Key Points
Question
Is nonmyeloablative autologous hematopoietic stem cell transplantation (HSCT) more effective than disease-modifying therapy for patients with highly active relapsing-remitting multiple sclerosis (MS)?
Findings
In this randomized clinical trial that included 110 patients with relapsing-remitting MS, treatment with nonmyeloablative HSCT compared with disease-modifying therapy resulted in a significantly prolonged time to disease progression (hazard ratio, 0.07).
Meaning
In this preliminary study, nonmyeloablative HSCT was more effective than disease-modifying therapy for patients with relapsing-remitting MS.
Abstract
Importance
Hematopoietic stem cell transplantation (HSCT) represents a potentially useful approach to slow or prevent progressive disability in relapsing-remitting multiple sclerosis (MS).
Objective
To compare the effect of nonmyeloablative HSCT vs disease-modifying therapy (DMT) on disease progression.
Design, Setting, and Participants
Between September 20, 2005, and July 7, 2016, a total of 110 patients with relapsing-remitting MS, at least 2 relapses while receiving DMT in the prior year, and an Expanded Disability Status Scale (EDSS; score range, 0-10 [10 = worst neurologic disability]) score of 2.0 to 6.0 were randomized at 4 US, European, and South American centers. Final follow-up occurred in January 2018 and database lock in February 2018.
Interventions
Patients were randomized to receive HSCT along with cyclophosphamide (200 mg/kg) and antithymocyte globulin (6 mg/kg) (n = 55) or DMT of higher efficacy or a different class than DMT taken during the previous year (n = 55).
Main Outcomes and Measures
The primary end point was disease progression, defined as an EDSS score increase after at least 1 year of 1.0 point or more (minimal clinically important difference, 0.5) on 2 evaluations 6 months apart, with differences in time to progression estimated as hazard ratios.
Results
Among 110 randomized patients (73 [66%] women; mean age, 36 [SD, 8.6] years), 103 remained in the trial, with 98 evaluated at 1 year and 23 evaluated yearly for 5 years (median follow-up, 2 years; mean, 2.8 years). Disease progression occurred in 3 patients in the HSCT group and 34 patients in the DMT group. Median time to progression could not be calculated in the HSCT group because of too few events; it was 24 months (interquartile range, 18-48 months) in the DMT group (hazard ratio, 0.07; 95% CI, 0.02-0.24; P < .001). During the first year, mean EDSS scores decreased (improved) from 3.38 to 2.36 in the HSCT group and increased (worsened) from 3.31 to 3.98 in the DMT group (between-group mean difference, −1.7; 95% CI, −2.03 to −1.29; P < .001). There were no deaths and no patients who received HSCT developed nonhematopoietic grade 4 toxicities (such as myocardial infarction, sepsis, or other disabling or potential life-threatening events).
Conclusions and Relevance
In this preliminary study of patients with relapsing-remitting MS, nonmyeloablative HSCT, compared with DMT, resulted in prolonged time to disease progression. Further research is needed to replicate these findings and to assess long-term outcomes and safety.
Trial Registration
ClinicalTrials.gov Identifier: NCT00273364
In this randomized trial, nonmyeloablative hematopoietic stem cell transplantation (HSCT) resulted in longer time to disease progression among patients with relapsing-remitting multiple sclerosis compared with continued disease-modifying therapy.
Introduction
Relapsing-remitting multiple sclerosis (MS) is an immune-mediated disorder of the central nervous system.1 Despite a 2013 annual cost of treatment with disease-modifying therapy (DMT, such as interferons, glatiramer acetate, fingolimod, natalizumab, or dimethyl fumarate) of approximately $65 000 per patient,2,3 the proportion of patients with no evidence of disease activity (defined as no progression, no relapses, and no new or enlarging lesions on magnetic resonance imaging [MRI]) is 30% to 50% after 2 years of treatment and approximately 18% after 4 years of treatment.4 The majority of patients with relapsing-remitting MS eventually enter an axonal degenerative phase of irreversible and progressive disability for which there are no significant efficacious therapies and during which there is an increase in disease-related mortality.5,6
Unlike DMT, hematopoietic stem cell transplantation (HSCT) is designed to eliminate autoreactive lymphocytes and restart a new immune system in a non-inflammatory environment without costimulatory signals.7,8,9 A previous case series of nonmyeloablative HSCT for relapsing-remitting MS found improvement in neurologic disability and a 4-year disease-free remission of 70%.10 The purpose of this study, the Multiple Sclerosis International Stem Cell Transplant (MIST) randomized clinical trial, was to compare the effects of nonmyeloablative HSCT with continued DMT treatment on disease progression among patients with highly active relapsing-remitting MS.
Methods
The trial was approved by the US Food and Drug Administration (FDA) and by institutional review boards and research ethics committees at each study site. All patients provided written informed consent.
Study Design and Inclusion Criteria
This open-label randomized clinical trial was conducted at 4 centers, and patients were enrolled between 2005 and 2016. Northwestern University (Chicago, Illinois) enrollment began in 2005, Sheffield Teaching Hospitals NHS Foundation Trust & University of Sheffield (Sheffield, England) enrollment began in 2014, University of Uppsala (Uppsala, Sweden) enrollment began in 2011, and University of São Paulo (Ribeirão Preto, Brazil) enrollment began in 2009. The study protocol was developed by the investigators in 2005 and was updated in 2017 for clarification after a scheduled institutional review board review. The updated protocol is available in Supplement 1. For this study, the final date of follow-up was January 31, 2018, and the database lock for data analysis was February 1, 2018.
Inclusion criteria were relapsing-remitting MS according to McDonald criteria,11 age 18 to 55 years, 2 or more clinical relapses or 1 relapse and MRI gadolinium-enhancing lesion(s) at a separate time within the previous 12 months despite receiving treatment with DMT, and an Expanded Disability Status Scale (EDSS) score between 2.0 and 6.0. Exclusion criteria were primary or secondary progressive multiple sclerosis; hereditary neurologic diseases; pregnancy; pulmonary, cardiac, renal, or liver dysfunction; abnormal platelet or white blood cell counts; active infection; prior treatment with alemtuzumab or mitoxantrone; or use of natalizumab within the prior 6 months, fingolimod within 3 months, or, for teriflunomide (which undergoes extensive enterohepatic recycling), failure of oral cholestyramine to decrease teriflunomide to a plasma concentration of less than 0.02 µg/mL.
Randomization and Interventions
Patients were randomized to receive DMT or HSCT. Study coordinators at each site were notified by email of randomization via computer-generated block sequences of size 4 and 6.
Patients randomized to the DMT group received an FDA-approved DMT of higher efficacy or a different class than the therapy they were taking at the time of randomization, based on the judgment of their treating neurologist. In addition to DMT, patients in this group could receive immune-modulating or immunosuppressive drugs such as methylprednisolone, rituximab, intravenous immunoglobulin, or cyclophosphamide. Ocrelizumab was excluded because the study completed enrollment in 2016 and ocrelizumab was not FDA licensed until 2017. Alemtuzumab was excluded because of drug-related persistent lymphopenia and autoimmune disorders12,13,14 that might increase complications and risk related to HSCT in the crossover group. After at least 1 year of treatment, patients in the DMT group who experienced progression of disability could cross over to receive HSCT.
For patients randomized to the HSCT group, use of DMT was discontinued and variable washout periods were observed before admission for HSCT (6 months for natalizumab, 3 months for fingolimod and dimethyl fumarate, and 4 months for rituximab). Patients who were receiving teriflunomide underwent either oral cholestyramine or activated charcoal clearance. Interferons and glatiramer acetate were continued until mobilization. After HSCT, patients did not receive immune-based therapies unless they experienced clinical relapse, new lesions on MRI, or both.
HSCT Procedures
Peripheral blood stem cells were collected 10 days after intravenous cyclophosphamide (2 g/m2) and 5 to 10 µg/kg per day of subcutaneous filgrastim beginning 5 days after cyclophosphamide. The immune ablative regimen was intravenous cyclophosphamide, 50 mg/kg per day on days −5 to day −2 before stem cell infusion (day 0) and rabbit antithymocyte globulin, 0.5 mg/kg on day −5, 1.0 mg/kg on day −4, and 1.5 mg/kg on days −3, −2, and −1. Methylprednisolone (1000 mg) was infused 30 minutes prior to rabbit antithymocyte globulin infusion. Beginning on day 0, daily oral prednisone was dosed at 60 mg for 3 days, 40 mg for 2 days, 20 mg for 2 days, and 10 mg for 2 days.
Blood products were irradiated, cytomegalovirus safe, and leukocyte depleted. Filgrastim (5-10 µg/kg per day) was started on day +4 and continued until engraftment. Hydration (125-150 mL normal saline per hour), diuretics, and intravenous mesna were continued until 24 hours after the last dose of cyclophosphamide. A Foley catheter was placed in patients with greater than 60 mL of postvoid urinary residual. Intravenous cephalosporin was started on day 0. Intravenous vancomycin was added for a febrile episode. Methylprednisolone (250 mg) was infused for rabbit antithymocyte globulin–related fever. Oral acyclovir was started on admission and continued for 1 year. Oral fluconazole was started on day +2, and oral trimethoprim-sulfamethoxazole or monthly nebulized pentamidine was started after platelet engraftment and continued for 3 months. Cytomegalovirus viral load was monitored for 90 days and was treated preemptively by switching from acyclovir to oral valganciclovir (900 mg twice daily) until testing negative by quantitative polymerase chain reaction.
Outcomes
The primary end point was time to disease progression, defined as an increase (worsening) in EDSS score of at least 1 point on 2 evaluations 6 months apart after at least 1 year of treatment,15 and not due to a non-MS disease process. If patient had a fever or infection, the evaluation was postponed until symptoms resolved. Progression may be defined as an increase in EDSS score of at least 0.5 points in patients with high disability scores (EDSS score >6.0) but an increase of at least 1.0 is generally used as in this study for moderate levels of disability (EDSS score 2-6).15,16 The numerical EDSS score ranges from 0 to 10 in 0.5-point increments from no neurologic disability (0) to worst neurologic disability (10) .17 A neurologist masked to treatment group assignment documented the EDSS evaluations. Because transient alopecia is common after HSCT, all patients, regardless of treatment allocation, wore a wig during the neurologic evaluation.
Prespecified secondary end points included survival; relapses; Neurologic Rating Scale (NRS) (range, 0-100 in 1-point increments from worst [0] to no [100] disability; minimal clinically important difference, 10)18; MRI T2-weighted lesion volume; Short Form 36 quality-of-life score (range, 1-100; higher scores indicate more favorable health state); Multiple Sclerosis Functional Composite (MSFC) score, which incorporates a timed 25-ft walk test; the 9-Hole Peg Test; and the Paced Auditory Serial Addition Test (PASAT).19 The NRS was assessed by a neurologist who was masked to treatment group assignment. Ambulation index was prespecified but is not reported because of quantitative documentation of gait via the timed 25-ft walk test.
Relapses were defined as neurologic symptoms lasting more than 24 hours; not associated with infection, fever, or heat intolerance; and deemed to require corticosteroids and were documented by an investigator who was not masked to treatment assignment. T2-weighted lesion volume on MRI was reported as percentage change from baseline.20
For the MRI assessments, patients underwent scanning procedures on the same type of scanner (General Electric or Siemens) and same magnet strength for their initial scan. Postcontrast T1-weighted imaging was obtained 5 minutes after intravenous infusion of gadolinium (a single dose of 0.1 mmol/kg). Patient positioning inside the scanner was standardized per the University of Texas MRI Analysis Center (Houston) imaging acquisition manual. T2-weighted lesion volume was determined using semiautomated contouring technique via J imaging software (National Institutes of Health; https://imagej.nih.gov/ij/docs/faqs.html). The same observer (X.H.) marked all lesions, and an experienced reader (F.N.) masked to treatment group assignment randomly reviewed MRI scans for accuracy.
Post hoc end points included evaluation of time to first relapse in the HSCT and DMT groups; outcomes in the subset of patients in the DMT group treated with natalizumab; evaluation for no evidence of disease activity (ie, no progression, no relapses, and no new or enlarging lesions on MRI in the entire study group); evaluation of clinical outcomes for patients in the DMT group who crossed over to receive HSCT; and evaluation of the entire study cohort to assess the effects of disease duration or study site on disease progression.
Transplantation-related adverse events were graded according to the National Cancer Institute’s Common Toxicity Criteria version 2.0 for grade 3 or 4 nonhematopoietic toxicity for all organs.21 For the DMT group and for the HSCT group after transplantation discharge, recorded adverse events included hospitalizations, emergency department visits, infections other than uncomplicated upper respiratory tract infections, and new medical problems or diagnoses.
Statistical Analysis
Sample size was determined a priori based on a study of mitoxantrone 2-year progression of 8% and control 2-year progression of 25%.22 Assuming a 5-year progression of 45% for mitoxantrone, it was estimated that a sample size of 110 patients (55 in each group) would provide at least 90% power to detect a difference of 30% or more if the corresponding progression rate in the HSCT group was 15%, with an α=.05.
Statistical differences in the primary outcome of time to progression between the DMT and HSCT group were estimated as hazard ratios. Between-group statistical differences in secondary outcomes (survival, number of relapses, EDSS, NRS, T2-weighted lesion volume, 25-ft walk, 9-Hole Peg Test, PASAT, and Short Form 36) were analyzed with the Wilcoxon rank sum test. The MSFC score and z scores were standardized to the patients within the trial and calculated in accordance with the National Multiple Sclerosis Society’s MSFC Administrative and Scoring Manual23 (eTable 1 in Supplement 2).
Post hoc analyses included time to first relapse and no evidence of disease activity for the entire group and for the subset of patients in the DMT group who were treated with natalizumab, which were estimated via the Kaplan-Meier method using the log-rank test. A mixed-effects analysis was performed on the primary end point of time to progression and adjusted by Cox proportional hazards modeling for duration of disease or transplantation site. The effect of site on disease progression was assessed by incorporating site as a random effect in the Cox regression model (ie, models were adjusted by site). The proportional hazards assumption was evaluated graphically by visualizing simulations of Schoenfeld residual patterns. These patterns appeared symmetric over time around 0, indicating that the proportional hazards assumptions were met. Two-tailed paired signed rank tests were used for comparison of prespecified EDSS, NRS, and T2-weighted lesion volume for all patients undergoing HSCT (including those who crossed over from the DMT group to receive HSCT) and followed up for up to 5 years.
During the first year, 3.8% of clinic visits were missed; thus, the number of individuals with missing data was small and was deemed unlikely to influence the results, and complete case analyses were performed without imputation for missing values. Because MS is more prevalent in white persons, race was documented based on physical examination and history. However, because this approach may be unreliable for determining some race/ethnicity categories, this variable was not reported.
Analyses were conducted using SAS version 9.4 (SAS Institute Inc) and Microsoft Excel 2007. For the primary and secondary outcomes, statistical significance was based on a 2-sided significance threshold of P < .05. The thresholds for significance were not adjusted for multiple comparisons; therefore, the findings from the secondary end points and post hoc analyses should be considered exploratory.
Results
Patient Characteristics
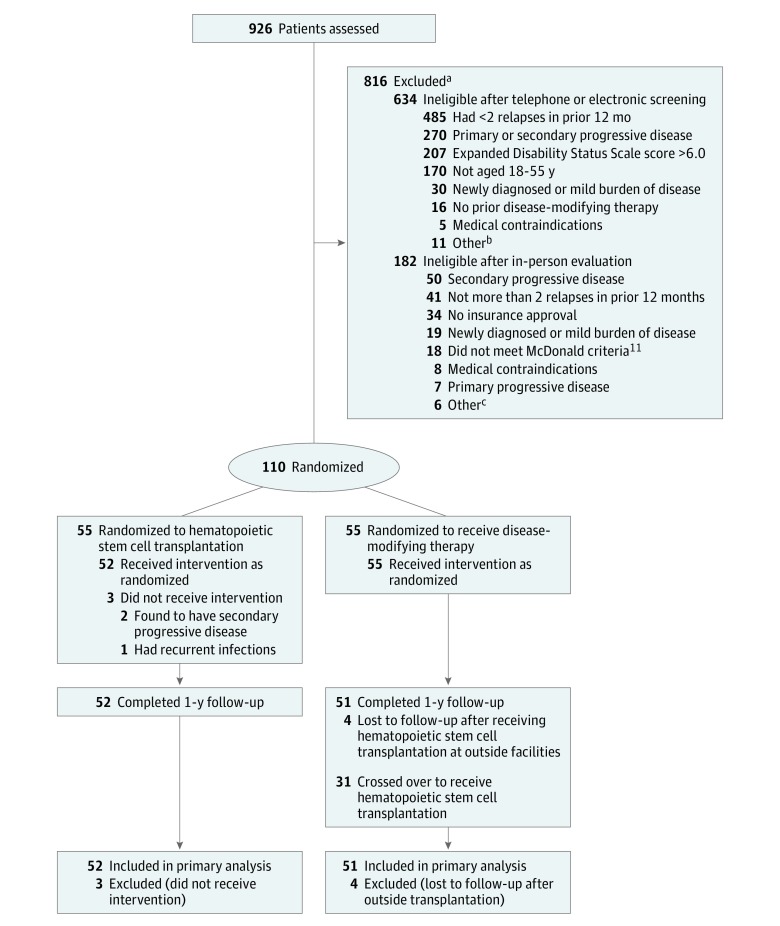
Among 926 patients who were physician- or self-referred for the trial, 634 were excluded after telephone screening, electronic evaluation, or both and 182 were excluded after history taking and physical examination. The most common reasons for exclusion were the absence of 2 relapses in the preceding 12 months or a diagnosis of progressive MS. Among the 110 patients enrolled in the study, 55 were randomized to each group. There were no significant differences between the HSCT and DMT groups in terms of sex, age, baseline EDSS scores, number of gadolinium-enhancing lesions, T2-weighted lesion volume on MRI before randomization, number of prior DMTs, or type of prior DMT or immune treatments except for interferon beta-1a, which was more common in the DMT group (Table 1). Duration of disease before enrollment was numerically longer in the DMT group, but the difference was not significant and duration was not related to disease progression in a mixed-effects model.
Table 1. Baseline Demographics and Disease Characteristics.
| Characteristics | Hematopoietic Stem Cell Transplantation (n = 55) | Disease-Modifying Therapy (n = 55) |
|---|---|---|
| Sex, No. (%) | ||
| Men | 21 (38) | 16 (34) |
| Women | 34 (62) | 39 (66) |
| Age, y | ||
| Mean (SD) | 35.6 (8.4) | 35.6 (8.2) |
| Median (range) | 34 (18-54) | 36 (19-52) |
| Duration of disease, mo | ||
| Mean (SD) | 63.1 (44.8) | 84.8 (61.2) |
| Median (range) | 56 (9-168) | 65 (8-255) |
| Prior immune modulation/suppression history, No. | ||
| Corticosteroids | 53 | 54 |
| Glatiramer acetate | 30 | 28 |
| Interferon beta-1a (Rebif) | 17 | 27 |
| Interferon beta-1a (Avonex) | 20 | 23 |
| Dimethyl fumarate | 12 | 12 |
| Interferon beta-1b | 15 | 11 |
| Natalizumab | 7 | 11 |
| Intravenous immunoglobulin | 3 | 3 |
| Fingolimod | 6 | 3 |
| Teriflunomide | 1 | 1 |
| Plasmapheresis | 0 | 1 |
| Azathioprine | 1 | 1 |
| Methotrexate | 1 | 0 |
| No. of different immune modulation/suppression treatments before hematopoietic stem cell transplantation | ||
| Mean (SD) | 3.2 (1.1) | 3.2 (1.2) |
| Median (range) | 3 (1-7) | 3 (1-7) |
| Baseline disability on Expanded Disability Status Scalea | ||
| Mean (SD) | 3.4 (1.2) | 3.3 (1.0) |
| Median (range)b | 3.0 (1.5-6.5) | 3.0 (1-6) |
| No. of gadolinium-enhancing lesions on baseline MRI | ||
| Mean (SD) | 4.5 (8.2) | 4.9 (8.4) |
| Median (range) | 2 (0-50) | 2 (0-41) |
| MRI T2-weighted lesion volume, cm3 | ||
| Mean (SD) | 16.4 (19.4) | 12.5 (13.6) |
| Median (range) | 8.2 (0.2-95) | 8.6 (0.1-58) |
Abbreviation: MRI, magnetic resonance imaging.
Score range, 0-10 in 0.5-point increments; higher scores indicate higher neurologic disability.10 An EDSS score of 3 indicates fully ambulatory but with moderate disability in 1 functional system or mild disability in 3 or 4 functional systems.
The EDSS scores at the time of enrollment were 2.0 to 6.0 in all patients.
Three patients in the HSCT group were subsequently withdrawn from the study, 2 for having secondary progressive MS and 1 for recurrent infections before receiving transplantation. The 2 cases of secondary progressive MS were identified shortly after enrollment, and these patients were censored after review by each study site’s principal investigator, the data and safety monitor, and FDA notification. Four patients in the DMT group were lost to follow-up after seeking HSCT at outside facilities (Figure 1).
Figure 1. Participant Flow in the Multiple Sclerosis International Stem Cell Transplant Randomized Clinical Trial.
aPatients could have been ineligible for more than 1 reason, so numbers do not add to total.
bFour patients did not meet McDonald criteria,11 3 had no insurance approval, 1 pregnancy, 1 prior hematopoietic stem cell transplantation in another country, 1 patient with psychosocial problems, and 1 inability to commit to follow-up.
cTwo patients with prior use of mitoxantrone, 1 died before enrollment, 1 patient with drug addiction, 1 patient with psychosocial problems, and 1 declined hematopoietic stem cell transplantation.
Patients in the DMT group were treated with DMT as deemed appropriate by their treating neurologist, with a mean of 1.3 different DMTs per patient: 21 patients received natalizumab, 14 dimethyl fumarate, 14 fingolimod, 9 glatiramer acetate, 7 interferon beta-1a, 6 mitoxantrone, and 1 teriflunomide. In addition to DMT, 38 patients received methylprednisolone, 2 received rituximab, and 1 patient each received plasmapheresis, intravenous cyclophosphamide, or intravenous immunoglobulins.
Primary End Point
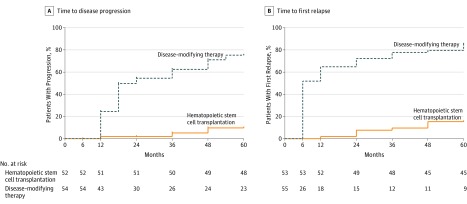
Disease progression (EDSS score increase of ≥1.0) occurred in 3 patients in the HSCT group and 34 patients in the DMT group, with a median follow-up of 2 years (mean, 2.8 years). Median time to progression could not be calculated in the HSCT group because of too few events and was 24 months (interquartile range, 18-48 months) in the DMT group (hazard ratio, 0.07; 95% CI, 0.02-0.24; P < .001) (Figure 2A). For the HSCT group, the proportion of patients with disease progression was 1.92% (95% CI, 0.27%-12.9%) at 1 year and 2 years, 5.19% (95% CI, 1.26%-20.1%) at 3 years, and 9.71% (95% CI, 3.0%-28.8%) at 4 and 5 years. For the DMT group, the proportion of patients with disease progression was 24.5% (95% CI, 14.7%-39.1%) at 1 year, 54.5% (95% CI, 40.7%-69.4%) at 2 years, 62.5% (95% CI, 48.3%-76.7%) at 3 years, 71.2% (95% CI, 56.8%-84.2%) at 4 years, and 75.3% (95% CI, 60.4%-87.8%) at 5 years. In the HSCT group, the mean EDSS score decreased (improved) from a pre-HSCT score of 3.38 to 2.36 at 1 year (mean change, −1.02 points). In the DMT group, the mean EDSS score increased (worsened) from 3.31 to 3.98 at 1 year (mean change, +0.67 points). The between-group difference in the change in EDSS scores from baseline to 1 year was −1.7 (95% CI, −2.03 to −1.29; P < .001).
Figure 2. Time to Disease Progression and First Relapse Among Patients Receiving Hematopoietic Stem Cell Transplantation vs Disease-Modifying Therapy.
Median follow-up time was 24 months (interquartile range, 12-48 months).
Secondary End Points
There were no deaths in the HSCT group or in the DMT group. In the first year, 36 (69%) of 52 patients relapsed in the DMT group compared with 1 (2%) of 51 patients who relapsed in the HSCT group (between-group difference, 78%; 95% CI, 64%-88%; P < .001). For the HSCT group, the proportion of patients with relapse was 0% at 6 months, 1.92% (95% CI, 0.27%-12.9%) at 1 year, 7.69% (95% CI, 2.96%-19.2%) at 2 years, 9.61% (95% CI, 4.1%-22%) at 3 years, and 15.4% (95% CI, 8.01%-28.4%) at 4 and 5 years. For the DMT group, the proportion of patients with relapse was 51.9% (95% CI, 39.9%-65.6%) at 6 months, 64.8% (95% CI, 52.2%-77.2%) at 1 year, 72.2% (95% CI, 66%-87.7%) at 2 years, 79.63% (95% CI, 68.1%-89.1%) at 3 years, 79.6% (95% CI, 68.1%-89.1%) at 4 years, and 85.2% (95% CI, 74.5%-93.1%) at 5 years.
At 6 months and 1 year after randomization, EDSS, NRS, and MRI T2-weighted lesion volume significantly improved following HSCT compared with the DMT group (all P < .001) (Table 2). The change in EDSS for each patient is shown in eFigure 1 in Supplement 2. In the HSCT group, the mean NRS score increased (improved) from a pre-HSCT score of 79.5 to 87.5 at 6 months and 88.3 at 1 year, and in the DMT group, the mean NRS decreased (worsened) from 81.1 to 78.2 at 6 months and 79.5 at 1 year. The between-group difference in change in NRS scores at 1 year was 11.2 (95% CI, 8.08-14.29). In the HSCT group, mean MRI T2-weighted lesion volume decreased from baseline 100% (16.2 cm3) to 75.5% (12.81 mm3) at 6 months and 68.3% (12.33 cm3) at 1 year compared with pre-HSCT baseline, whereas in the DMT group baseline, mean MRI T2-weighted lesion volume increased from 100% (12.54 cm3) to 129% (14.04 cm3) at 6 months and 134.3% (15.14 cm3) at 1 year. The between-group difference in change in T2-weighted lesion volume at 1 year was −66% (95% CI, −70.6% to −61.3%).
Table 2. Secondary Outcomes for Hematopoietic Stem Cell Transplantation vs Disease-Modifying Therapy During the First Year of the Trial.
| Outcomes | Hematopoietic Stem Cell Transplantation | Disease-Modifying Therapy | Between-Group Difference in Means (95% CI) | P Value | Between-Group Difference in Differences From Baseline to 1 y (95% CI) | P Valuea | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Participants | Mean (SD) | Median (IQR) | No. of Participants | Mean (SD) | Median (IQR) | |||||
| EDSS scoreb | ||||||||||
| Baseline | 52 | 3.4 (1.2) | 3 (2.5-4) | 54 | 3.3 (1.0) | 3 (2.5-4) | 0.07 (−0.35 to 0.49) | .97 | ||
| 6 mo | 52 | 2.5 (1.4) | 2.5 (2-3) | 54 | 3.7 (1.5) | 3.5 (3-4) | −1.17 (−1.76 to −0.58) | <.001 | ||
| 1 y | 50 | 2.4 (1.4) | 2 (1.5-3.4) | 48 | 4 (1.7) | 4 (2.5-5.5) | −1.62 (−2.24 to −0.99) | <.001 | −1.7 (−2.03 to −1.29) | <.001 |
| NRS scorec | ||||||||||
| Baseline | 53 | 79.5 (10.2) | 80 (74-88) | 54 | 81.1 (10.9) | 82 (75-89) | −1.6 (−4.48 to 3.66) | .56 | ||
| 6 mo | 53 | 87.5 (9.2) | 86.5 (81-96) | 53 | 78.2 (11.6) | 80.5 (69-86) | 9.28 (5.02 to 13.53) | <.001 | ||
| 1 y | 50 | 88.3 (9.15) | 92 (83-96) | 48 | 79.5 (11.8) | 83 (72-87) | 9.8 (6.26 to 14.72) | <.001 | 11.2 (8.08 to 14.29) | .001 |
| MRI T2-weighted lesion volume, % | ||||||||||
| Baseline | 53 | 100 | 52 | 100 | ||||||
| 6 mo | 51 | 75.5 (16) | 79.2 (67-86) | 46 | 129 (53.9) | 123.8 (98-126) | −53.11 (−62.6 to −43.6) | <.001 | ||
| 1 y | 48 | 68.3 (20.7) | 70.2 (61-82) | 49 | 134.3 (45.6) | 123.7 (105-123) | −66.19 (−75.17 to −57.21) | <.001 | −66 (−70.6 to −61.3) | <.001 |
| Timed 25-ft walk, s | ||||||||||
| Baseline | 51 | 6.5 (3.16) | 5.8 (4.4-7.3) | 55 | 5.6 (1.7) | 4.9 (4.3-6.6) | 0.89 (−0.06 to 1.84) | .35 | ||
| 6 mo | 49 | 5.9 (3.6) | 4.6 (4-5.5) | 43 | 7 (4) | 6 (4.2-8.8) | −1.13 (−2.68 to 0.42) | .04 | ||
| 1 y | 49 | 6 (4.5) | 4.6 (3.9-6.2) | 48 | 8 (6.2) | 6.2 (4.7-8.5) | −1.95 (−4.09 to 0.19) | .001 | −2.85 (−3.92 to −1.77) | <.001 |
| 9-Hole Peg Test, sd | ||||||||||
| Baseline | 50 | 30.8 (23.2) | 22.5 (20.6-30.7) | 55 | 24.7 (6.3) | 22.9 (20.7-26.3) | 6.12 (−0.27 to 12.51) | .72 | ||
| 6 mo | 43 | 26 (19.2) | 21 (18.5-25.6) | 44 | 26.3 (8.1) | 23.7 (20.7-29.9) | −0.28 (−6.46 to 5.92) | .05 | ||
| 1 y | 49 | 24 (9.5) | 21 (18.9-24.7) | 49 | 25.6 (8.2) | 23 (20.8-27) | −1.64 (−5.43 to 1.61) | .01 | −8.03 (–11.3 to −4.76) | <.001 |
| PASAT, %e | ||||||||||
| Baseline | 54 | 67.4 (20.9) | 70 (50.4-85.9) | 54 | 65.2 (21.5) | 70 (51.5-81.3) | 2.17 (−6 to 10.3) | .68 | ||
| 6 mo | 48 | 73.3 (20.2) | 79.2 (57.7-90) | 42 | 73.5 (20.8) | 80 (64.2-90) | −0.24 (−8.74 to 8.26) | .97 | ||
| 1 y | 48 | 77.8 (21.1) | 83.3 (69-94) | 48 | 75.4 (22.5) | 81.7 (62-89) | 2.39 (−5.65 to 10.4) | .36 | 0.22 (−72.4 to 72.9) | .61 |
| Short Form 36 quality-of-life scoref | ||||||||||
| Baseline | 51 | 50.5 (20.1) | 50.12 (32.8-64.9) | 51 | 49.5 (18) | 47 (38.2-60.9) | 1.04 (−6.36 to 8.44) | .92 | ||
| 6 mo | 49 | 68 (21.3) | 73 (50.7-86.1) | 42 | 45.2 (19.4) | 42.8 (31.8-54.6) | 22.71 (14.2 8 to 31.14) | <.001 | ||
| 1 y | 49 | 70 (21.3) | 76 (58.7-87.3) | 49 | 46.1 (22.5) | 44 (28.8-57.4) | 23.9 (16.63 to 33.03) | <.001 | 23 (17.6 to 28.9) | <.001 |
Abbreviations: IQR, interquartile range; MRI, magnetic resonance imaging.
Between-group P value calculated by Wilcoxon rank sum test.
The Expanded Disability Status Scale (EDSS) score ranges from 0 to 10 in 0.5-increments; higher scores indicate worse neurologic disability.10
The Neurologic Rating Scale (NRS) score ranges from 0 (worst) to 100 (best) in 1-point increments.
The 9-Hole Peg Test is a measure of arm function.
The Paced Auditory Serial Addition Test (PASAT) is scored as the total number correct out of 60 possible, and data shown are percentage of correct answers.
The Short Form 36 score ranges from 1 to 100; higher scores indicate more favorable health state.
Total Short Form 36 quality-of-life scores significantly increased following HSCT compared with the DMT group (P < .001) (Table 2). In the HSCT group, the mean total Short Form 36 score increased from 50.5 at baseline to 67.9 at 6 months and 70.3 at 1 year, whereas in the DMT group, the mean total Short Form 36 score decreased from 49.5 at baseline to 45.2 at 6 months and 46.1 at 1 year. The between-group difference in change in Short Form 36 scores at 1 year was 23 (95% CI, 17.6-28.9). The mean MSFC score increased (improved) in the HSCT group (+0.32 at 1 year) and decreased (worsened) in the DMT group (−0.31 at 1 year), for a between-group difference in change in MSFC scores at 1 year of 0.51 (95% CI, 0.28-0.72; P < .001). In the DMT group, the mean 25-ft walk time increased (worsened) from 5.59 seconds to 7.01 seconds at 6 months and 7.96 seconds at 1 year, whereas in the HSCT group, the mean 25-ft walk time decreased (improved) from 6.48 seconds at baseline to 5.88 seconds at 6 months and 6.01 seconds at 1 year, for a between-group difference in change in 25-ft walk scores at 1 year of −2.85 seconds (95% CI, −3.92 to −1.77 seconds; P < .001). In the HSCT group, mean 9-Hole Peg Test scores decreased (improved) from 30.81 seconds at baseline to 26 seconds at 6 months and 24 seconds at 1 year, but in the DMT group, 9-Hole Peg Test scores increased (worsened) from 24.69 seconds at baseline to 26.28 seconds at 6 months and 25.64 seconds at 1 year, for a between-group difference in change in scores at 1 year of −8.03 seconds (95% CI, −11.3 to −4.76 seconds; P < .001). The mean PASAT scores increased (improved) in both the DMT and HSCT groups, and the between-group difference in change in scores at 1 year was 0.22% (95% CI, −72.4% to 72.9%), which was not statistically significant (P = .61).
Post Hoc Analysis
Median time to first relapse in the DMT group was 6 months (interquartile range, 6-36 months), but data could not be calculated in the HSCT group because of too few events. The hazard ratio for time to first relapse was 0.097 (95% CI, 0.045-0.208; P < .001) (Figure 2B).
Proportions of patients with no evidence of disease activity were significantly different (P < .001) between the DMT and HSCT groups (eFigure 2 in Supplement 2). For the HSCT group, the proportion with no evidence of disease activity was 98.1% (95% CI, 87.4%-99.7%) at 6 months and 1 year, 93.3% (95% CI, 80.6%-97.8%) at 2 years, 90.3% (95% CI, 75.9%-96.3%) at 3 years, and 78.5% (95% CI, 59.8%-89.5%) at 4 and 5 years. The proportion with no evidence of disease activity in the DMT group was 39.6% (95% CI, 26.6%-52.39%) at 6 months, 20.8% (95% CI, 11%-32.5%) at 1 year, 11.9% (95% CI, 4.3%-23.6%) at 2 years, 5.93% (95% CI, 1.17%-16.6%) at 3 years, and 2.97% (95% CI, 0.24%-12.8%) at 4 and 5 years.
In the DMT group, 21 patients received natalizumab. Two left the study to receive HSCT at other sites and 19 were evaluable. For patients in the DMT group who received natalizumab, the proportion with progression (worsening on EDSS) was 5.3% at 1 year, 24.3% at 2 years, 30.5% at 3 years, 46% at 4 years, and 67.6% at 5 years (eFigure 3 in Supplement 2). The proportion of patients with relapse was 42.1% at 6 months, 69.3% at 1 year, 75.4% at 2 years, 81.6% at 4 years, and 100% at 5 years. The proportion with no evidence of disease activity was 52.6% at 6 months, 31.6% at 1 year, 25.3% at 2 years, 12.6% at 3 years, and 6.3% at 4 years (eFigure 2 in Supplement 2).
A mixed-effects analysis was performed on the primary end point of time to progression and adjusted by Cox model for duration of disease or transplantation site. Duration of disease had no effect on progression in the HSCT group (adjusted hazard ratio, 0.99; 95% CI, 0.95-1.03; P = .47) or in the DMT group (adjusted hazard ratio, 1.00; 95% CI, 0.99-1.03; P = .98). There was no significant difference between sites in the primary end point of progression, except for in Sweden, which had a smaller difference in the rate of progression between HSCT and DMT (P = .05). The patients in Sweden had lower disability at the time of enrollment (EDSS score, 2.7) compared with in the United States (EDSS score, 3.4), England (EDSS score, 3.7), or Brazil (EDSS score, 4.2).
Five-year outcomes after transplantation in the 52 patients in the HSCT group, the 31 patients in the DMT group who crossed over to HSCT, and all 83 patients who underwent HSCT are shown in eTable 2 in Supplement 2. For the 31 patients who crossed over from the DMT group to receive HCST, there was significant improvement in EDSS scores, NRS scores, and T2-weighted lesion volume percentages. The outcomes for the combined group of all 83 patients who underwent HSCT were comparable with the outcomes for the HSCT group alone (eTable 2).
Adverse Events
The median day of white blood cell engraftment (absolute neutrophil count >1000/μL) and day of hospital discharge after HSCT were day +9 and +10, respectively. In the HSCT group, there were no Common Toxicity Criteria grade 4 nonhematopoietic toxicities such as myocardial infarction, embolism, dialysis, sepsis, or need for pressor support; transfer to intensive care unit; parenteral nutrition; surgery; or other disabling or potential life-threatening events. Inpatient grade 3 toxicities are shown in eTable 3 in Supplement 2. Inpatient infections included 1 case of Clostridium difficile diarrhea, 1 Escherichia coli urinary tract infection, and 1 culture-negative pneumonia. There were no early or late fungal, Pneumocystis jirovecii, cytomegalovirus, Epstein-Barr, or JC virus infections in either group.
In the HSCT group, posttransplantation infections were 16 upper respiratory tract infections (7 sinusitis; 2 each of bronchitis, undefined pneumonia, and streptococcal pharyngitis; and 1 each of influenza, respiratory syncytial virus, and Mycoplasma pneumoniae), 6 urinary tract infections, 2 C difficile diarrhea, and 7 dermatomal varicella zoster reactivations. In the DMT group, posttransplantation infections were 15 upper respiratory tract infections (6 sinusitis; 2 bronchitis; 2 influenza; and 1 each of streptococcal pharyngitis, undefined pneumonia, M pneumoniae, tooth abscess, and otitis media), 8 urinary tract infections, and 2 varicella zoster reactivations. The rate of infection per patient per year was 0.19 in the HSCT group and 0.23 in the DMT group.
Idiopathic thrombocytopenic purpura occurred in 1 HSCT patient and in 1 other patient in the HSCT group who relapsed and started fingolimod and in whom idiopathic thrombocytopenic purpura recurred after a fingolimod rechallenge. One thyroid nodule was reported in the DMT group. Three cases of hyperthyroidism and 1 case of hypothyroidism were observed in the HSCT group; all of these patients had received interferon therapy before HSCT.
Discussion
Approximately 85% of patients with MS have relapsing-remitting MS.1 In this preliminary study of patients with relapsing-remitting MS, nonmyeloablative HSCT, compared with DMT, resulted in prolonged time to disease progression. Patients in the HSCT group also experienced improvement in other outcomes including the EDSS, NRS, and MSFC and quality of life as well as a decrease in MRI T2-weighted lesion volume.
To our knowledge, this is the first randomized trial of HSCT in patients with relapsing-remitting MS. Other centers have reported observational studies showing similar improvements in EDSS, no evidence of disease activity, or both in patients with relapsing-remitting MS undergoing HSCT.10,24,25,26 This degree of improvement has not been demonstrated in pharmaceutical trials even with more intensive DMT such as alemtuzumab.12,13
In addition, in this study, the EDSS scores for patients whose disease worsened while receiving DMT also improved after crossover to receive HSCT. Since most patients who progressed and crossed over to HSCT subsequently improved in EDSS disability, the current definitions of progression for relapsing-remitting MS may not accurately assess irreversible disease progression for all patients.15
Limitations
This study has several limitations. First, a relatively small number of patients were treated compared with pharmaceutical-sponsored trials, and the relatively small sample size resulted in small numbers of patients available to assess longer-term outcomes. The number of patients needed in randomized trials with active comparators depends on the known or expected treatment effect of the 2 treatments. Previous DMT trials for relapsing-remitting MS needed a fairly large number of patients to show the superiority of a new DMT compared with interferons or glatiramer acetate. Because HSCT efficacy was assumed to be superior to DMT, the MIST trial was designed with a smaller number of patients.
Second, the study design allowed patients in the DMT group in whom that treatment failed to cross over to receive HSCT, which also limited the ability to collect follow-up data for patients receiving DMT and to assess longer-term secondary outcomes. Because of ethical concerns of treatment equipoise between the 2 groups, the crossover option was included for patients whose EDSS worsened with continued DMT treatment. The crossover prevented comparison of the HSCT and DMT groups after 1 year but did not affect the primary end point of time to progression or the end points of time to first relapse or no evidence of disease activity, and allowed completion of the study. Even with the provision for crossover, 4 patients in the DMT group left the study to receive HSCT at other sites. Crossover did allow patients in whom DMT continued to fail to be their own control, demonstrating, despite continued failure of DMT, marked improvement after HSCT (eTable 2 in Supplement 2).
Third, alemtuzumab and ocrelizumab were excluded from use in the DMT group. Ocrelizumab was not included as DMT because the study completed enrollment in 2016 and ocrelizumab was not FDA licensed until 2017. Alemtuzumab was FDA licensed in 2014 but was excluded because prolonged alemtuzumab-induced lymphopenias and secondary autoimmune disorders14 could contribute to or cause post-HSCT infections or autoimmune diseases in the crossover group. In comparison with this study, which allowed multiple DMTs in the control group, controls for pharmaceutical DMT trials have been limited to placebo, interferon, and glatiramer acetate.
Fourth, although the evaluating neurologist for EDSS and NRS was masked to treatment assignment, the physician who recorded relapses was not masked. Fifth, although patients at all sites had significant improvement in EDSS after HSCT and the study was numerically weighted toward the US site, 1 site enrolled patients with lower disability scores, and at that site the primary outcome of progression between HSCT and DMT groups was less pronounced. However, it would be anticipated that the rate of progression would be slower in less disabled patients.27
Conclusions
In this preliminary study of patients with highly active relapsing-remitting MS and moderate disability, nonmyeloablative HSCT, compared with DMT, resulted in prolonged time to disease progression. Further research is needed to replicate the findings and to assess long-term outcomes and safety.
Trial Protocol
eFigure 1. Lineplot of EDSS Change in Each Patient
eFigure 2. NEDA (Disease Free Survival) of Disease Modifying Therapy Versus Hematopoietic Stem Cell Transplantation
eFigure 3. Time to Progression, Time to First Relapse, and NEDA (Disease Free Survival) for Control Patients Treated With Natalizumab
eTable 1. Comparison of Expanded Disability Status Scale (EDSS), Neurologic Rating Scale (NRS) and Multiple Sclerosis Functional Composite (MSFC) Score
eTable 2. Secondary Outcomes of All Patients Getting HSCT, Original HSCT Group, and DMT That Crossed Over to HSCT
eTable 3. Transplant Toxicities
Data Sharing Statement
References
- 1.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169-180. doi: 10.1056/NEJMra1401483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartung DM, Bourdette DN, Ahmed SM, Whitham RH. The cost of multiple sclerosis drugs in the US and the pharmaceutical industry: too big to fail? Neurology. 2015;84(21):2185-2192. doi: 10.1212/WNL.0000000000001608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozkaya D, Livingston T, Migliaccio-Walle K, Odom T. The cost-effectiveness of disease-modifying therapies for the treatment of relapsing-remitting multiple sclerosis. J Med Econ. 2017;20(3):297-302. doi: 10.1080/13696998.2016.1258366 [DOI] [PubMed] [Google Scholar]
- 4.Sormani MP, Muraro PA, Saccardi R, Mancardi G. NEDA status in highly active MS can be more easily obtained with autologous hematopoietic stem cell transplantation than other drugs. Mult Scler. 2017;23(2):201-204. doi: 10.1177/1352458516645670 [DOI] [PubMed] [Google Scholar]
- 5.Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343(20):1430-1438. doi: 10.1056/NEJM200011163432001 [DOI] [PubMed] [Google Scholar]
- 6.Leray E, Vukusic S, Debouverie M, et al. Excess mortality in patients with multiple sclerosis starts at 20 years from clinical onset: data from a large-scale French observational study. PLoS One. 2015;10(7):e0132033. doi: 10.1371/journal.pone.0132033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abrahamsson SV, Angelini DF, Dubinsky AN, et al. Non-myeloablative autologous haematopoietic stem cell transplantation expands regulatory cells and depletes IL-17 producing mucosal-associated invariant T cells in multiple sclerosis. Brain. 2013;136(pt 9):2888-2903. doi: 10.1093/brain/awt182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muraro PA, Douek DC, Packer A, et al. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J Exp Med. 2005;201(5):805-816. doi: 10.1084/jem.20041679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burman J, Fransson M, Tötterman TH, Fagius J, Mangsbo SM, Loskog AS. T-cell responses after haematopoietic stem cell transplantation for aggressive relapsing-remitting multiple sclerosis. Immunology. 2013;140(2):211-219. doi: 10.1111/imm.12129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burt RK, Balabanov R, Han X, et al. Association of nonmyeloablative hematopoietic stem cell transplantation with neurological disability in patients with relapsing-remitting multiple sclerosis. JAMA. 2015;313(3):275-284. doi: 10.1001/jama.2014.17986 [DOI] [PubMed] [Google Scholar]
- 11.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald criteria.” Ann Neurol. 2005;58(6):840-846. doi: 10.1002/ana.20703 [DOI] [PubMed] [Google Scholar]
- 12.Cohen JA, Coles AJ, Arnold DL, et al. ; CARE-MS I Investigators . Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819-1828. doi: 10.1016/S0140-6736(12)61769-3 [DOI] [PubMed] [Google Scholar]
- 13.Coles AJ, Twyman CL, Arnold DL, et al. ; CARE-MS II Investigators . Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1829-1839. doi: 10.1016/S0140-6736(12)61768-1 [DOI] [PubMed] [Google Scholar]
- 14.Baker D, Herrod SS, Alvarez-Gonzalez C, Giovannoni G, Schmierer K. Interpreting lymphocyte reconstitution data from the pivotal phase 3 trials of alemtuzumab. JAMA Neurol. 2017;74(8):961-969. doi: 10.1001/jamaneurol.2017.0676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Healy BC, Engler D, Glanz B, Musallam A, Chitnis T. Assessment of definitions of sustained disease progression in relapsing-remitting multiple sclerosis. Mult Scler Int. 2013;2013:189624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodkin DE. EDSS reliability. Neurology. 1991;41(2, pt1):332. doi: 10.1212/WNL.41.2_Part_1.332 [DOI] [PubMed] [Google Scholar]
- 17.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452. doi: 10.1212/WNL.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 18.Sipe JC, Knobler RL, Braheny SL, Rice GP, Panitch HS, Oldstone MB. A neurologic rating scale (NRS) for use in multiple sclerosis. Neurology. 1984;34(10):1368-1372. doi: 10.1212/WNL.34.10.1368 [DOI] [PubMed] [Google Scholar]
- 19.Fischer JS, Rudick RA, Cutter GR, Reingold SC; National MS Society Clinical Outcomes Assessment Task Force . The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. Mult Scler. 1999;5(4):244-250. doi: 10.1177/135245859900500409 [DOI] [PubMed] [Google Scholar]
- 20.Fahrbach K, Huelin R, Martin AL, et al. Relating relapse and T2 lesion changes to disability progression in multiple sclerosis: a systematic literature review and regression analysis. BMC Neurol. 2013;13:180. doi: 10.1186/1471-2377-13-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Common Toxicity Criteria. Version 2.0. April 30, 1999. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf.
- 22.Millefiorini E, Gasperini C, Pozzilli C, et al. Randomized placebo-controlled trial of mitoxantrone in relapsing-remitting multiple sclerosis: 24-month clinical and MRI outcome. J Neurol. 1997;244(3):153-159. doi: 10.1007/s004150050066 [DOI] [PubMed] [Google Scholar]
- 23.National MS Society Multiple Sclerosis Functional Composite Administrative and Scoring Manual October 2001. http://main.nationalmssociety.org/docs/HOM/MSFC_Manual_and_Forms.pdf.
- 24.Nash RA, Hutton GJ, Racke MK, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for relapsing-remitting multiple sclerosis (HALT-MS): a 3-year interim report. JAMA Neurol. 2015;72(2):159-169. doi: 10.1001/jamaneurol.2014.3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burman J, Iacobaeus E, Svenningsson A, et al. Autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: the Swedish experience. J Neurol Neurosurg Psychiatry. 2014;85(10):1116-1121. doi: 10.1136/jnnp-2013-307207 [DOI] [PubMed] [Google Scholar]
- 26.Sormani MP, Muraro PA, Saccardi R, Mancardi G. NEDA status in highly active MS can be more easily obtained with autologous hematopoietic stem cell transplantation than other drugs. Mult Scler. 2017;23(2):201-204. doi: 10.1177/1352458516645670 [DOI] [PubMed] [Google Scholar]
- 27.Roxburgh RH, Seaman SR, Masterman T, et al. Multiple Sclerosis Severity Score: using disability and disease duration to rate disease severity. Neurology. 2005;64(7):1144-1151. doi: 10.1212/01.WNL.0000156155.19270.F8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Lineplot of EDSS Change in Each Patient
eFigure 2. NEDA (Disease Free Survival) of Disease Modifying Therapy Versus Hematopoietic Stem Cell Transplantation
eFigure 3. Time to Progression, Time to First Relapse, and NEDA (Disease Free Survival) for Control Patients Treated With Natalizumab
eTable 1. Comparison of Expanded Disability Status Scale (EDSS), Neurologic Rating Scale (NRS) and Multiple Sclerosis Functional Composite (MSFC) Score
eTable 2. Secondary Outcomes of All Patients Getting HSCT, Original HSCT Group, and DMT That Crossed Over to HSCT
eTable 3. Transplant Toxicities
Data Sharing Statement