Key Points
Question
Are entecavir and tenofovir disoproxil fumarate, the first-line antiviral agents for chronic hepatitis B infection, associated with similar risks of hepatocellular carcinoma during long-term treatment?
Findings
In a Korean nationwide population cohort of 24 156 treatment-naive adult patients with chronic hepatitis B infection, tenofovir treatment was associated with a significantly lower risk of hepatocellular carcinoma and mortality compared with entecavir treatment. The data were validated in a hospital cohort of 2701 treatment-naive adult patients with chronic hepatitis B infection.
Meaning
Given the poor prognosis of patients who developed hepatocellular carcinoma, this study’s findings may have considerable clinical implications in the prevention of this cancer in patients with chronic hepatitis B infection.
Abstract
Importance
Entecavir and tenofovir disoproxil fumarate have comparable efficacy in achieving surrogate end points, including virologic response, and are equally recommended as first-line treatments for patients with chronic hepatitis B (CHB). However, it is unclear whether treatment with these drugs is associated with equivalent clinical outcomes, especially development of hepatocellular carcinoma (HCC).
Objective
To compare entecavir and tenofovir in terms of the risk of HCC and death or liver transplant in patients with CHB infection.
Design, Setting, and Participants
A nationwide historical population cohort study involving treatment-naive adult patients with CHB who started treatment with entecavir (n = 11 464) or tenofovir disoproxil fumarate (n = 12 692) between January 1, 2012, and December 31, 2014, using data from the Korean National Health Insurance Service database. As validation, a hospital cohort of patients with CHB treated with entecavir (n = 1560) or tenofovir (n = 1141) in a tertiary referral center between January 1, 2010, and December 31, 2016, were analyzed. Nationwide cohort data were retrieved from January 1, 2010, to December 31, 2016, and hospital cohort data from January 1, 2010, to October 31, 2017.
Main Outcomes and Measures
Cumulative incidence rates of HCC and death and transplant rates.
Results
Among the population cohort of 24 156, the mean (SD) age was 48.9 (9.8) years, and 15 120 patients (62.6%) were male. Among the hospital cohort of 2701, the mean (SD) age was 48.8 (10.5) years and 1657 patients (61.3%) were male. In the population cohort, the annual incidence rate of HCC was significantly lower in the tenofovir group (0.64 per 100 person-years [PY]) than in the entecavir group (1.06 per 100 PY). By multivariable-adjusted analysis, tenofovir therapy was associated with a significantly lower risk of HCC (hazard ratio [HR], 0.61; 95% CI, 0.54-0.70) and all-cause mortality or transplant (HR, 0.77; 95% CI, 0.65-0.92) compared with entecavir. The tenofovir group also showed a significantly lower risk of HCC in the 10 923–pair propensity score–matched population cohort (HR, 0.62; 95% CI, 0.54-0.70) and 869-pair propensity score–matched hospital cohort (HR, 0.68; 95% CI, 0.46-0.99) compared with the entecavir group.
Conclusions and Relevance
This study suggests that tenofovir treatment was associated with a significantly lower risk of HCC compared with entecavir treatment in a population-based cohort of adults with CHB; these findings were validated in a hospital cohort. Given the poor prognosis of patients with HCC, these findings may have considerable clinical implications in prevention of this cancer in patients with CHB infection.
This cohort study uses data from the Korean National Health Insurance Service database to compare the risk of hepatocellular cancer associated with entecavir vs tenofovir disoproxil fumarate in patients receiving long-term treatment for chronic hepatitis B infection.
Introduction
Chronic hepatitis B (CHB) infection is the most common chronic viral infection worldwide, with approximately 250 million people carrying hepatitis B virus (HBV).1,2 Hepatitis B virus accounts for approximately 45% of cases of hepatocellular carcinoma (HCC) and 30% of cases of cirrhosis3 and causes nearly 1 million deaths each year.4
Persistent replication of HBV is an independent risk factor for progression of CHB to cirrhosis and HCC,5 and reducing HBV DNA concentrations through long-term nucleoside or nucleotide analogue therapy is associated with reduced risk of HCC and/or mortality in patients with CHB.6,7 However, HBV is rarely eradicated, and most patients with CHB require long-term nucleoside or nucleotide analogue therapy.8
Entecavir and tenofovir disoproxil fumarate are potent nucleoside or nucleotide analogues, respectively, with high genetic barriers to resistance. They are equally recommended as first-line treatment for CHB by practice guidelines9,10,11 because they show similar efficacy in intermediate surrogate end points, such as virologic, biochemical, serologic, and histologic responses. Nevertheless, to our knowledge, there have been no randomized trials comparing the efficacy of entecavir and tenofovir in reducing HCC risk. A few cohort studies found no significant difference in HCC risk between entecavir and tenofovir12,13; however, those studies were not designed to directly compare the clinical outcomes of the 2 drugs and were underpowered owing to small numbers of patients and events.
In the present nationwide population cohort study, we compared the effectiveness of entecavir and tenofovir on the risk of HCC in patients with CHB and validated the results in a hospital cohort.
Methods
Study Population
This study was a nationwide, population-level, historical cohort study of adult patients with CHB who started treatment with either entecavir or tenofovir. The data were obtained from the claims database of the National Health Insurance Service (NHIS) of the Republic of Korea, an HBV-endemic country. Korea has a single-payer, universal health coverage system, and the NHIS provides health insurance to more than 99% of the population. Accordingly, NHIS has a comprehensive health database for diagnoses, treatments, procedures, and prescriptions. The database also provides information on sociodemographic status, comorbidities, severity of liver disease, and concomitant medications, lifestyle, and health-related behaviors (eg, smoking status). As a validation, a hospital cohort of patients with CHB treated with entecavir or tenofovir in a tertiary referral center were analyzed. The institutional review boards of the Korean National Evidence-Based Healthcare Collaborating Agency and Asan Medical Center approved this study and granted a waiver of informed consent from study participants owing to the historical cohort nature of the study.
Between January 1, 2010, and December 31, 2016, we collected from the NHIS database historical cohort of 41 962 treatment-naive adults with CHB who started treatment with entecavir, 0.5 mg/d, or tenofovir disoproxil fumarate, 300 mg/d, between January 1, 2012, and December 31, 2014, as the source population with predefined inclusion and exclusion criteria (eFigure 1A in the Supplement). We limited the study period because the NHIS first reimbursed entecavir in January 2007 and tenofovir in December 2012. All patients had International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) code B181 for CHB. Cirrhosis was defined as ICD-10 code K74 and regarded as a decompensated state when the patient received any medication or had undergone any procedure to manage complications (ie, ascites or varices; eTable 1 in the Supplement). Patients meeting 1 or more of the following criteria were excluded: being younger than 30 years or 80 years or older at baseline; received a diagnosis of hepatitis C, hepatitis D, acute viral hepatitis, or HIV infection; received an organ transplant; received other anti-HBV treatments; received a diagnosis of HCC or other malignant neoplasm; or experienced clinical events (HCC, death, or transplant) within 6 months after treatment.
The reimbursement criteria for entecavir and tenofovir were identical and did not change during the study period: serum HBV DNA levels of 20 000 IU/mL or greater for hepatitis B e antigen (HBeAg)–positive patients, of 2000 IU/mL or greater for HBeAg-negative patients, and alanine aminotransferase (ALT) levels of 80 U/mL or greater in the absence of cirrhosis (to convert ALT levels to microkatals per liter, multiply by 0.0167). In the presence of cirrhosis, criteria were HBV DNA levels of 2000 IU/mL or greater and ALT levels of 40 U/mL or greater.
Outcomes and Follow-up Evaluation
The primary outcome was development of HCC. Secondary outcomes were all-cause mortality and liver transplant. The index date was the date a patient first received a prescription for entecavir or tenofovir. The follow-up period for each patient was calculated from the index date to the date of HCC diagnosis, death, transplant, or the last follow-up (December 31, 2016). Patients whose treatment regimen was changed were censored at 6 months after the change was made.
Information on baseline characteristics, concomitant medications, and clinical outcomes were obtained from the NHIS database. Diagnostic and procedural codes based on the NHIS database are provided in eTable 1 in the Supplement. This database has a high HCC registration rate (ICD-10 code C22, 96.5%) and highly accurate diagnoses and has previously been validated as a reliable resource for research.14 Liver transplant was defined by ICD-10 codes Z944 and T864 and ICD-10 reimbursement benefit extension coverage code V013. To verify the complete set of follow-up data, information on the vital status and primary diagnosis of HCC in all patients was validated by accessing the death certificate database from the National Population Registry of the Korea National Statistical Office using unique personal identification numbers. The death registration was nearly complete, and 98.3% of deaths were confirmed by physician’s diagnoses.15
Validation Hospital Cohort
A limitation of the NHIS database was that we could not collect detailed individual laboratory data, such as baseline levels of HBV DNA and ALT and hepatic functional status. Therefore, we separately performed validation analyses using a hospital-based cohort of 2701 consecutive treatment-naive adult patients with CHB who initiated treatment with entecavir or tenofovir between January 1, 2010, and December 31, 2016, at Asan Medical Center, a 2700-bed academic tertiary care center in Seoul, Republic of Korea (eFigure 1B in the Supplement). All patients were positive for hepatitis B surface antigen (HBsAg) for at least 6 months at baseline. Cirrhosis was radiologically defined by findings of a coarse liver echotexture and nodular liver surface on ultrasonography and clinically by clinical features of portal hypertension (eg, ascites, splenomegaly, or varices), thrombocytopenia (platelet count, <150 × 103/μL [conversion to ×109/L is 1:1]), or both. All patients were advised to continue treatment after HBeAg seroconversion until HBsAg seroclearance. The patients were followed up every 3 to 6 months from the index date to the time of death, transplant, or last follow-up (October 31, 2017). Verification methods were identical to those used in the nationwide cohort.
Statistical Analysis
All patients who met the eligibility criteria at baseline were included in the analyses, and their data were analyzed based on intention to treat.
Categorical variables were compared using a χ2 test and continuous variables were compared using an unpaired 2-tailed t test. Cumulative incidence curves for HCC and death or transplant were estimated using the Kaplan-Meier method and compared using a log-rank test. Univariate and multivariable Cox proportional hazards models were used to compare clinical outcomes between groups.
Propensity score–matching analysis was used to reduce the effect of selection bias and potential confounding between the 2 groups. Propensity scores were computed by using the following variables: age, sex, socioeconomic status, level of health care, smoking, cirrhosis, diabetes, and hypertension for the nationwide cohort. In the validation hospital cohort, propensity scores were computed from age; sex; HBeAg positivity; serum levels of HBV DNA, ALT, albumin, total bilirubin, international normalized ratio, platelet count, and creatinine concentration; diabetes; hypertension; cirrhosis; ascites; Child-Pugh score; Chinese University HCC score; Guide With Age, Gender, HBV DNA, Core Promoter Mutations, and Cirrhosis–HCC score; platelet age gender B score; and Risk Estimation for Hepatocellular Carcinoma in Chronic Hepatitis B score. In the validation hospital cohort, multiple imputation was used to estimate the missing values, which comprised 0.02% to 3.7% of the baseline laboratory data. For propensity score matching, a nearest-neighbor 1:1 matching scheme with a caliper size of 0.1 was used. Inverse probability treatment weighting (IPTW) was also used (eMethods in the Supplement). Competing risk analysis was conducted for the interpretation of the cumulative incidence of HCC after adjusting for the probability of death and liver transplant.
All statistical analyses were performed using SAS software, version 9.1 (SAS Institute Inc) and R statistical software, version 3.3.1, (R Foundation Inc; http://cran.r-project.org/). All reported P values are 2-sided, and P values less than .05 were considered statistically significant.
Results
Baseline Characteristics of the Nationwide Cohort
Among the population cohort of 24 156, the mean (SD) age was 48.9 (9.8) years, and 15 120 patients (62.6%) were male. Owing to the homogenous nature of the study population, data on race/ethnicity were not collected. Data from a total of 24 156 treatment-naive adult patients with CHB were analyzed (entecavir group, 11 464 and tenofovir group, 12 692). The mean (SD) ages of entecavir and tenofovir groups were 49.3 (9.8) and 48.6 (9.8) years, respectively (eTable 2 in the Supplement). Among the patients, 2991 (26.1%) of the entecavir group and 3488 (27.5%) of the tenofovir group had cirrhosis. Propensity score–matching analysis generated 10 923 pairs, and the characteristics of pairs were balanced, with the standardized difference less than 10% for all baseline variables (eTable 2 in the Supplement).
Clinical Outcomes in the Nationwide Cohort
During follow-up, HCC developed in 984 patients (annual incidence, 0.84 per 100 person-years [PY]; 590 in the entecavir group vs 394 in the tenofovir group), and 509 patients died or received a transplant (annual incidence, 0.43 per 100 PY; 281 in the entecavir group vs 228 in the tenofovir group, as shown in eTable 3 in the Supplement).
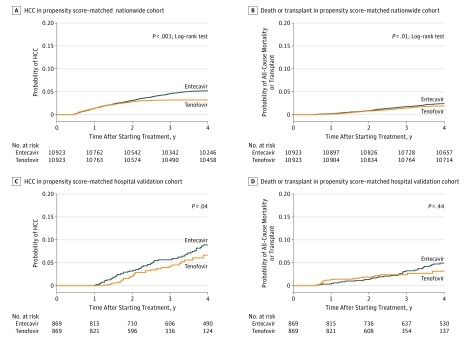
The annual incidence rate of HCC was significantly lower in the tenofovir group (0.64 per 100 PY) than in the entecavir group (1.06 per 100 PY) by multivariable-adjusted analysis (hazard ratio [HR], 0.61; 95% CI, 0.54-0.70); P < .001; eTable 3 in the Supplement). In the propensity score–matched analysis, the risk of HCC diverged between the 2 groups after 2 years’ follow-up and was significantly lower in the tenofovir group than in the entecavir group (HR, 0.62; 95% CI, 0.54-0.70; P < .001; eTable 3 in the Supplement and Figure, A).
Figure. Cumulative Incidence of Hepatocellular Carcinoma (HCC) and Death or Transplant in Propensity Score–Matched Pairs of Patients With Chronic Hepatitis B Infection Treated With Entecavir or Tenofovir.
A, Hepatocellular carcinoma in the nationwide population cohort. B, Death or transplant in the nationwide population cohort. C, Hepatocellular carcinoma in the validation hospital cohort. D, Death or transplant in the validation hospital cohort. Tenofovir was given as tenofovir disoproxil fumarate.
The annual rate of all-cause mortality or liver transplant was also significantly lower in the tenofovir group than in the entecavir group (0.36 per 100 PY vs 0.50 per 100 PY) by multivariable analysis (HR, 0.77; 95% CI, 0.65-0.92; P = .004) and by propensity score–matched analysis (HR, 0.79; 95% CI, 0.66-0.94; P = .01; eTable 3 in the Supplement and Figure, B).
By competing risk analysis in the propensity score–matched pairs adjusting for the risk of death and transplantation, tenofovir treatment was associated with a significantly lower risk of HCC than entecavir treatment (HR, 0.62; 95% CI, 0.54-0.70; P < .001; eTable 3 in the Supplement).
Baseline Characteristics of the Validation Hospital Cohort
The validation cohort was composed of 2701 eligible patients with CHB who commenced treatment with entecavir or tenofovir. For the entire hospital cohort, the mean (SD) age was 48.8 (10.5) years. The mean (SD) age of the entecavir group was 49.2 (10.5) years and of the tenofovir group was 48.1 (10.5) years (P = .01; eTable 4 in the Supplement); 1657 patients (61.3%) were male. The proportion of patients with cirrhosis did not significantly differ between the 2 groups (59.9% vs 57.2%; P = .17).
Propensity score–matching analysis generated 869 pairs, and baseline characteristics were balanced (eTable 4 and eFigure 2 in the Supplement). After IPTW, baseline characteristics between the 2 groups were balanced (eTable 5 and eFigure 3 in the Supplement).
Virologic Response in the Validation Hospital Cohort
Virologic response (VR) was defined as a serum HBV DNA level less than 60 IU/mL at 1 year of treatment. Missing or unavailable VR data were considered as a failure of VR.
The proportion of patients experiencing VR was significantly lower in the entecavir group than in the tenofovir group in both the entire cohort (1227 of 1560 patients [78.7%] vs 972 of 1141 [85.2%]; P < .001) and in the propensity score–matched cohort (670 of 869 [77.1%] vs 737 of 869 [84.8%]; P < .001; eTable 6 in the Supplement). During follow-up, the proportion of patients whose treatment was modified (ie, switched to or added other antiviral agents for any reason) was significantly higher in the entecavir group than in the tenofovir group (182 of 1560 patients [11.7%] vs 2 of 1141 [0.2%]; P < .001; eTable 6 in the Supplement). The proportion of patients with ALT level normalization at 1 year of treatment was significantly lower in the entecavir group than in the tenofovir group (604 of 1560 patients [38.7%] vs 506 of 1141 [44.3%]; P = .002).
Virologic response at 1 year of treatment was not independently associated with HCC (HR, 1.13; 95% CI, 0.69-1.84; P = .63), although it was significantly associated with a reduced rate of death or transplant (HR, 0.55; 95% CI, 0.33-0.91; P = .02; eTable 7 in the Supplement).
Clinical Outcomes in the Validation Hospital Cohort
During 8267 PY of follow-up, 154 of the 2701 patients (5.7%) developed HCC, and 91 (3.4%) died or received a liver transplant.
By multivariable analysis, tenofovir treatment was independently associated with a significantly lower risk of HCC (HR, 0.66; 95% CI, 0.46-0.96; P = .03; eTables 7 and 8 in the Supplement). Propensity score–matched analysis adjusted for VR also showed that the risk of HCC was significantly lower in the tenofovir group (HR, 0.68; 95% CI, 0.46-0.99; P = .04; eTable 3 in the Supplement and Figure, C). This result was consistently found in a separate propensity score–matched cohort after adding VR at 1 year of treatment as a matching covariate (eFigure 4 in the Supplement). In the competing risk analysis, tenofovir treatment was again associated with a significantly lower risk of HCC (HR, 0.65; 95% CI, 0.44-0.95; P = .03; eTable 9 and eFigure 5 in the Supplement). After adjustment with IPTW, tenofovir treatment was associated with a significantly lower risk of HCC (HR, 0.68; 95% CI, 0.46-0.99; P = .045; eTable 9 in the Supplement).
In contrast, the risk of death or transplant was not significantly different between the 2 treatment groups by multivariable analysis (HR, 0.79; 95% CI, 0.48-1.28; P = .33; eTable 7 in the Supplement), by propensity score–matched analysis adjusted for VR (HR, 0.80; 95% CI, 0.46-1.41; P = .44; Figure, D), and by IPTW analysis (HR, 0.71; 95% CI, 0.43-1.16; P = .17; eTable 9 in the Supplement).
Subcohort Analyses
In the nationwide cohort, propensity-score matching was separately performed for patients without cirrhosis (8003 pairs) and with cirrhosis (2914 pairs). Tenofovir treatment was associated with a lower risk for HCC regardless of cirrhosis (eTable 3 in the Supplement), which was also identified by stratified analyses for the levels of health care, socioeconomic status, and the year of first prescription (eTables 10-12 in the Supplement).
Among the patients with cirrhosis in the validation hospital cohort, tenofovir treatment was associated with a significantly lower risk of HCC by multivariable analysis (HR, 0.64; 95% CI, 0.43-0.95; P = .03; eTable 13-14 in the Supplement) and in the 510 propensity score–matched pairs after adjustment for VR (HR, 0.65; 95% CI, 0.45-0.94; P = .02; eTable 13 and eFigures 6 and 7 in the Supplement).
The outcomes of patients with and without treatment modifications in the entecavir group in the hospital cohort were not significantly different (eFigure 8 in the Supplement). After excluding the patients with treatment modifications during follow-up, the tenofovir group showed a significantly lower risk of HCC compared with the entecavir group (HR, 0.63; 95% CI, 0.43-0.91; P = .01; eFigure 9 in the Supplement).
Discussion
In this nationwide population-based study, clinical outcomes of 24 156 patients with CHB infection after initiation of treatment with entecavir or tenofovir were compared. We found that, compared with entecavir treatment, tenofovir treatment was associated with a significantly lower risk of HCC, which was consistently observed in unadjusted, multivariable-adjusted, propensity score–matched, and competing risk analysis in the entire cohort as well as in the cirrhosis and noncirrhosis subcohorts. This finding was also validated in a large hospital cohort, which showed a similar magnitude of lowered risk of HCC in the tenofovir group on application of the same robust statistical methods.
Current American, European, and Asian-Pacific clinical practice guidelines for CHB do not state any preference between entecavir and tenofovir.9,10,11 However, given the necessity of long-term treatment in most patients with CHB, the importance of comparative data on the effectiveness and safety of entecavir and tenofovir is immense.
The mechanism of our main finding on the association of tenofovir with a significantly lower risk of HCC compared with entecavir might be explained in part by the better VR profiles of the tenofovir group as shown in the hospital cohort, which are in line with the results of previous studies.16,17,18 However, considering that VR was not an independent risk factor for HCC, the differences in HCC risk between the 2 treatments cannot be fully explained by their antiviral potency. A recent study showed that higher serum interferon λ3 levels were induced in patients treated with nucleotide analogues (adefovir dipivoxil and tenofovir), but not in those treated with nucleoside analogues (lamivudine and entecavir).19 Interferon lambda showed potent antitumor activity in murine models of cancer, including hepatoma,20,21 and this antitumor activity could presumably contribute to the difference in the HCC risk that we observed.
Entecavir was shown to be carcinogenic in mice and rats when administered at doses higher than those used in humans.22 It was also shown to potentially incorporate into the human genome, which may contribute to a putative mechanism of carcinogenicity, especially if the embedded genomes have higher error rates during subsequent rounds of replication.23,24,25 These data raise concern about the carcinogenic potential of entecavir even at clinical doses during long-term treatment, especially in patients with cirrhosis who have increased chromosomal instability of hepatocytes.26,27
Nevertheless, given that HBV replication itself is a strong independent risk factor for HCC, assessing the carcinogenic potential of entecavir should be balanced by considering its protective role against HCC development via suppression of HBV replication. Many observational studies and meta-analyses have shown that in patients with CHB, entecavir therapy significantly reduced HCC risk compared with no treatment.18,22,28 However, a previous cohort study demonstrated that HCC risk with entecavir was not lower than that with lamivudine.7 Nonetheless, the effect of entecavir on HCC risk would be better compared with tenofovir because both drugs have similar potencies in suppressing HBV replication.16,18,28 Based on our data, 185 HCC cases were estimated to have been preventable at 4 years’ follow-up if the 11 464 patients in the entecavir group had used tenofovir (eTable 15 in the Supplement).
Limitations
The major limitation of the present study is that it was based on observational data, which may be subject to bias and confounding. To overcome this issue, we used multiple rigorous strategies (multivariable adjustment, propensity-score matching, IPTW, competing risk analysis, and stratified analyses) to adjust for the differences in baseline susceptibility to the tested outcomes. Given the low incidence of clinical events, our population-level historical cohort study can be considered a proper approach to assess the comparative effectiveness of the drugs.29 Second, there was a disparity in the follow-up periods between the 2 groups owing to the later approval of tenofovir. However, the yearly risk of HCC was shown to gradually decrease with prolonged treatment with entecavir or tenofovir, particularly in patients with cirrhosis.12,30,31 In fact, the risk of HCC could only be reduced after achieving a decrease in HBV DNA and ALT levels by years of nucleoside or nucleotide analogue therapy.30,31 Therefore, the shorter duration of treatment in the tenofovir group is not likely to have resulted in a decrease of HCC risk. Third, as a single-nation study, our results are limited in terms of generalization. Most patients in our study were assumed to be infected with genotype C HBV acquired through vertical transmission,32 which may be associated with enhanced risk of HCC. However, genotype C is also predominant in many Asian countries, where the majority of people in need of HBV treatment live.1,2 Finally, the criterion to initiate treatment in our patients (ALT level ≥80 U/mL) was stricter than recommended elsewhere, and this criterion may influence replicability if the differential effectiveness of tenofovir is only relevant for those with high ALT concentrations.
Conclusions
Our data suggest that tenofovir treatment may be associated with a significantly lower risk of HCC in patients with CHB compared with entecavir treatment. Given the poor prognosis of HCC, our findings might have considerable clinical implications for preventing the occurrence of HCC in patients with CHB. Further studies are needed to ensure the replicability of our findings.
eMethods. Supplemental Methods
eTable 1. Definition of Diagnostic and Procedural Codes Based on Korea National Health Insurance Service (NHIS) Database
eTable 2. Baseline Characteristics of the Nationwide Cohort of Chronic Hepatitis B Patients Treated With Entecavir vs Tenofovir
eTable 3. Clinical Outcomes of the Nationwide Cohort of Chronic Hepatitis B Patients Treated With Entecavir vs Tenofovir
eTable 4. Baseline Characteristics of the Patients Treated With Entecavir or Tenofovir in the Validation Hospital Cohort
eTable 5. Baseline Characteristics of the Validation Hospital Cohort After Inverse Probability Treatment Weighting
eTable 6. Virologic, Biochemical, and Serologic Responses in the Validation Hospital Cohort
eTable 7. Multivariable Analysis in the Validation Hospital Cohort
eTable 8. Univariate Analysis to Identify Independent Factors of Hepatocellular Carcinoma and Death or Transplantation in the Hospital Cohort of Patients Treated With Entecavir or Tenofovir
eTable 9. Comparison of Outcomes by Unadjusted and Adjusted Analyses in the Hospital Cohort
eTable 10. Stratified Analysis by Levels of Health Care in the Nationwide Cohort of CHB Patients Treated With entecavir vs TDF
eTable 11. Stratified Analysis by Socioeconomic Status in the Nationwide Cohort of CHB Patients Treated With ETV vs TDF
eTable 12. Stratified Analysis by the Year of First Prescription in the Nationwide Cohort of CHB Patients Treated With ETV vs TDF
eTable 13. Baseline Characteristics of the Propensity Score−Matched Cirrhosis Subcohorts of the Hospital Cohort
eTable 14. Multivariable Analysis in the Cirrhosis Subcohort of the Validation Hospital Cohort
eTable 15. Estimation for the Number of HCC Cases Preventable When Using TDF as Opposed to ETV
eFigure 1. Patient Flow Diagrams
eFigure 2. Distribution of the Propensity Scores and Standardized Differences in the Mean or Proportion of Variables Before and After Matching in the Validation Hospital Cohort
eFigure 3. Distribution of Standardized Differences in the Mean or Proportion of Variables Before and After Weighting in the Validation Hospital Cohort
eFigure 4. Hepatocellular Carcinoma in the Propensity Score-Matched Cohort Adding Virologic Response at 1-Year of Treatment as a Matching Covariate in the Validation Hospital Cohort (HR 0.69, 95% CI 0.47 − 0.99, P = .047)
eFigure 5. Competing Risk Analysis for Hepatocellular Carcinoma Adjusting for the Possibility of Death or Transplantation in the Validation Hospital Cohort
eFigure 6. Distribution of Propensity Scores and Standardized Differences in the Mean or Proportion of Variables Before and After Matching in Cirrhosis Subcohort of the Hospital Cohort
eFigure 7. Hepatocellular Carcinoma in the Propensity Score-Matched Cirrhosis Subcohort of the Validation Hospital Cohort
eFigure 8. Cumulative Incidence of Clinical Outcomes Between Patients With and Without Treatment Modifications in the ETV Group in the Hospital Cohort
eFigure 9. Cumulative Incidence of Clinical Outcomes Between ETV and TDF Groups After Exclusion of Patients Whose Treatment Was Modified During Follow-up
References
- 1.Polaris Observatory Collaborators Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3(6):383-403.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29599078&dopt=Abstract doi: 10.1016/S2468-1253(18)30056-6 [DOI] [PubMed] [Google Scholar]
- 2.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546-1555.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26231459&dopt=Abstract doi: 10.1016/S0140-6736(15)61412-X [DOI] [PubMed] [Google Scholar]
- 3.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211-1259.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28919117&dopt=Abstract doi: 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nayagam S, Thursz M, Sicuri E, et al. Requirements for global elimination of hepatitis B: a modelling study. Lancet Infect Dis. 2016;16(12):1399-1408.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27638356&dopt=Abstract doi: 10.1016/S1473-3099(16)30204-3 [DOI] [PubMed] [Google Scholar]
- 5.Chen CJ, Yang HI, Su J, et al. ; REVEAL-HBV Study Group . Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295(1):65-73.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16391218&dopt=Abstract doi: 10.1001/jama.295.1.65 [DOI] [PubMed] [Google Scholar]
- 6.Liaw YF, Sung JJ, Chow WC, et al. ; Cirrhosis Asian Lamivudine Multicentre Study Group . Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351(15):1521-1531.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15470215&dopt=Abstract doi: 10.1056/NEJMoa033364 [DOI] [PubMed] [Google Scholar]
- 7.Lim YS, Han S, Heo NY, Shim JH, Lee HC, Suh DJ. Mortality, liver transplantation, and hepatocellular carcinoma among patients with chronic hepatitis B treated with entecavir vs lamivudine. Gastroenterology. 2014;147(1):152-161.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24583062&dopt=Abstract doi: 10.1053/j.gastro.2014.02.033 [DOI] [PubMed] [Google Scholar]
- 8.Kim GA, Lim YS, An J, et al. HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: clinical outcomes and durability. Gut. 2014;63(8):1325-1332.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24162593&dopt=Abstract doi: 10.1136/gutjnl-2013-305517 [DOI] [PubMed] [Google Scholar]
- 9.European Association for the Study of the Liver EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370-398.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28427875&dopt=Abstract doi: 10.1016/j.jhep.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 10.Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1-98.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26563120&dopt=Abstract doi: 10.1007/s12072-015-9675-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560-1599.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29405329&dopt=Abstract doi: 10.1002/hep.29800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papatheodoridis GV, Idilman R, Dalekos GN, et al. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B. Hepatology. 2017;66(5):1444-1453.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28622419&dopt=Abstract doi: 10.1002/hep.29320 [DOI] [PubMed] [Google Scholar]
- 13.Köklü S, Tuna Y, Gülşen MT, et al. Long-term efficacy and safety of lamivudine, entecavir, and tenofovir for treatment of hepatitis B virus–related cirrhosis. Clin Gastroenterol Hepatol. 2013;11(1):88-94.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23063679&dopt=Abstract doi: 10.1016/j.cgh.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 14.Seo HJ, Oh IH, Yoon SJ. A comparison of the cancer incidence rates between the national cancer registry and insurance claims data in Korea. Asian Pac J Cancer Prev. 2012;13(12):6163-6168.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23464424&dopt=Abstract doi: 10.7314/APJCP.2012.13.12.6163 [DOI] [PubMed] [Google Scholar]
- 15.Choi KH, Kim DH. Trend of suicide rates according to urbanity among adolescents by gender and suicide method in Korea, 1997-2012. Int J Environ Res Public Health. 2015;12(5):5129-5142.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25985313&dopt=Abstract doi: 10.3390/ijerph120505129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuo SR, Zuo XC, Wang CJ, et al. A meta-analysis comparing the efficacy of entecavir and tenofovir for the treatment of chronic hepatitis B infection. J Clin Pharmacol. 2015;55(3):288-297.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25293471&dopt=Abstract doi: 10.1002/jcph.409 [DOI] [PubMed] [Google Scholar]
- 17.Batirel A, Guclu E, Arslan F, et al. Comparable efficacy of tenofovir versus entecavir and predictors of response in treatment-naïve patients with chronic hepatitis B: a multicenter real-life study. Int J Infect Dis. 2014;28:153-159.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25286184&dopt=Abstract doi: 10.1016/j.ijid.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 18.Woo G, Tomlinson G, Nishikawa Y, et al. Tenofovir and entecavir are the most effective antiviral agents for chronic hepatitis B: a systematic review and Bayesian meta-analyses. Gastroenterology. 2010;139(4):1218-1229.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20600036&dopt=Abstract doi: 10.1053/j.gastro.2010.06.042 [DOI] [PubMed] [Google Scholar]
- 19.Murata K, Asano M, Matsumoto A, et al. Induction of IFN-λ3 as an additional effect of nucleotide, not nucleoside, analogues: a new potential target for HBV infection. Gut. 2018;67(2):362-371.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27789659&dopt=Abstract doi: 10.1136/gutjnl-2016-312653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abushahba W, Balan M, Castaneda I, et al. Antitumor activity of type I and type III interferons in BNL hepatoma model. Cancer Immunol Immunother. 2010;59(7):1059-1071.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20217081&dopt=Abstract doi: 10.1007/s00262-010-0831-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato A, Ohtsuki M, Hata M, Kobayashi E, Murakami T. Antitumor activity of IFN-lambda in murine tumor models. J Immunol. 2006;176(12):7686-7694.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16751416&dopt=Abstract doi: 10.4049/jimmunol.176.12.7686 [DOI] [PubMed] [Google Scholar]
- 22.US Food and Drug Administration NDA Review pharmacology/toxicology review and evaluation: NDA No. 21-797: submitted September 30, 2004. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/21797_BARACLUDE_pharmr.PDF. Accessed August 21, 2018.
- 23.Brown JA, Pack LR, Fowler JD, Suo Z. Presteady state kinetic investigation of the incorporation of anti-hepatitis B nucleotide analogues catalyzed by noncanonical human DNA polymerases. Chem Res Toxicol. 2012;25(1):225-233.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22132702&dopt=Abstract doi: 10.1021/tx200458s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang L, Wu X, He F, et al. Genetic evidence for genotoxic effect of entecavir, an anti-hepatitis B virus nucleotide analog. PLoS One. 2016;11(1):e0147440.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26800464&dopt=Abstract doi: 10.1371/journal.pone.0147440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brambilla G, Mattioli F, Robbiano L, Martelli A. Studies on genotoxicity and carcinogenicity of antibacterial, antiviral, antimalarial and antifungal drugs. Mutagenesis. 2012;27(4):387-413.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22228823&dopt=Abstract doi: 10.1093/mutage/ger094 [DOI] [PubMed] [Google Scholar]
- 26.Wilkens L, Flemming P, Gebel M, et al. Induction of aneuploidy by increasing chromosomal instability during dedifferentiation of hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2004;101(5):1309-1314.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14745031&dopt=Abstract doi: 10.1073/pnas.0305817101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiemann SU, Satyanarayana A, Tsahuridu M, et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 2002;16(9):935-942.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12087054&dopt=Abstract doi: 10.1096/fj.01-0977com [DOI] [PubMed] [Google Scholar]
- 28.Lok AS, McMahon BJ, Brown RS Jr, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta-analysis. Hepatology. 2016;63(1):284-306.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26566246&dopt=Abstract doi: 10.1002/hep.28280 [DOI] [PubMed] [Google Scholar]
- 29.Varbobitis I, Papatheodoridis GV. The assessment of hepatocellular carcinoma risk in patients with chronic hepatitis B under antiviral therapy. Clin Mol Hepatol. 2016;22(3):319-326.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27729632&dopt=Abstract doi: 10.3350/cmh.2016.0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim WR, Loomba R, Berg T, et al. Impact of long-term tenofovir disoproxil fumarate on incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer. 2015;121(20):3631-3638.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26177866&dopt=Abstract doi: 10.1002/cncr.29537 [DOI] [PubMed] [Google Scholar]
- 31.Ahn J, Lim JK, Lee HM, et al. Lower observed hepatocellular carcinoma incidence in chronic hepatitis B patients treated with entecavir: results of the ENUMERATE study. Am J Gastroenterol. 2016;111(9):1297-1304. doi: 10.1038/ajg.2016.257 [DOI] [PubMed] [Google Scholar]
- 32.Kim H, Jee YM, Song BC, et al. Molecular epidemiology of hepatitis B virus (HBV) genotypes and serotypes in patients with chronic HBV infection in Korea. Intervirology. 2007;50(1):52-57.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17164558&dopt=Abstract doi: 10.1159/000096313 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods
eTable 1. Definition of Diagnostic and Procedural Codes Based on Korea National Health Insurance Service (NHIS) Database
eTable 2. Baseline Characteristics of the Nationwide Cohort of Chronic Hepatitis B Patients Treated With Entecavir vs Tenofovir
eTable 3. Clinical Outcomes of the Nationwide Cohort of Chronic Hepatitis B Patients Treated With Entecavir vs Tenofovir
eTable 4. Baseline Characteristics of the Patients Treated With Entecavir or Tenofovir in the Validation Hospital Cohort
eTable 5. Baseline Characteristics of the Validation Hospital Cohort After Inverse Probability Treatment Weighting
eTable 6. Virologic, Biochemical, and Serologic Responses in the Validation Hospital Cohort
eTable 7. Multivariable Analysis in the Validation Hospital Cohort
eTable 8. Univariate Analysis to Identify Independent Factors of Hepatocellular Carcinoma and Death or Transplantation in the Hospital Cohort of Patients Treated With Entecavir or Tenofovir
eTable 9. Comparison of Outcomes by Unadjusted and Adjusted Analyses in the Hospital Cohort
eTable 10. Stratified Analysis by Levels of Health Care in the Nationwide Cohort of CHB Patients Treated With entecavir vs TDF
eTable 11. Stratified Analysis by Socioeconomic Status in the Nationwide Cohort of CHB Patients Treated With ETV vs TDF
eTable 12. Stratified Analysis by the Year of First Prescription in the Nationwide Cohort of CHB Patients Treated With ETV vs TDF
eTable 13. Baseline Characteristics of the Propensity Score−Matched Cirrhosis Subcohorts of the Hospital Cohort
eTable 14. Multivariable Analysis in the Cirrhosis Subcohort of the Validation Hospital Cohort
eTable 15. Estimation for the Number of HCC Cases Preventable When Using TDF as Opposed to ETV
eFigure 1. Patient Flow Diagrams
eFigure 2. Distribution of the Propensity Scores and Standardized Differences in the Mean or Proportion of Variables Before and After Matching in the Validation Hospital Cohort
eFigure 3. Distribution of Standardized Differences in the Mean or Proportion of Variables Before and After Weighting in the Validation Hospital Cohort
eFigure 4. Hepatocellular Carcinoma in the Propensity Score-Matched Cohort Adding Virologic Response at 1-Year of Treatment as a Matching Covariate in the Validation Hospital Cohort (HR 0.69, 95% CI 0.47 − 0.99, P = .047)
eFigure 5. Competing Risk Analysis for Hepatocellular Carcinoma Adjusting for the Possibility of Death or Transplantation in the Validation Hospital Cohort
eFigure 6. Distribution of Propensity Scores and Standardized Differences in the Mean or Proportion of Variables Before and After Matching in Cirrhosis Subcohort of the Hospital Cohort
eFigure 7. Hepatocellular Carcinoma in the Propensity Score-Matched Cirrhosis Subcohort of the Validation Hospital Cohort
eFigure 8. Cumulative Incidence of Clinical Outcomes Between Patients With and Without Treatment Modifications in the ETV Group in the Hospital Cohort
eFigure 9. Cumulative Incidence of Clinical Outcomes Between ETV and TDF Groups After Exclusion of Patients Whose Treatment Was Modified During Follow-up