Key Points
Question
What is the clinical benefit of lowering plasma triglyceride levels compared with lowering low-density lipoprotein cholesterol levels?
Findings
In mendelian randomization analyses involving 654 783 participants, triglyceride-lowering variants in the lipoprotein lipase gene and low-density lipoprotein cholesterol (LDL-C)–lowering variants in the LDL receptor gene were associated with similar lower risk of coronary heart disease per 10-mg/dL lower level of apolipoprotein B (ApoB)–containing lipoproteins (odds ratios of 0.771 and 0.773, respectively).
Meaning
The clinical benefit of lower triglyceride levels was similar to the clinical benefit of lower LDL-C levels per unit difference in ApoB and may be related to the absolute reduction in ApoB-containing lipoprotein particles.
Abstract
Importance
Triglycerides and cholesterol are both carried in plasma by apolipoprotein B (ApoB)–containing lipoprotein particles. It is unknown whether lowering plasma triglyceride levels reduces the risk of cardiovascular events to the same extent as lowering low-density lipoprotein cholesterol (LDL-C) levels.
Objective
To compare the association of triglyceride-lowering variants in the lipoprotein lipase (LPL) gene and LDL-C–lowering variants in the LDL receptor gene (LDLR) with the risk of cardiovascular disease per unit change in ApoB.
Design, Setting, and Participants
Mendelian randomization analyses evaluating the associations of genetic scores composed of triglyceride-lowering variants in the LPL gene and LDL-C–lowering variants in the LDLR gene, respectively, with the risk of cardiovascular events among participants enrolled in 63 cohort or case-control studies conducted in North America or Europe between 1948 and 2017.
Exposures
Differences in plasma triglyceride, LDL-C, and ApoB levels associated with the LPL and LDLR genetic scores.
Main Outcomes and Measures
Odds ratio (OR) for coronary heart disease (CHD)—defined as coronary death, myocardial infarction, or coronary revascularization—per 10-mg/dL lower concentration of ApoB-containing lipoproteins.
Results
A total of 654 783 participants, including 91 129 cases of CHD, were included (mean age, 62.7 years; 51.4% women). For each 10-mg/dL lower level of ApoB-containing lipoproteins, the LPL score was associated with 69.9-mg/dL (95% CI, 68.1-71.6; P = 7.1 × 10−1363) lower triglyceride levels and 0.7-mg/dL (95% CI, 0.03-1.4; P = .04) higher LDL-C levels; while the LDLR score was associated with 14.2-mg/dL (95% CI, 13.6-14.8; P = 1.4 × 10−465) lower LDL-C and 1.9-mg/dL (95% CI, 0.1-3.9; P = .04) lower triglyceride levels. Despite these differences in associated lipid levels, the LPL and LDLR scores were associated with similar lower risk of CHD per 10-mg/dL lower level of ApoB-containing lipoproteins (OR, 0.771 [95% CI, 0.741-0.802], P = 3.9 × 10−38 and OR, 0.773 [95% CI, 0.747-0.801], P = 1.1 × 10−46, respectively). In multivariable mendelian randomization analyses, the associations between triglyceride and LDL-C levels with the risk of CHD became null after adjusting for differences in ApoB (triglycerides: OR, 1.014 [95% CI, 0.965-1.065], P = .19; LDL-C: OR, 1.010 [95% CI, 0.967-1.055], P = .19; ApoB: OR, 0.761 [95% CI, 0.723-0.798], P = 7.51 × 10−20).
Conclusions and Relevance
Triglyceride-lowering LPL variants and LDL-C–lowering LDLR variants were associated with similar lower risk of CHD per unit difference in ApoB. Therefore, the clinical benefit of lowering triglyceride and LDL-C levels may be proportional to the absolute change in ApoB.
This mendelian randomization analysis compares the association of triglyceride-lowering variants in the lipoprotein lipase (LPL) gene and of LDL-C–lowering variants in the LDL receptor gene (LDLR) with coronary heart disease (CHD) risk per unit change in apolipoprotein B.
Introduction
All major clinical guidelines recommend treatment to lower plasma low-density lipoprotein cholesterol (LDL-C) because numerous randomized trials have demonstrated that therapies that lower LDL-C levels by reducing LDL particles through upregulation of the LDL receptor (LDLR) reduce the risk of cardiovascular events.1,2,3,4,5 By contrast, the guidelines do not recommend treatment to lower plasma triglyceride levels because randomized trials have not provided consistent evidence that lowering plasma triglyceride levels reduces the risk of cardiovascular events.1,2
Several novel therapies that potently reduce triglyceride levels are currently in development.6,7,8 The development of these therapies has been motivated in part by the observation that rare loss-of-function mutations in the lipoprotein lipase (LPL) gene are associated with higher plasma triglyceride levels and a higher risk of cardiovascular disease; while rare, loss-of-function mutations in the APOC3, ANGPTL3, and ANGPTL4 genes, which encode for natural inhibitors of LPL, are associated with lower triglyceride levels and a corresponding lower risk of cardiovascular disease.9,10,11,12,13 However, it is unknown whether lowering plasma triglyceride levels by targeting the LPL pathway will reduce the risk of cardiovascular events.
Both triglycerides and cholesterol are carried in plasma by apolipoprotein B (ApoB)–containing lipoprotein particles. Because all ApoB-containing lipoproteins, including triglyceride-rich lipoprotein particles and LDL particles, have a single ApoB molecule the clinical benefit of lowering triglyceride levels can be compared with the clinical benefit of lowering LDL-C levels by estimating their effects per unit change in ApoB. Therefore, the objective of this study was to use mendelian randomization to compare the association of triglyceride-lowering LPL variants and LDL-C–lowering LDLR variants with the risk of cardiovascular disease per unit difference in ApoB to make inferences about the potential clinical benefit of lowering plasma triglyceride levels as compared with lowering LDL-C levels.
Methods
Study Population
The study included individual participant data from 367 641 participants enrolled in the UK Biobank study, individual participant data from 102 837 participants enrolled in 1 of 14 prospective cohort or case-control studies that reported data on cardiovascular outcomes in the US National Center for Biotechnology Information Database of Genotypes and Phenotypes program (dbGAP), and summary-level data from 184 305 participants enrolled in 1 of 48 prospective cohort, case-control, or cross-sectional studies included in the Coronary Artery Disease Genomewide Replication and Meta-Analysis plus the Coronary Artery Disease (CARDIoGRAMplusC4D) Consortium.14,15,16 Participants of European descent in the UK Biobank, and all racial/ethnic groups for which cardiovascular data were reported in the dbGAP and CARDIoGRAMplusC4D Consortium studies, were included in the analysis. In each included study, race/ethnicity was self-identified using a study-specific fixed-category questionnaire and was recorded to allow assessment of potential heterogeneity of effect estimates by ethnicity.
Contributing studies received ethical approval from their respective institutional review boards, and written informed consent was obtained from all participants. A description of the included studies and the genotyping platforms used in each study is provided in eTable 1 in the Supplement.
Genetic Instruments
The LPL genetic score was constructed by combining all variants within 100kb on either side of the LPL gene that were associated with plasma triglyceride levels at genome-wide level of significance (P < 5.0 × 10−8) as reported by the Global Lipids Genetics Consortium and that were in low linkage disequilibrium (r2 < 0.3) with all other variants included in the score.17,18 The LDLR genetic score was constructed similarly by combining all variants within 100kb on either side of the LDLR gene that were associated with plasma LDL-C levels at genome-wide level of significance and that were in low linkage disequilibrium (r2 < 0.3) with all other variants included in the score. The exposure allele for each LPL variant was defined as the allele associated with lower plasma triglyceride levels, and the exposure allele for each LDLR variant was defined as the allele associated with lower LDL-C levels.17,18 For each participant, an LPL genetic score was calculated by summing the number of triglyceride-lowering alleles that participants inherited at each variant included in the LPL score and an LDLR score was calculated by summing the number of LDL-C–lowering alleles that participants inherited at each variant included in the LDLR score. Participants were excluded if they had missing data for 1 or more variants included in either genetic score.
Study Outcomes
The primary clinical outcome was coronary heart disease (CHD) defined as a composite of prevalent or the first incident occurrence of myocardial infarction (MI), coronary revascularization, or coronary death. For analyses involving individual participant data, the primary clinical outcome was harmonized across all included studies. For analyses involving summary-level data, the definition of CHD was defined by each study included in the CARDIoGRAMplusC4D consortium, which included CHD death, MI, and coronary revascularization but in some studies also included chronic stable angina or more than 50% stenosis in a major epicardial coronary artery.16
Study Design and Statistical Analysis
A description of the study design, analyses performed, and data used for each analysis is provided in eFigures 1-3 in the Supplement. The association of each genetic score with plasma triglycerides, LDL-C, and ApoB was evaluated using linear regression and with CHD risk using logistic regression. All regression analyses were performed separately in each of the included studies adjusting for age, sex, and the first 5 principal components of ancestry. To directly compare the clinical benefit of lower triglyceride levels due to the LPL score with lower LDL-C levels due to the LDLR genetic score, the associations of each score with risk of CHD was scaled for a common 10-mg/dL lower level of ApoB-containing lipoproteins. For individual participant data, the scaled point estimates were obtained by weighting each variant included in either genetic score by its associated change in ApoB. For summary-level data, the scaled associations were obtained by dividing the reported point estimate (and standard error) for an outcome by the reported point estimate for ApoB (measured in mg/dL). The scaled summary point estimates for all variants included in a score were then combined in a fixed-effect inverse variance-weighted meta-analysis to estimate the association between that genetic score generated using summary data and the outcome for a 10-mg/dL lower level of ApoB-containing lipoproteins.
The point estimates derived from the individual participant data and the summary data were then combined across studies in a fixed-effect inverse variance-weighted meta-analysis to produce an overall summary point estimate using a previously reported method that accounts for correlation between variants.19
Effect modification between lowering triglyceride levels through the LPL pathway and lower LDL-C levels through the LDL receptor pathway was assessed by comparing the associations of each genetic score with the risk of CHD stratified by the other genetic score. The association of combined exposure to triglyceride-lowering LPL variants and LDL-C–lowering LDLR variants with the risk of CHD was evaluated in a 2 × 2 factorial mendelian randomization analysis.20,21,22,23 For both the stratified and factorial analyses, associations with the risk of CHD were necessarily restricted to participants with individual data and associations with changes in triglycerides, LDL-C, and ApoB were necessarily restricted to participants with individual data for whom 1 or more lipid measurements were available.
Sensitivity Analyses
To compare the potential clinical benefit of pharmacologically lowering triglyceride and LDL-C levels, the associations of the LDLR and LPL scores with the risk of CHD per unit difference in ApoB were compared with variants in the genes that encode the targets of current therapies that lower LDL-C through the LDL receptor pathway; variants in the genes that encode the targets of potential therapies that lower triglycerides through the LPL pathway; and variants in the APOB gene. To compare the association of triglyceride and LDL-C levels with the risk of CHD per unit difference in ApoB not related to the LPL and LDLR genes, several additional genetic scores were constructed using up to 178 genetic variants associated with either triglycerides, LDL-C, or both at genome-wide significance as reported by the Global Lipids Genetics Consortium.17,18
To further assess the independent associations of lower triglycerides, lower LDL-C, and lower ApoB on the risk of CHD, a multivariable mendelian randomization analysis was performed using these 178 genetic variants combined with the LPL and LDLR variants. This analysis was performed using meta-regression analyses in which the dependent variable was the associated log-odds for the risk of CHD, and the independent variables were the reported differences in plasma triglycerides, LDL-C, and ApoB for each variant included in the analysis, weighted by the inverse of the squared standard error for the association of each variant with CHD and forced to pass through the origin.
All analyses were performed using Stata (version 14.2; StataCorp), R (version 3.2.2; R Project for Statistical Computing), or Golden Helix SNP & Variation Suite software (version 8.1.4). A 2-tailed P value less than .05 was considered statistically significant. A detailed description is provided in eMethods in the Supplement.
Results
Participant Characteristics
A total of 654 783 participants, including 91 129 cases of CHD, were included in the analysis (mean age, 62.7 years; 51.4% women). Individual participant data were available for 470 478 participants including 30 328 cases of CHD (Table 1). Summary-level data were available for a further 184 305 participants, including 60 801 cases of CHD.
Table 1. Baseline Characteristics of Study Participantsa.
| Characteristic | Participant Baseline Characteristics | No. of Participants With Available Data for Each Baseline Characteristic |
|---|---|---|
| Sample size, No. | 654 783 | 654 783 |
| CHD cases, No. | 91 129 | 654 783 |
| Age, mean (SD), y | 62.7 (8.1) | 654 783 |
| Sex | ||
| Women, No. (%) | 336 462 (51.4) | 654 783 |
| Men, No. (%) | 318 321 (48.6) | 654 783 |
| Blood pressure, mean (SD), mm Hg | ||
| Systolic | 132.1 (18.2) | 470 478 |
| Diastolic | 80.9 (9.3) | 470 478 |
| Body mass index, mean (SD)b | 27.5 (4.9) | 470 478 |
| Prevalent diabetes, No. (%) | 21 642 (4.6) | 470 478 |
| Current smoker, No. (%) | 43 284 (9.2) | 470 478 |
| Cholesterol, mean (SD), mg/dL | ||
| Total | 206.6 (39.4) | 31 221 |
| Low-density lipoprotein | 129.7 (32.1) | 31 221 |
| High-density lipoprotein | 52.0 (15.4) | 31 221 |
| Triglycerides, median (IQR), mg/dL | 117.6 (84.1-163.3) | 31 221 |
| Apolipoprotein B, mean (SD), mg/dL | 101.4 (27.3) | 31 221 |
| Non–high-density lipoprotein cholesterol (total cholesterol−HDL-C), mean (SD), mg/dL | 154.9 (38.3) | 31 221 |
| Remnant cholesterol (total cholesterol−HDL-C−LDL-C), median (IQR), mg/dL | 23.9 (15.9-32.8) | 31 221 |
Abbreviations: CHD, coronary heart disease; IQR, interquartile range.
SI conversion factors: To convert cholesterol to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113.
Data for age and sex were available for all 654 783 participants included in the primary analysis. Data for other nonlipid baseline characteristics were available for 470 478 participants with individual-level data enrolled in the UK Biobank or 1 of the 14 case-control or cohort studies that reported cardiovascular outcomes in the database of genotypes and phenotypes program (dbGAP). Data for baseline lipid measurements were available for 31 221 participants with individual data enrolled in 1 of the dbGAP studies and for whom lipid measurements were available (the UK Biobank has not yet released lipid measurements of enrolled participants). Non–high-density lipoprotein cholesterol and remnant cholesterol levels are calculated values.
Calculated as weight in kilograms divided by height in meters squared.
LPL and LDLR Genetic Scores
A total of 5 independently inherited variants were included in the LPL score (eTables 2 and 3 in the Supplement) and 3 independently inherited variants were included in the LDLR score (eTables 4 and 5 in the Supplement). Each exposure allele in the LPL score was associated with an inverse variance-weighted mean of 11.64-mg/dL (95% CI, 10.38-10.90; P = 8.3 × 10−1365) lower plasma triglyceride level (to convert to mmol/L, multiply by 0.0113), 0.11-mg/dL (95% CI, 0.00-0.21; P = .04) higher plasma LDL-C level (to convert to mmol/L, multiply by 0.0259, and a 1.72-mg/dL (95% CI, 1.30-2.14; P = 5.5 × 10−16) lower level of ApoB-containing lipoproteins. By contrast, each exposure allele in the LDLR score was associated with an inverse variance-weighted mean of 3.42-mg/dL (95% CI, 3.27-3.57; P = 2.3 × 10−464) lower plasma LDL-C level, 0.48-mg/dL (95% CI, 0.03-0.93; P = .04) lower plasma triglyceride level, and a 2.40-mg/dL (95% CI, 2.02-2.79; P = 3.9 × 10−34) lower level of ApoB-containing lipoproteins.
Association of Genetic Scores With Lipids and CHD per Unit Change in ApoB
For each 10-mg/dL lower level of ApoB-containing lipoproteins, the LPL score was associated with 69.9-mg/dL (95% CI, 68.1-71.6; P = 7.1 × 10−1363) lower plasma triglyceride levels and 0.7-mg/dL (95% CI, 0.03-1.4; P = .04) higher plasma LDL-C level (Figure 1). By contrast, for the same 10-mg/dL lower level of ApoB-containing lipoproteins, the LDLR score was associated with 14.2-mg/dL (95% CI, 13.6-14.8; P = 1.4 × 10−465) lower plasma LDL-C level and 1.9-mg/dL (95% CI, 0.1-3.9; P = .04) lower plasma triglyceride level. Despite these differences in associated lipid levels, the LPL and LDLR scores were associated with similar lower risk of CHD per 10-mg/dL lower level of ApoB-containing lipoproteins (odds ratio [OR], 0.771 [95% CI, 0.741-0.802], P = 3.9 × 10−38 for the LPL score and OR, 0.773 [95% CI, 0.747-0.801], P = 1.1 × 10−46 for the LDLR score). The associations of the LPL and LDLR scores with the risk of CHD per unit lower ApoB was consistent between studies that contributed individual participant data and studies that contributed summary data (eTable 6 in the Supplement).
Figure 1. Associations Between the Lipoprotein Lipase (LPL) and LDL Receptor Gene (LDLR) Genetic Scores With Triglycerides, Low-Density Lipoprotein Cholesterol (LDL-C), and Risk of Coronary Heart Disease (CHD) per 10-mg/dL Lower Concentration of Apolipoprotein B (ApoB)–Containing Lipoproteins.
Triglycerides are carried in plasma by ApoB-containing triglyceride-rich lipoproteins while cholesterol is carried predominantly by ApoB-containing low-density lipoproteins. Changes in plasma triglycerides and LDL-C concentration are thus markers of the corresponding changes in the concentration of the ApoB-containing lipoproteins that transport these lipids. Variants in the LPL gene that increase LPL activity are associated with lower triglycerides and a corresponding lower ApoB concentration, while variants in the LDLR gene that increase activity of the LDL receptor are associated with lower LDL-C and a corresponding lower ApoB. The figure shows that for each 10-mg/dL lower plasma ApoB concentration associated with variants in the LPL score, there is a corresponding 69.9-mg/dL lower triglyceride level, no change in LDL-C, and a lower risk of CHD (odds ratio, 0.771 [95% CI, 0.741-0.802]). By contrast, for the same 10-mg/dL lower plasma ApoB concentration associated with variants in the LDLR score, there is a corresponding 14.1-mg/dL lower LDL-C level, no change in triglycerides, and a similar lower risk of CHD (odds ratio, 0.773 [95% CI, 0.747-0.801]). Therefore, despite being associated with changes in different lipids, the LPL and LDLR scores were associated with similar lower risk of CHD for the same lower plasma ApoB concentration. The data presented are for the associations of the LPL and LDLR genetic scores with risk of CHD per 10-mg/dL decrease in ApoB-containing lipoproteins in all 654 783 participants included in the study. The associations of either score with changes in triglycerides and LDL-C per 10-mg/dL lower level of ApoB-containing lipoproteins are from up to 305 699 participants enrolled in the Global Lipid Genetics Consortium. Boxes represent effect size estimates and lines represent 95% CIs.
In stratified analyses, the associations of the LPL and LDLR scores with plasma lipids, lipoproteins, and the risk of CHD appeared to be independent of each other (LPL score OR for CHD per 10-mg/dL lower ApoB, 0.771 [95% CI, 0.714-0.832] for participants with LDLR scores below the median and 0.769 [95% CI, 0.709-0.834] for participants with LDLR scores above the median) (eFigure 4 in the Supplement). In a 2 × 2 factorial mendelian randomization analysis, combined exposure to both the LPL and LDLR genetic scores was associated with linearly additive lower levels of triglyceride (LPL score alone: −20.1 mg/dL [95% CI, −28.8 to −13.3]; LDLR score alone: −3.8 mg/dL [95% CI, −15.1 to 7.3]; combined exposure to both scores: −24.3 mg/dL [95% CI, −32.4 to −16.2]), LDL-C levels (LPL score alone: −0.1 mg/dL [95% CI, −0.5 to 0.3]; LDLR score alone: −4.8 mg/dL [95% CI, −7.6 to −2.0]; combined exposure to both scores: −4.9 mg/dL [95% CI, −7.7 to −2.1]), and ApoB (LPL score alone: −3.0 mg/dL [95% CI, −4.9 to −1.2]; LDLR score alone: −3.4 mg/dL [95% CI, −5.2 to −1.5]; combined exposure to both scores: −6.4 mg/dL [95% CI, −8.5 to −4.4]), as well as a log-linearly additive decreases in the risk of CHD (LPL score alone: OR, 0.924 [95% CI, 0.889-0.960]; LDLR score alone: OR. 0.921 [95% CI: 0.885-0.958]; combined exposure to both scores: OR, 0.842 [95% CI, 0.811-0.874]) that was proportional to the absolute difference in ApoB but not to differences in either triglycerides or LDL-C (eFigure 5 in the Supplement).
Sensitivity Analyses
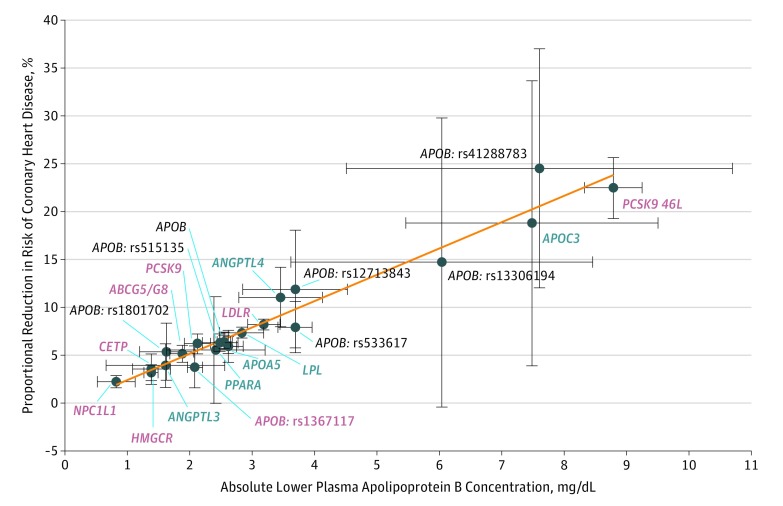
In additional analyses, variants in the genes that encode the targets for several potential therapies that lower triglycerides through the LPL pathway, and variants in the genes that encode the targets of several current therapies that lower LDL-C through the LDLR pathway, were also associated with similar lower risk of CHD per unit difference in ApoB as compared with the LPL and LDLR scores and as compared with an APOB score composed of 8 independently inherited variants in the APOB gene (Figure 2). Furthermore, the associated lower CHD risk for each of these variants and genetic scores was log-linearly proportional to their associated absolute lower level of ApoB-containing lipoproteins (Figure 3).
Figure 2. Association of Genetic Variants and Genetics Scores With Triglycerides, Low-Density Lipoprotein Cholesterol (LDL-C), and Risk of Coronary Heart Disease (CHD) per 10-mg/dL Lower Concentration of Apolipoprotein B (ApoB)–Containing Lipoproteins.
The figure shows the associations with triglycerides, LDL-C, and risk of CHD for the same 10-mg/dL lower ApoB-containing lipoprotein concentration for variants in the LPL and LDLR scores as compared with variants in the genes that encode the targets of current therapies that lower LDL-C through the LDL receptor pathway; variants in the genes that encode the targets of potential therapies that lower triglycerides through the LPL pathway; and variants in the APOB gene. For example, each 10-mg/dL lower plasma ApoB concentration associated with the partial loss-of-function rs11591147 variant in the PCSK9 gene was associated with a corresponding 18.0-mg/dL lower LDL-C level, no change in triglycerides, and a lower risk of CHD (odds ratio, 0.774 [95% CI, 0.721-0.832]). By contrast, for the same 10-mg/dL lower plasma ApoB concentration associated with the functional rs116843064 variant in the ANGPTL4 gene, there was a corresponding 69.4-mg/dL lower triglyceride level, no change in LDL-C, and a similar lower risk of CHD (odds ratio, 0.726 [95% CI, 0.609-0.865]). Furthermore, for the same 10-mg/dL lower plasma ApoB concentration associated with variants in the APOB gene score, there was a corresponding 9.7-mg/dL lower triglyceride level, 15.6-mg/dL lower LDL-C level, and a similar lower risk of CHD (odds ratio, 0.778 [95% CI, 0.737-0.821]). Despite a range of associated changes in triglycerides, LDL-C, or both, all genetic variants and genetic scores were associated with similar lower risk of CHD for the same 10-mg/dL lower plasma ApoB concentration. The APOB score is composed of the 8 independently inherited variants in the APOB gene listed in the figure. Boxes represent effect size estimates and lines represent 95% CIs. Associations with CHD per 10-mg/dL lower ApoB were measured in all 654 783 participants included in the study; associations with changes in triglycerides and LDL-C per 10-mg/dL lower ApoB were measured in up to 305 699 participants from the Global Lipid Genetics Consortium.
Figure 3. Log-Linear Association Between Absolute Differences in Apolipoprotein B (ApoB) and Lower Risk of Coronary Heart Disease (CHD).
The associations of each genetic variant with ApoB concentration is plotted against its unadjusted association with CHD, expressed as a proportional lower risk (calculated as [1−ORCHD]×100). Variants in the genes that encode the targets of therapies that lower triglycerides through the LPL pathway are marked with blue labels, and variants in the genes that encode the targets of therapies that lower LDL-C through upregulation of the LDL receptor are marked by red labels. Circles represent the associated absolute change in ApoB and corresponding proportional lower risk of CHD for each variant. The horizontal lines through each circle represents ±1 standard errors for the associated absolute change in ApoB for each variant; and the vertical line through each circle represents ±1 standard errors for the associated proportional lower risk of CHD. Associations with CHD were measured in all 654 783 participants included in the study; associations with ApoB were measured in a meta-analysis of 14 studies including up to 84 324 participants.
Several additional genetic scores consisting of other variants associated with triglycerides or LDL-C at genome-wide level of significance (excluding variants in the LPL and LDLR genes)—including scores consisting of variants associated with either triglycerides or LDL-C; triglycerides but not LDL-C; LDL-C but not triglycerides; both triglycerides and LDL-C with the same direction of effect; and both triglycerides and LDL-C with opposite directions of effect—were also associated with similar lower risk of CHD per 10-mg/dL lower level of ApoB-containing lipoproteins (Table 2). In multivariable mendelian randomization analyses that included both triglycerides and LDL-C in the same model, the associations between plasma triglycerides and LDL-C with the risk of CHD were independent and genome-wide significant. However, when changes in ApoB were included in these analyses, the associations between both plasma triglycerides and LDL-C with the risk of CHD became null (triglycerides: OR, 1.014 [95% CI, 0.965-1.065], P = .19; LDL-C: OR, 1.010 [95% CI, 0.967-1.055], P = .19; and ApoB: OR, 0.761 [95% CI, 0.723-0.798], P = 7.51 × 10−20) (Table 3; eTable 8 in the Supplement).
Table 2. Association of Additional Genetic Scores With Triglycerides, LDL-C, and Risk of CHD per 10-mg/dL Lower Concentration of ApoB-Containing Lipoproteinsa.
| Composition of Genetic Score | ∆ Triglycerides (95% CI) | ∆ LDL-C (95% CI) | Odds Ratio for CHD (95% CI) per 10-mg/dL Lower ApoB |
|---|---|---|---|
| 51 Variants associated with triglycerides at P < 5.0×10−8, but not LDL-C (P > .001) | −43.1 (−44.5 to −41.7) | −2.1 (−2.6 to −1.6) | 0.762 (0.724 to 0.803) |
| 59 Variants associated with LDL-C at P < 5.0×10−8, but not triglycerides (P > .001) | −2.1 (−3.0 to −1.1) | −15.5 (−15.8 to −15.1) | 0.774 (0.748 to 0.800) |
| 168 Variants associated with either triglycerides or LDL-C at P < 5.0×10−8 | −21.6 (−22.1 to −21.1) | −11.8 (−12.0 to −11.6) | 0.770 (0.757 to 0.783) |
| 91 Variants associated with triglycerides at P < 5.0×10−8 | −35.3 (−35.9 to −34.6) | −9.3 (−9.6 to −9.1) | 0.776 (0.758 to 0.795) |
| 100 Variants associated with LDL-C at P < 5.0×10−8 | −17.5 (−18.0 to −17.0) | −13.5 (−13.7 to −13.3) | 0.776 (0.762 to 0.791) |
| 23 Variants associated with both triglycerides and LDL-C, both at P < 5.0×10−8, in same direction of effect | −32.3 (−33.0 to −31.5) | −12.0 (−12.3 to −11.7) | 0.793 (0.771 to 0.815) |
| 10 Variants associated with both triglycerides and LDL-C, both at P < 5.0×10−8, with opposite directions of effect | 17.2 (16.0 to 18.4) | −22.5 (−23.0 to −22.1) | 0.798 (0.767 to 0.830) |
| 9 Variants associated with both triglycerides and LDL-C, both at P < 5.0×10−8, with opposite directions of effect (excluding APOE variant rs7412) | 26.0 (23.7 to 28.3) | −20.3 (−21.2 to −19.4) | 0.770 (0.711 to 0.833) |
Abbreviations: ApoB, apolipoprotein B; CHD, coronary heart disease; LDL-C, low-density lipoprotein cholesterol; OR, odds ratio.
To compare the association of triglycerides and LDL-C with the risk of CHD for the same lower concentration of ApoB-containing lipoproteins not related to the LPL and LDLR genes, several additional genetic scores were constructed using up to 178 genetic variants associated with either triglycerides, LDL-C, or both at genome-wide significance as reported by the Global Lipids Genetics Consortium (GLGC). The data presented are for the associations of each genetic score with changes in triglycerides and LDL-C per 10-mg/dL lower ApoB in up to 305 699 participants in the GLGC and with the risk of CHD per 10-mg/dL lower ApoB in all 654 783 participants included in this study. For example, for each 10-mg/dL lower plasma ApoB concentration associated with a genetic score consisting of 51 variants associated with triglycerides but not LDL-C at genome-wide level of significance, there was a corresponding 41.3-mg/dL lower triglyceride level, 2.1-mg/dL lower LDL-C level, and a lower risk of CHD (odds ratio [OR], 0.762 [95% CI, 0.724-0.803]). By contrast, for the same 10-mg/dL lower plasma ApoB concentration associated with a genetic score consisting of 59 variants associated with LDL-C but not triglycerides at genome-wide level of significance, there was a corresponding 15.5-mg/dL lower LDL-C level, 2.1-mg/dL lower triglyceride level, and a similar lower risk of CHD (OR, 0.774 [95% CI, 0.748-0.800]). Furthermore, for the same 10-mg/dL lower plasma ApoB concentration associated with a genetic score consisting of 168 variants associated with either triglycerides or LDL-C at genome-wide level of significance, there was a corresponding 21.6-mg/dL lower triglyceride level, an 11.8-mg/dL lower LDL-C level, and a similar lower risk of CHD (OR, 0.770 [95% CI, 0.757-0.783]). Despite being associated with different changes in lipids, all genetic scores were associated with similar lower risk of CHD for the same 10-mg/dL lower plasma ApoB concentration. The unadjusted associations with triglycerides, LDL-C, ApoB, and CHD for each variant included in the genetic scores are provided in eTable 7 in the Supplement.
Table 3. Multivariable Mendelian Randomization Analysis of the Association Between Plasma Triglycerides, LDL-C, and ApoB With the Risk of CHDa.
| Analysis | Variables | Odds Ratio for CHD (95% CI) |
P Value |
|---|---|---|---|
| Association of 10-mg/dL lower ApoB with risk of CHD | ApoB | 0.770 (0.760-0.781) | 1.42E-170 |
| Association of 10-mg/dL lower LDL-C with risk of CHD | LDL-C | 0.846 (0.833-0.858) | 8.16E-77 |
| Association of 50-mg/dL lower triglycerides with risk of CHD | Triglycerides | 0.815 (0.785-0.846) | 1.37E-18 |
| Association of 10-mg/dL lower LDL-C and 50-mg/dL lower triglycerides with risk of CHD included in same model | LDL-C | 0.862 (0.849-0.875) | 6.92E-65 |
| Triglycerides | 0.876 (0.850-0.902) | 1.36E-14 | |
| Association of 10-mg/dL lower LDL-C, 50-mg/dL lower triglycerides, and 10-mg/dL lower ApoB with risk of CHD included in same model | ApoB | 0.761 (0.723-0.798) | 7.51E-20 |
| LDL-C | 1.010 (0.967-1.055) | .19 | |
| Triglycerides | 1.014 (0.965-1.065) | .19 |
Abbreviations: ApoB, apolipoprotein B; CHD, coronary heart disease; LDL-C, low-density lipoprotein cholesterol.
Data presented are derived from a multivariable meta-regression analysis of 186 genetic variants, including the 5 variants included in the LPL score, 3 variants included in the LDLR score, and 178 variants associated with either triglycerides, LDL-C, or both at genome-wide significance as reported by the Global Lipids Genetics Consortium. Effect sizes for the associated risk of CHD are reported per 10-mg/dL lower ApoB concentration, per 10-mg/dL lower LDL-C level, or per 50-mg/dL lower triglyceride level (because dividing triglyceride concentration by 5 estimates the cholesterol content carried by triglyceride-rich ApoB-containing lipoproteins as estimated by the Friedewald formula). In these analyses, the dependent variable was the effect estimate for risk of CHD in all 654 783 participants included in the study for each variant and the independent variables were the effect estimates for the associated changes in plasma triglycerides, LDL-C, and ApoB, measured in up to 305 699 participants in Global Lipids Genetics Consortium for each variant. The analysis was weighted by the inverse squared standard error of the associated risk of CHD for each variant and forced to pass through the origin. For example, in multivariable mendelian randomization analyses involving these 186 genetic variants, both triglycerides (odds ratio [OR], 0.876 per 50-mg/dL lower triglycerides) and LDL-C (OR, 0.862 per 10-mg/dL lower LDL-C) were independently associated with a lower risk of CHD at genome-wide level of significance. By contrast, when ApoB was included in the multivariable mendelian randomization analyses, the associations with CHD for both triglycerides (OR, 1.014 per 50-mg/dL lower triglycerides) and LDL-C (OR, 1.010 per 10-mg/dL lower LDL-C) became null, but the association per 10-mg/dL lower ApoB remained unchanged (OR, 0.761 per 10-mg/dL lower ApoB). The unadjusted associations with triglycerides, LDL-C, ApoB, and CHD for each variant included in the analysis are provided in eTable 7 in the Supplement. Additional multivariable meta-regression analyses for various combinations of these variants is provided in eTable 8 in the Supplement.
Discussion
In this study, triglyceride-lowering LPL variants and LDL-C–lowering LDLR variants were associated with similar lower CHD risk per unit lower level of ApoB-containing lipoproteins. The associations between lower triglyceride level and lower LDL-C level with risk of CHD due to these variants appeared to be independent, additive, and proportional to the absolute change in ApoB. In addition, numerous variants in the genes that encode the targets of potential therapies that lower triglyceride levels through the LPL pathway and current therapies that lower LDL-C levels through the LDLR pathway were also associated with similar lower CHD risk per unit lower plasma ApoB levels. Furthermore, multiple genetic scores composed of other variants associated with either triglycerides, LDL-C, or both were also associated with similar lower risk of CHD per unit lower level of ApoB-containing particles, even when the associated changes in triglyceride and LDL-C levels were in opposite directions. In multivariable mendelian randomization analyses, the independent and genome-wide significant associations between triglycerides and LDL-C with the risk of CHD became null after adjusting for changes in ApoB.
The results of this study suggest that the clinical benefit of lowering triglyceride levels is similar to the clinical benefit of lowering LDL-C levels per unit change in ApoB and is proportional to the net absolute reduction in ApoB-containing lipoproteins. The results of this study therefore suggest that all ApoB-containing lipoprotein particles, including triglyceride-rich very-low-density lipoprotein (VLDL) particles and their metabolic remnants as well as LDL particles, have approximately the same effect on the risk of cardiovascular disease per particle. As a result, the clinical benefit of lowering triglyceride levels, lowering LDL-C levels, or lowering both may be proportional to the absolute change in ApoB-containing lipoproteins, regardless of the observed changes in plasma triglycerides or LDL-C.
The results of this study are consistent with the current understanding of the biology of lipids and atherosclerosis. Both triglycerides and cholesterol are carried in plasma by ApoB-containing lipoprotein particles. These particles are secreted by the liver as VLDL particles, which principally contain triglycerides, some cholesterol, and 1 molecule of ApoB. Lipoprotein lipase removes most of the triglycerides from these particles to convert the triglyceride-rich VLDL particles into triglyceride-depleted cholesterol-carrying LDL particles, which are then removed from plasma by hepatic LDL receptors. All ApoB-containing lipoproteins less than 70 nm in diameter, including triglyceride-rich VLDL remnants and LDL particles, freely flux across the endothelial barrier where they can become retained in the artery wall.24 The cholesterol, and perhaps triglyceride, content of the ApoB particles retained in the artery wall provokes an inflammatory response that leads to the initiation and progression of atherosclerotic plaque.25 The results of this study suggest that the effect of ApoB-containing particles on the risk of atherosclerotic cardiovascular disease appears to be determined largely by the concentration of circulating ApoB particles, which in turn determines the number of particles that become retained in the artery wall, regardless of whether those particles principally contain cholesterol or triglycerides. The present findings and interpretations based on mendelian randomization confirm and extend the initial findings and interpretations in 1980 of Sniderman and colleagues,26 which were based on cross-sectional coronary angiographic studies.
The results of this study are also consistent with prior mendelian randomization studies demonstrating that triglyceride-rich ApoB-containing remnant particles appear to be causally associated with the risk of cardiovascular disease.27,28 The results of the current study extend those findings by suggesting that triglyceride-rich remnant particles have approximately the same effect on the risk of cardiovascular disease as LDL particles. Furthermore, the results of this study are consistent with a recent mendelian randomization study that demonstrated that the causal effect of LDL particles on the risk of cardiovascular disease appears to be determined by the concentration of circulating LDL particles as measured by ApoB rather than by the mass of cholesterol carried by those particles as measured by LDL-C.23 The results of the current study confirm and extend those findings by suggesting that the causal effect of all ApoB-containing lipoprotein particles on the risk of cardiovascular disease appears to be determined by the circulating concentration of those particles rather than by the mass of cholesterol or triglyceride that they carry.
The results of this study may also help to explain why prior randomized trials evaluating fibrates, which lower plasma triglyceride levels at least partially through the LPL pathway, have failed to consistently demonstrate that lowering triglyceride levels reduces the risk of cardiovascular events.29,30,31,32,33 The concentration of triglyceride-rich lipoproteins can be estimated by dividing plasma triglyceride concentration by 5 (on the mg/dL scale). Therefore, if all ApoB-containing particles have approximately the same atherogenic effect as suggested by this study, then to reduce the risk of cardiovascular events by 20% as can be achieved by lowering LDL-C levels by 40 mg/dL,3,4 triglyceride levels must be reduced by 5-fold this quantity, or approximately 200 mg/dL, to achieve the same corresponding reduction in ApoB-containing lipoproteins. However, the mean reduction in plasma triglyceride concentration in the fibrate trials was only 20 mg/dL to 50 mg/dL, a fraction of what would be needed to significantly reduce the risk of major vascular events within a short-term trial. Therefore, the results of the fibrate trials appear to be explained by the modest reductions in triglyceride level and therefore the modest corresponding reductions in ApoB-containing lipoproteins observed in these studies. Future randomized trials evaluating novel therapies that lower plasma triglyceride levels should be designed based on the net absolute reductions in ApoB-containing lipoproteins that can be achieved with those therapies, rather than on the corresponding therapeutic changes in triglycerides or LDL-C, particularly for therapies that alter plasma concentrations of both triglycerides and LDL-C either in the same or competing directions.
Limitations
This study has several limitations. First, this study compared triglyceride- and LDL-C–lowering genetic variants not lipid-lowering therapies. Second, genetic variants reflect the effect of lifelong changes in ApoB-containing lipoproteins on the risk of cardiovascular disease, which appear to be cumulative over time.5,34 As a result, the lower risk associated with lower triglycerides, LDL-C, and ApoB reported in this study is much larger than what have been reported for lipid-lowering therapies in randomized trials. However, having first established that the association between lifetime exposure to lower triglycerides and LDL-C on the risk of cardiovascular disease is approximately the same per unit lower level of ApoB-containing particles, it is reasonable to then anticipate that short-term pharmacologic reductions in plasma triglyceride and LDL-C levels will be associated with the same lower risk of cardiovascular events per unit change in ApoB.21 Third, this study specifically estimates the clinical benefit of the lipid-lowering effect of therapies that reduce plasma triglycerides, LDL-C, or both, but not the other potential pleiotropic effects that a therapy may have on the risk of cardiovascular disease. Indeed, the reported reductions in cardiovascular events in the JELIS and REDUCE-IT trials were far greater than what would have been expected from the modest observed changes in plasma lipid levels, thus suggesting that the observed clinical benefit of the omega-3 fatty acid eicosapentaenoic acid may be largely due to its non–lipid-related effects.35,36
Conclusions
Triglyceride-lowering LPL variants and LDL-C–lowering LDLR variants were associated with similar lower risk of CHD per unit difference in ApoB. Therefore, the clinical benefit of lowering triglyceride and LDL-C levels may be proportional to the absolute change in ApoB.
eMethods.
eTable 1. Included Studies and Genotyping Platforms
eTable 2. LPL Variants Included in LPL Score and Associations With Plasma Triglycerides and LDL-C in Global Lipids Genetics Consortium
eTable 3. Linkage Disequilibrium Matrix for Variants Included in the LPL Genetic Score
eTable 4. LDLR Variants Included in LDLR Genetic Score and Their Associations With Plasma Triglycerides and LDL-C in Global Lipids Genetics Consortium
eTable 5. Linkage Disequilibrium Matrix for Variants Included in the LDLR Genetic Score
eTable 6. Association of LPL and LDLR Scores With CHD Risk per 10 mg/dl Lower ApoB Among Studies Contributing Individual Participant Data and Summary Level Data
eTable 7. Variants Associated With Triglycerides or LDL-C in the Global Lipids Genetics Consortium
eTable 8. Additional Multivariable Mendelian Randomization Analyses for the Association Between Changes in Triglycerides, LDL-C, ApoB and the Risk of Coronary Heart Disease
eFigure 1. Study Design and Analyses
eFigure 2. Plot of LPL Variants Included in LPL Score
eFigure 3. Plot of LDLR Variants Included in LDLR Score
eFigure 4. Associations of the LPL and LDLR scores with plasma lipid levels and risk of Coronary Heart Disease for the Same Change in Plasma Concentration of ApoB-Containing Lipoproteins Stratified by the Other Score
eFigure 5. 2x2 Factorial Mendelian Randomization Analysis Evaluating the Separate and Combined Effects of the LPL and LDLR Scores on Plasma Lipids, Lipoproteins, and Risk of Coronary Heart Disease
eReferences
References
- 1.Catapano AL, Graham I, De Backer G, et al. ; ESC Scientific Document Group . 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37(39):2999-3058. [DOI] [PubMed] [Google Scholar]
- 2.Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934. [DOI] [PubMed] [Google Scholar]
- 3.Baigent C, Blackwell L, Emberson J, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316(12):1289-1297. [DOI] [PubMed] [Google Scholar]
- 5.Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: 1, Evidence from genetic, epidemiologic, and clinical studies: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dewey FE, Gusarova V, Dunbar RL, et al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med. 2017;377(3):211-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaudet D, Gipe DA, Pordy R, et al. ANGPTL3 inhibition in homozygous familial hypercholesterolemia. N Engl J Med. 2017;377(3):296-297. [DOI] [PubMed] [Google Scholar]
- 8.Gaudet D, Alexander VJ, Baker BF, et al. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med. 2015;373(5):438-447. [DOI] [PubMed] [Google Scholar]
- 9.Nordestgaard BG, Abildgaard S, Wittrup HH, Steffensen R, Jensen G, Tybjaerg-Hansen A. Heterozygous lipoprotein lipase deficiency: frequency in the general population, effect on plasma lipid levels, and risk of ischemic heart disease. Circulation. 1997;96(6):1737-1744. [DOI] [PubMed] [Google Scholar]
- 10.Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371(1):32-41. [DOI] [PubMed] [Google Scholar]
- 11.Khera AV, Won HH, Peloso GM, et al. ; Myocardial Infarction Genetics Consortium, DiscovEHR Study Group, CARDIoGRAM Exome Consortium, and Global Lipids Genetics Consortium . Association of rare and common variation in the lipoprotein lipase gene with coronary artery disease. JAMA. 2017;317(9):937-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dewey FE, Gusarova V, O’Dushlaine C, et al. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N Engl J Med. 2016;374(12):1123-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stitziel NO, Stirrups KE, Masca NG, et al. ; Myocardial Infarction Genetics and CARDIoGRAM Exome Consortia Investigators . Coding variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. N Engl J Med. 2016;374(12):1134-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sudlow C, Gallacher J, Allen N, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mailman MD, Feolo M, Jin Y, et al. The NCBI dbGaP database of genotypes and phenotypes. Nat Genet. 2007;39(10):1181-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikpay M, Goel A, Won HH, et al. A comprehensive 1000 genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47(10):1121-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willer CJ, Schmidt EM, Sengupta S, et al. ; Global Lipids Genetics Consortium . Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45(11):1274-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu DJ, Peloso GM, Yu H, et al. ; Charge Diabetes Working Group; EPIC-InterAct Consortium; EPIC-CVD Consortium; GOLD Consortium; VA Million Veteran Program . Exome-wide association study of plasma lipids in >300,000 individuals. Nat Genet. 2017;49(12):1758-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2016;35(11):1880-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ference BA. How to use mendelian randomization to anticipate the results of randomized trials. Eur Heart J. 2018;39(5):360-362. [DOI] [PubMed] [Google Scholar]
- 21.Ference BA, Majeed F, Penumetcha R, Flack JM, Brook RD. Effect of naturally random allocation to lower low-density lipoprotein cholesterol on the risk of coronary heart disease mediated by polymorphisms in NPC1L1, HMGCR, or both: a 2 × 2 factorial mendelian randomization study. J Am Coll Cardiol. 2015;65(15):1552-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ference BA, Robinson JG, Brook RD, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375(22):2144-2153. [DOI] [PubMed] [Google Scholar]
- 23.Ference BA, Kastelein JJP, Ginsberg HN, et al. Association of genetic variants related to CETP inhibitors and statins with lipoprotein levels and cardiovascular risk. JAMA. 2017;318(10):947-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borén J, Williams KJ. The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: a triumph of simplicity. Curr Opin Lipidol. 2016;27(5):473-483. [DOI] [PubMed] [Google Scholar]
- 25.Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384(9943):626-635. [DOI] [PubMed] [Google Scholar]
- 26.Sniderman A, Shapiro S, Marpole D, Skinner B, Teng B, Kwiterovich PO Jr. Association of coronary atherosclerosis with hyperapobetalipoproteinemia [increased protein but normal cholesterol levels in human plasma low density (beta) lipoproteins]. Proc Natl Acad Sci U S A. 1980;77(1):604-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61(4):427-436. [DOI] [PubMed] [Google Scholar]
- 28.Sarwar N, Sandhu MS, Ricketts SL, et al. ; Triglyceride Coronary Disease Genetics Consortium and Emerging Risk Factors Collaboration . Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies [published correction appears in Lancet. 2010;376(9735):90]. Lancet. 2010;375(9726):1634-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia: safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317(20):1237-1245. [DOI] [PubMed] [Google Scholar]
- 30.Rubins HB, Robins SJ, Collins D, et al. ; Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group . Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341(6):410-418. [DOI] [PubMed] [Google Scholar]
- 31.Bezafibrate Infarction Prevention (BIP) Study Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. Circulation. 2000;102(1):21-27. [DOI] [PubMed] [Google Scholar]
- 32.Keech A, Simes RJ, Barter P, et al. ; FIELD study investigators . Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849-1861. [DOI] [PubMed] [Google Scholar]
- 33.Ginsberg HN, Elam MB, Lovato LC, et al. ; ACCORD Study Group . Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ference BA, Yoo W, Alesh I, et al. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a mendelian randomization analysis. J Am Coll Cardiol. 2012;60(25):2631-2639. [DOI] [PubMed] [Google Scholar]
- 35.Yokoyama M, Origasa H, Matsuzaki M, et al. ; Japan EPA lipid intervention study (JELIS) Investigators . Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090-1098. [DOI] [PubMed] [Google Scholar]
- 36.Bhatt DL, Steg PG, Miller M, et al. ; REDUCE-IT Investigators . Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia [published online November 10, 2018]. N Engl J Med. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Included Studies and Genotyping Platforms
eTable 2. LPL Variants Included in LPL Score and Associations With Plasma Triglycerides and LDL-C in Global Lipids Genetics Consortium
eTable 3. Linkage Disequilibrium Matrix for Variants Included in the LPL Genetic Score
eTable 4. LDLR Variants Included in LDLR Genetic Score and Their Associations With Plasma Triglycerides and LDL-C in Global Lipids Genetics Consortium
eTable 5. Linkage Disequilibrium Matrix for Variants Included in the LDLR Genetic Score
eTable 6. Association of LPL and LDLR Scores With CHD Risk per 10 mg/dl Lower ApoB Among Studies Contributing Individual Participant Data and Summary Level Data
eTable 7. Variants Associated With Triglycerides or LDL-C in the Global Lipids Genetics Consortium
eTable 8. Additional Multivariable Mendelian Randomization Analyses for the Association Between Changes in Triglycerides, LDL-C, ApoB and the Risk of Coronary Heart Disease
eFigure 1. Study Design and Analyses
eFigure 2. Plot of LPL Variants Included in LPL Score
eFigure 3. Plot of LDLR Variants Included in LDLR Score
eFigure 4. Associations of the LPL and LDLR scores with plasma lipid levels and risk of Coronary Heart Disease for the Same Change in Plasma Concentration of ApoB-Containing Lipoproteins Stratified by the Other Score
eFigure 5. 2x2 Factorial Mendelian Randomization Analysis Evaluating the Separate and Combined Effects of the LPL and LDLR Scores on Plasma Lipids, Lipoproteins, and Risk of Coronary Heart Disease
eReferences