.38; P = .54) (Table 2).
Table 2. Effect of Study Treatment on Primary Outcome and Secondary Outcomes From Randomization to Initial Hospital Discharge Using Intention-to-Treat Analysis.
| Outcomes | No./Total (%) | Difference, % (95% CI)a | Odds Ratio or Sub–Hazard Ratio (95% CI)b | P Value | |
|---|---|---|---|---|---|
| Hydrocortisone | Placebo | ||||
| Primary Outcome | |||||
| Death or bronchopulmonary dysplasia at 36 wk postmenstrual agec | 128/181 (70.7) | 140/190 (73.7) | |||
| Crude analysis | −3.0 (−12.0 to 6.1) | 0.86 (0.55-1.36) | |||
| Adjusted analysisd | −3.6 (−12.7 to 5.4) | 0.87 (0.54-1.38) | .54 | ||
| Components of Primary Outcome | |||||
| Death at 36 wk postmenstrual age | 28/181 (15.5) | 45/190 (23.7) | −8.2 (−16.2 to −0.1) | 0.59 (0.35-0.995)e | .048 |
| Bronchopulmonary dysplasia at 36 wk postmenstrual age | 100/181 (55.2) | 95/190 (50.0) | 5.2 (−4.9 to 15.2) | 1.24 (0.82-1.86)e | .31 |
| Other Outcomes | |||||
| Death at 28 d postnatal age | 17/181 (9.4) | 23/190 (12.1) | −2.7 (−9.1 to 3.7) | 0.75 (0.39-1.46)e | .40 |
| Death before hospital discharge | 36/181 (19.9) | 54/190 (28.4) | −8.5 (−17.1 to 0.2) | 0.63 (0.39-1.01)e | .06 |
| Bronchopulmonary dysplasia at 28 d postnatal age | 134/181 (74.0) | 151/190 (79.5) | −5.4 (−14.0 to 3.2) | 0.74 (0.45-1.20)e | .22 |
| Severity of bronchopulmonary dysplasia at 36 wk postmenstrual age in survivors | 0.89 (0.56-1.40)f | .60 | |||
| Moderate | 13/153 (8.5) | 6/145 (4.1) | 4.4 (−1.4 to 10.3) | ||
| Severe | 87/153 (56.9) | 89/145 (61.4) | −4.5 (−15.4 to 6.6) | ||
| Failure to extubate after start of study medication in survivors | |||||
| Day 3 | 146/173 (84.4) | 170/183 (92.9) | −8.5 (−15.3 to −1.9) | 0.41 (0.21-0.83)e | .01 |
| Day 7 | 92/169 (54.4) | 139/178 (78.1) | −23.7 (−32.9 to −13.8) | 0.34 (0.21-0.54)e | <.001 |
| Day 14 | 56/166 (33.7) | 86/168 (51.2) | −17.5 (−27.5 to −6.9) | 0.49 (0.31-0.76)e | .001 |
| Day 21 | 37/160 (23.1) | 50/160 (31.3) | −8.1 (−17.7 to 1.6) | 0.66 (0.40-1.09)e | .10 |
| Duration of mechanical ventilation, median (IQR), d | 11 (5, 25) | 13 (7-27) | −2 (−4 to 0)g | .12h | |
| Duration of supplemental oxygen, median (IQR), di | 61 (39-82) | 59 (40-80) | 1 (−5 to 8)g | .70h | |
| Use of open-label medication | 51/181 (28.2) | 108/190 (56.8) | −28.7 (−37.8 to −18.7) | 0.36 (0.26-0.50)j | <.001 |
| Intraventricular hemorrhage grade >2k | 2/181 (1.1) | 3/190 (1.6) | −0.5 (−3.5 to 2.5) | >.99l | |
| Periventricular leukomalaciak | 7/181 (3.9) | 9/190 (4.7) | −0.9 (−5.4 to 4.7) | .80l | |
| Patent ductus arteriosusm | 72/181 (39.8) | 78/190 (41.1) | −1.3 (−11.1 to 8.6) | 0.92 (0.68-1.27)j | .62 |
| Necrotizing enterocolitis grade ≥2n | 15/181 (8.3) | 19/190 (10.0) | −1.7 (−7.7 to 4.3) | 0.80 (0.41-1.55)j | .50 |
| Gastrointestinal bleeding | 8/181 (4.4) | 8/190 (4.2) | 0.2 (−4.2 to 4.8) | >.99l | |
| Spontaneous intestinal perforation | 4/181 (2.2) | 9/190 (4.7) | −2.5 (−6.8 to 1.5) | .26l | |
| Hypertensiono | 10/181 (5.5) | 13/190 (6.8) | −1.3 (−6.5 to 3.9) | 0.82 (0.36-1.86)j | .63 |
| Hyperglycemia requiring insulin therapy | 33/181 (18.2) | 15/190 (7.9) | 10.3 (3.5 to 17.3) | 2.44 (1.34-4.47)j | .004 |
| Sepsis, clinical or culture proven | 91/181 (50.3) | 111/190 (58.4) | −8.1 (−18.0 to 2.0) | 0.80 (0.61-1.05)j | .11 |
| Meningitis | 4/181 (2.2) | 8/190 (4.2) | −2.0 (−6.1 to 1.9) | .38l | |
| Pneumonia | 45/181 (24.9) | 64/190 (33.7) | −8.8 (−17.8 to 0.0) | 0.68 (0.47-0.997)j | .048 |
| Retinopathy of prematurity grade >2p | 44/181 (24.3) | 42/190 (22.1) | 2.2 (−6.4 to 10.8) | 1.13 (0.70-1.83)e | .62 |
| Time to hospital discharge in survivors, median (IQR), dq | 108 (93-130) | 101 (91-120) | 5 (1 to 11)g | .10h | |
| Weight at 36 wk postmenstrual age in survivors, mean (SD), gr | 2235 (395) | 2125 (468) | 109 (9 to 209)s | .03b | |
| Head circumference at 36 wk postmenstrual age in survivors, mean (SD), cmt | 30.8 (1.6) | 30.6 (1.9) | 0.3 (−0.1 to 0.7)s | .18b | |
| Length at 36 wk postmenstrual age in survivors, mean (SD), cmu | 42.7 (3.8) | 42.3 (3.2) | 0.4 (−0.5 to 1.4)s | .70b | |
Abbreviation: IQR, interquartile range.
Data are percentages unless otherwise indicated. Crude data are given unless otherwise indicated.
Linear, logistic, or competing risk regression analysis as appropriate. Crude data are given unless otherwise indicated.
Bronchopulmonary dysplasia was defined as requiring positive pressure support, requiring supplemental oxygen with a fraction of inspired oxygen exceeding 0.30 (severe BPD), or having a fraction of inspired oxygen of 0.22 to 0.29 with a failed oxygen reduction test (moderate BPD).16,17
For the primary outcome, absolute risk reduction and odds ratio are adjusted for the stratification variables of gestational age and study center using a logistic regression model.
Odds ratio from logistic regression analysis.
Common odds ratio from ordinal logistic regression analysis.
Crude estimate of the treatment group difference calculated using Hodges-Lehman estimate and using a distribution-free 2-sided 95% CI.
Mann-Whitney U test.
Duration of supplemental oxygen since birth.
Sub–hazard ratio from competing risks analysis with death occurring before the event of interest treated as a competing risk.
Grading on cerebral ultrasonography according to protocol as defined by Ment et al.33 Intraventricular hemorrhage grade 2 was defined as blood within the ventricular system but not distending it.
Fisher exact test when less than 20 (non)events.
Patent ductus arteriosus requiring medical intervention and/or surgical ligation.
Grading according to Bell stages.34 Grade 2 was defined as presence of pneumatosis intestinalis.
Hypertension was defined as a systolic blood pressure greater than 80 mm Hg for infants with a gestational age of less than 26 weeks, greater than 90 mm Hg for infants between 26 and 28 weeks’ gestation, and greater than 100 mm Hg for infants with a gestational age of greater than 28 weeks.
Grading according to international classification.35 Grade 2 was defined as presence of a ridge in the region of the demarcation line.
Total number of survivors: hydrocortisone group, n=145; placebo group, n=136.
Number of participants assessed at 36 weeks’ postmenstrual age: hydrocortisone group, n=148; placebo group, n=139.
Crude mean difference with 95% CI.
Number of survivors assessed for head circumference at 36 weeks’ postmenstrual age: hydrocortisone group, n=140; placebo group, n=129.
Number of survivors assessed for length at 36 weeks’ postmenstrual age: hydrocortisone group, n=105; placebo group, n=99.
Secondary Outcomes
There were no missing data for secondary outcomes except for growth variables at 36 weeks’ postmenstrual age (Table 2).
The rate of BPD was not significantly different between the hydrocortisone group (55.2%) and the placebo group (50.0%) (crude risk difference, 5.2% [95% CI, −4.9% to 15.2%]; crude odds ratio, 1.24 [95% CI, 0.82-1.86]; P = .31) (Table 2). The rate of death, however, was significantly decreased in the hydrocortisone group (28/181 infants [15.5%]) compared with the placebo group (45/190 infants [23.7%]) (crude risk difference, −8.2% [95% CI, −16.2% to −0.1%]; crude odds ratio, 0.59 [95% CI, 0.35-0.995]; P = .048). Reported causes of death were similar between the 2 groups (eTable 1 in Supplement 3).
Analyses of the prespecified secondary outcomes showed that the lower death rate in the hydrocortisone group at 36 weeks’ postmenstrual age was no longer significantly different at hospital discharge (hydrocortisone vs placebo, 19.9% vs 28.4%; risk difference, −8.5% [95% CI, −17.1% to 0.2%]; crude odds ratio, 0.63 [95% CI, 0.39-1.01]; P = .06) (Table 2). No significant difference in the distribution of severity of BPD was seen between treatment groups.
Significantly more infants were successfully extubated in the hydrocortisone than in the placebo group on day 3 (84.4% vs 92.9%; crude risk difference, −8.5% [95% CI, −15.3% to −1.9%]; crude odds ratio, 0.41 [95% CI, 0.21-0.83]; P = .01), day 7 (54.4% vs 78.1%; crude risk difference, −23.7% [95% CI, −32.9% to −13.8%]; crude odds ratio, 0.34 [95% CI, 0.21-0.54]; P < .001), and day 14 (33.7% vs 51.2%; crude risk difference, −17.5% [95% CI, −27.5% to −6.9%]; crude odds ratio, 0.49 [95% CI, 0.31-0.76]; P = .001) after initiating therapy (Table 2). The incidence of hyperglycemia requiring insulin therapy was higher in the hydrocortisone group compared with the placebo group (18.2% vs 7.9%; crude risk difference, 10.3% [95% CI, 3.5%-17.3%]; sub–hazard ratio, 2.44 [95% CI, 1.34-4.47]; P = .004). There was a significantly lower rate of pneumonia (24.9% vs 33.7%; crude risk difference, −8.8% [95% CI, −17.5% to 0.0%]; sub–hazard ratio, 0.68 [95% CI, 0.47-0.997]; P = .048) and significantly greater mean weight at 36 weeks’ postmenstrual age (2235 g vs 2125 g; P = .03) in the hydrocortisone group compared with the placebo group (Table 2). There were no significant differences between the groups for other secondary outcomes. The rate of open-label glucocorticoid use in the hydrocortisone group was 28.2% compared with 56.8% in the placebo group (crude risk difference, −28.7% [95% CI, −37.8% to −18.7%]; sub–hazard ratio, 0.36 [95% CI, 0.26-0.50]; P < .001).
Adverse Events
The most common adverse events related to preterm birth in the hydrocortisone and placebo groups, respectively, were patent ductus arteriosus (39.8% vs 41.1%), clinical or culture-proven sepsis (50.3% vs 58.4%), retinopathy of prematurity higher than grade 2 (24.3% vs 22.1%), and pneumonia (24.9% vs 33.7%). No serious adverse drug reactions were reported, and the observed rate of severe adverse events and number of study medication protocol deviations were low and similar in treatment groups (eTable 2 in Supplement 3).
Prespecified Sensitivity and Subgroup Analyses
Neither the prespecified sensitivity nor subgroup analyses revealed a significant effect of hydrocortisone on the primary composite outcome (eTable 3 and eTable 4 in Supplement 3).
Post Hoc Analyses
The post hoc mixed-effects logistic regression model with site as random effect showed similar results for the treatment effect on the primary composite outcome (adjusted odds ratio, 0.82; 95% CI, 0.51-1.32; P = .41).
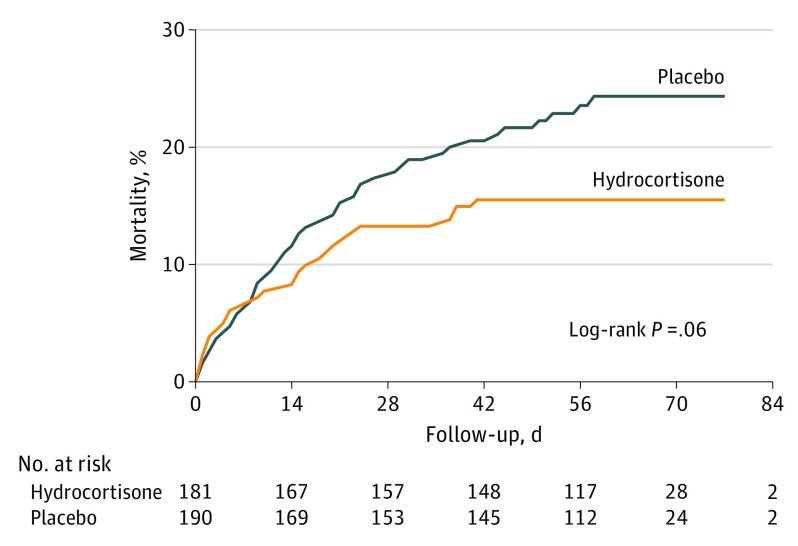
The post hoc per-protocol analysis of the primary outcome component of death showed a similar reduction as the intention-to-treat analysis (eTable 5 in Supplement 3). Adjustment for risk factors for death showed no substantive change in treatment effect (adjusted odds ratio, 0.56; 95% CI, 0.32-0.96; P = .03). Post hoc subgroup analyses did not find differential treatment effects across subgroups, except for the subgroup of infants born at a gestational age of less than 27 weeks, which showed a reduced death rate in the hydrocortisone group (14.1%) compared with the placebo group (26.4%) (crude risk difference, −12.3% [95% CI, −21.0% to −3.3%]; crude relative risk, 0.53 [95% CI, 0.33-0.86]; P = .03 for interaction) (eTable 6 in Supplement 3). Kaplan-Meier curves for survival until 36 weeks’ postmenstrual age were not significantly different (log-rank test, P = .06) (Figure 2).
Figure 2. Overall Cumulative Mortality From Randomization to 36 Weeks’ Postmenstrual Age.
Median follow-up to 36 weeks’ postmenstrual age was 61 days (interquartile range, 50-67 days) for hydrocortisone and 58 days (interquartile range, 44-67 days) for placebo.
Discussion
This multicenter randomized trial found no significant difference in the primary composite outcome of death or BPD at 36 weeks’ postmenstrual age among infants randomized to hydrocortisone compared with those randomized to placebo at 7 to 14 days. Hydrocortisone has mainly been studied as a prophylactic treatment started in the first days of life independent of the patient’s pulmonary condition and treatment. Some of these trials, such as the PREMILOC trial, showed a benefit on the combined outcome of death or BPD, while others did not.7,28 With the exception of a small pilot study assessing changes in brain volume,29 no other randomized trial has addressed the effect of hydrocortisone initiated between 7 and 14 days after birth on death or BPD. In contrast to the finding of this trial, a systematic review on dexamethasone treatment initiated after the first week of life found a reduction in the composite outcome of death or BPD, with the association mainly driven by a reduction in BPD.8 The absence of a clear effect of hydrocortisone on the primary outcome component of BPD in this study may have several explanations. First, hydrocortisone may have a different effect on lung inflammation than dexamethasone. Hydrocortisone targets both the glucocorticoid and mineralocorticoid receptors, whereas dexamethasone solely targets glucocorticoid receptors. This dual receptor activity may cause less brain toxicity30 but may also result in less potency to reduce pulmonary inflammation. Second, the administered cumulative dose of hydrocortisone used may have been too low to affect the development of BPD. This explanation is less likely, as a meta-analysis of the dexamethasone trials initiated after the first week of life showed that a cumulative dose of more than 2 mg/kg resulted in a significant beneficial effect on the outcome of death or BPD,19 and the administered cumulative hydrocortisone dose in this trial was equivalent to a dexamethasone dose of 2.9 mg/kg. Third, the higher use of open-label corticosteroids in the placebo group may have diluted a possible effect of hydrocortisone on BPD in surviving children. The rate of open-label use of corticosteroids in this trial was similar to most dexamethasone trials, so this also seems an unlikely explanation for the apparent difference in effect on BPD between hydrocortisone and dexamethasone.31
Exploratory analysis showed that hydrocortisone may reduce the risk of the primary outcome component of death at 36 weeks’ postmenstrual age. However, this finding must be interpreted cautiously and considered exploratory, as the study was not powered to adjust for multiple secondary outcome comparisons. Furthermore, although the effect size changed little, the observed reduction did not remain statistically significant up to hospital discharge. For this reason, a careful assessment of the plausibility of this finding using post hoc sensitivity analyses was performed.24,25 These analyses support the robustness of the hydrocortisone effect on death at 36 weeks’ postmenstrual age. The exploratory observation of reduced risk of death is consistent with studies assessing prophylactic use of hydrocortisone. A meta-analysis of these studies showed that the reduced risk of the outcome of death or BPD in favor of hydrocortisone was mainly driven by the component of death at hospital discharge.7 Consistent with this study, the meta-analysis revealed no significant association of prophylactic hydrocortisone with BPD.
There was no significant difference in causes of death between groups, including pulmonary deterioration. Subclinical adrenocortical insufficiency has been reported in sick preterm infants, especially those with a gestational age of less than 28 weeks.32 Supplementation with hydrocortisone might reduce the risk of mortality in this high-risk population by improving pulmonary and hemodynamic stability and damping the systemic inflammatory response.32 The finding that the reduced risk of death was larger in the group born before 27 weeks’ gestation supports this suggested mechanism.
The results of this trial may have important implications for clinical practice. Currently, the main reasons for clinicians to treat preterm infants with corticosteroids are to expedite weaning from mechanical ventilation and reduce the risk of BPD. Treatment is usually started late in the disease course, ie, after several weeks of mechanical ventilation, and despite the lack of randomized evidence to date, many centers have changed from treating with dexamethasone to hydrocortisone.9,13 These results suggest that hydrocortisone facilitates extubation but, in contrast to dexamethasone, does not reduce BPD. Therefore, this finding does not support the use of hydrocortisone for this indication. Yet hydrocortisone initiated between 7 and 14 days after birth may reduce mortality, and this benefit is not outweighed by an increase in short-term morbidity, as reported by previous studies initiating corticosteroid treatment before or after the first week of life.7,8 Although many infants in this trial experienced adverse effects of preterm birth, the only adverse effect ascribed specifically to hydrocortisone treatment was hyperglycemia requiring insulin. Information on short-term outcomes is insufficient to assess the long-term safety of hydrocortisone treatment. Therefore, follow-up of the study cohort, including assessment of neurodevelopmental outcome at 24 months’ corrected age, is currently under way.
Limitations
This study has several limitations. First, this study was not powered to detect smaller differences in the primary outcome. Considering that previous studies showed that dexamethasone reduced the rate of death or BPD almost 25% compared with placebo,19 it is unlikely that differences smaller than 15% favoring hydrocortisone treatment would change current practice. Second, the higher use of open-label corticosteroids in the placebo group may have diluted a possible effect of hydrocortisone on BPD in the surviving children. Third, lowering the respiratory index in the first year of the study could have affected the sample size calculation by including patients with a lower a priori risk of the primary outcome. However, the observed high rate of the primary outcome in the placebo group does not support this reasoning.
Conclusions
Among mechanically ventilated very preterm infants, administration of hydrocortisone between 7 and 14 days after birth, compared with placebo, did not improve the composite outcome of death or BPD at 36 weeks’ postmenstrual age. These findings do not support the use of hydrocortisone for this indication.
Trial Protocol
Statistical Analysis Plan
eTable 1. Reported Causes of Death
eTable 2. SUSARs, and SAEs and Protocol Deviations Reported Until Discharge Home
eTable 3. Pre-specified Sensitivity Analyses of the Primary Composite Outcome Death or Bronchopulmonary Dysplasia at 36 Weeks Postmenstrual Age
eTable 4. Pre-specified Exploratory Subgroup Analyses of the Primary Composite Outcome Death or Bronchopulmonary Dysplasia at 36 Weeks Postmenstrual Age in the Intention-to-Treat Population
eTable 5. Post Hoc Sensitivity Analyses for Death at 36 Weeks Postmenstrual Age
eTable 6. Post Hoc Exploratory Subgroup Analyses of Death at 36 Weeks Postmenstrual Age for Postulated Risk Factors Associated With Death in the Intention-to-Treat Population
eFigure. Supplemental Flow of Infants for the Per-Protocol and As-Treated Analysis Populations
eReferences
Data Sharing Statement
References
- 1.Horbar JD, Carpenter JH, Badger GJ, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012;129(6):1019-1026. doi: 10.1542/peds.2011-3028 [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Bell EF, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314(10):1039-1051. doi: 10.1001/jama.2015.10244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Jesus LC, Pappas A, Shankaran S, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Risk factors for post-neonatal intensive care unit discharge mortality among extremely low birth weight infants. J Pediatr. 2012;161(1):70-74. doi: 10.1016/j.jpeds.2011.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malleske DT, Chorna O, Maitre NL. Pulmonary sequelae and functional limitations in children and adults with bronchopulmonary dysplasia. Paediatr Respir Rev. 2018;26:55-59. [DOI] [PubMed] [Google Scholar]
- 5.Twilhaar ES, Wade RM, de Kieviet JF, van Goudoever JB, van Elburg RM, Oosterlaan J. Cognitive outcomes of children born extremely or very preterm since the 1990s and associated risk factors: a meta-analysis and meta-regression. JAMA Pediatr. 2018;172(4):361-367. doi: 10.1001/jamapediatrics.2017.5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins RD, Jobe AH, Koso-Thomas M, et al. Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr. 2018;197:300-308. doi: 10.1016/j.jpeds.2018.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyle LW, Cheong JL, Ehrenkranz RA, Halliday HL. Early (<8 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2017;10:CD001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doyle LW, Cheong JL, Ehrenkranz RA, Halliday HL. Late (>7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2017;10:CD001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nuytten A, Behal H, Duhamel A, et al. ; EPICE (Effective Perinatal Intensive Care in Europe) Research Group . Evidence-based neonatal unit practices and determinants of postnatal corticosteroid-use in preterm births below 30 weeks GA in Europe: a population-based cohort study. PLoS One. 2017;12(1):e0170234. doi: 10.1371/journal.pone.0170234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Virkud YV, Hornik CP, Benjamin DK, et al. Respiratory support for very low birth weight infants receiving dexamethasone. J Pediatr. 2017;183:26-30. doi: 10.1016/j.jpeds.2016.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Committee on Fetus and Newborn Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics. 2002;109(2):330-338. doi: 10.1542/peds.109.2.330 [DOI] [PubMed] [Google Scholar]
- 12.Watterberg KL; American Academy of Pediatrics Committee on Fetus and Newborn . Policy statement–postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics. 2010;126(4):800-808. doi: 10.1542/peds.2010-1534 [DOI] [PubMed] [Google Scholar]
- 13.Parat S, Mhanna MJ. Respiratory management of extremely low birth weight infants: survey of neonatal specialists. World J Pediatr. 2016;12(3):314-319. doi: 10.1007/s12519-016-0024-z [DOI] [PubMed] [Google Scholar]
- 14.Onland W, Offringa M, Cools F, et al. Systemic hydrocortisone to prevent bronchopulmonary dysplasia in preterm infants (the STOP-BPD study): a multicenter randomized placebo controlled trial. BMC Pediatr. 2011;11:102. doi: 10.1186/1471-2431-11-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janevic T, Zeitlin J, Auger N, et al. Association of race/ethnicity with very preterm neonatal morbidities. JAMA Pediatr. 2018;172(11):1061-1069. doi: 10.1001/jamapediatrics.2018.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onland W, Merkus MP, Nuytemans DH, Jansen-van der Weide MC, Holman R, van Kaam AH; STOP-BPD Study Group . Systemic hydrocortisone to prevent bronchopulmonary dysplasia in preterm infants (the STOP-BPD study): statistical analysis plan. Trials. 2018;19(1):178. doi: 10.1186/s13063-018-2505-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723-1729. doi: 10.1164/ajrccm.163.7.2011060 [DOI] [PubMed] [Google Scholar]
- 18.Walsh MC, Wilson-Costello D, Zadell A, Newman N, Fanaroff A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J Perinatol. 2003;23(6):451-456. doi: 10.1038/sj.jp.7210963 [DOI] [PubMed] [Google Scholar]
- 19.Onland W, Offringa M, De Jaegere AP, van Kaam AH. Finding the optimal postnatal dexamethasone regimen for preterm infants at risk of bronchopulmonary dysplasia: a systematic review of placebo-controlled trials. Pediatrics. 2009;123(1):367-377. doi: 10.1542/peds.2008-0016 [DOI] [PubMed] [Google Scholar]
- 20.Pugni L, Pietrasanta C, Acaia B, et al. Chorioamnionitis and neonatal outcome in preterm infants: a clinical overview. J Matern Fetal Neonatal Med. 2016;29(9):1525-1529. doi: 10.3109/14767058.2015.1053862 [DOI] [PubMed] [Google Scholar]
- 21.Travers CP, Carlo WA, McDonald SA, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Mortality and pulmonary outcomes of extremely preterm infants exposed to antenatal corticosteroids. Am J Obstet Gynecol. 2018;218(1):130.e1-130.e13. doi: 10.1016/j.ajog.2017.11.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Townsel CD, Emmer SF, Campbell WA, Hussain N. Gender differences in respiratory morbidity and mortality of preterm neonates. Front Pediatr. 2017;5:6. doi: 10.3389/fped.2017.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander GR, Slay Wingate M, Salihu H, Kirby RS. Fetal and neonatal mortality risks of multiple births. Obstet Gynecol Clin North Am. 2005;32(1):1-16. doi: 10.1016/j.ogc.2004.10.005 [DOI] [PubMed] [Google Scholar]
- 24.European Medicines Agency Guideline on the Investigation of Subgroups in Confirmatory Clinical Trials January 23, 2014. https://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/02/WC500160523.pdf.
- 25.DeMets DL, Cook TD, Buhr KA. Guidelines for statistical analysis plans. JAMA. 2017;318(23):2301-2303. doi: 10.1001/jama.2017.18954 [DOI] [PubMed] [Google Scholar]
- 26.Baer RJ, Rogers EE, Partridge JC, et al. Population-based risks of mortality and preterm morbidity by gestational age and birth weight. J Perinatol. 2016;36(11):1008-1013. doi: 10.1038/jp.2016.118 [DOI] [PubMed] [Google Scholar]
- 27.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. doi: 10.1186/1471-2431-13-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baud O, Maury L, Lebail F, et al. ; PREMILOC Trial Study Group . Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): a double-blind, placebo-controlled, multicentre, randomised trial. Lancet. 2016;387(10030):1827-1836. doi: 10.1016/S0140-6736(16)00202-6 [DOI] [PubMed] [Google Scholar]
- 29.Parikh NA, Kennedy KA, Lasky RE, McDavid GE, Tyson JE. Pilot randomized trial of hydrocortisone in ventilator-dependent extremely preterm infants: effects on regional brain volumes. J Pediatr. 2013;162(4):685-690. doi: 10.1016/j.jpeds.2012.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang CC, Lin HR, Liang YC, Hsu KS. Effects of neonatal corticosteroid treatment on hippocampal synaptic function. Pediatr Res. 2007;62(3):267-270. doi: 10.1203/PDR.0b013e318123f744 [DOI] [PubMed] [Google Scholar]
- 31.Onland W, van Kaam AH, De Jaegere AP, Offringa M. Open-label glucocorticoids modulate dexamethasone trial results in preterm infants. Pediatrics. 2010;126(4):e954-e964. doi: 10.1542/peds.2010-0597 [DOI] [PubMed] [Google Scholar]
- 32.Fernandez EF, Watterberg KL. Relative adrenal insufficiency in the preterm and term infant. J Perinatol. 2009;29(suppl 2):S44-S49. doi: 10.1038/jp.2009.24 [DOI] [PubMed] [Google Scholar]
- 33.Ment LR, Bada HS, Barnes P, et al. Practice parameter: neuroimaging of the neonate: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;58(12):1726-1738. doi: 10.1212/WNL.58.12.1726 [DOI] [PubMed] [Google Scholar]
- 34.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255-264. doi: 10.1056/NEJMra1005408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.International Committee for the Classification of Retinopathy of Prematurity The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123(7):991-999. doi: 10.1001/archopht.123.7.991 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eTable 1. Reported Causes of Death
eTable 2. SUSARs, and SAEs and Protocol Deviations Reported Until Discharge Home
eTable 3. Pre-specified Sensitivity Analyses of the Primary Composite Outcome Death or Bronchopulmonary Dysplasia at 36 Weeks Postmenstrual Age
eTable 4. Pre-specified Exploratory Subgroup Analyses of the Primary Composite Outcome Death or Bronchopulmonary Dysplasia at 36 Weeks Postmenstrual Age in the Intention-to-Treat Population
eTable 5. Post Hoc Sensitivity Analyses for Death at 36 Weeks Postmenstrual Age
eTable 6. Post Hoc Exploratory Subgroup Analyses of Death at 36 Weeks Postmenstrual Age for Postulated Risk Factors Associated With Death in the Intention-to-Treat Population
eFigure. Supplemental Flow of Infants for the Per-Protocol and As-Treated Analysis Populations
eReferences
Data Sharing Statement