Key Points
Question
Does iStent implantation concurrent with cataract surgery have a greater association with reduced use of ocular antihypertensive medications postoperatively compared with cataract surgery alone?
Findings
In this cohort study of enrollees in a US managed care network, patients who underwent bilateral iStent implantation concurrent with cataract surgery had a greater mean reduction in drops used (0.99 vs 0.49) and a higher proportion using no drops postoperatively (73.5% vs 55.3%) compared with matched control individuals who underwent cataract surgery only, by 20 months to 24 months postoperatively.
Meaning
These data suggest iStents are associated with reduced medication dependence in patients with glaucoma.
Abstract
Importance
The iStent Trabecular Micro-Bypass (Glaukos Corporation) is a minimally invasive glaucoma implant used in conjunction with cataract surgery to lower intraocular pressure.
Objective
To determine whether implantation of the iStent concurrent with cataract surgery is associated with reduced use of ocular antihypertensive medications in a US health care claims database.
Design, Setting, and Participants
Retrospective, observational longitudinal cohort study of individuals enrolled in a US managed care network who underwent iStent implantation with cataract surgery (iStent/CEIOL) from 2012 to 2016 (n = 1509 bilateral and n = 1462 unilateral surgery). A control group of individuals who underwent bilateral cataract surgery only (CEIOL) were matched 1:1 to patients undergoing bilateral iStent/CEIOL on baseline demographic and clinical factors. Data were analyzed between November 1, 2017, and January 31, 2018.
Main Outcomes and Measures
The number of topical ocular antihypertensive agents used postoperatively by patients undergoing iStent/CEIOL compared with baseline and with matched CEIOL control individuals, and hazard ratios with 95% confidence intervals for sustained reduced use of at least 1 topical ocular antihypertensive agent postoperatively.
Results
Of the 2971 eligible enrollees, mean age at first surgery was 74.3 years, and 1659 (55.8%) were women. Patients undergoing iStent/CEIOL had diagnoses that included primary open-angle glaucoma (n = 2329; 78.4%), narrow angles (n = 381; 12.8%), and secondary glaucomas (n = 261; 8.8%). At baseline, 1223 (41.2%) were receiving no topical glaucoma agents; 876 (29.5%), 437 (14.7%), and 435 (14.6%) were receiving 1, 2, or at least 3 agents, respectively. Although only 678 persons (22.8%) completed at least 2 years of postoperative follow-up, the proportion of patients receiving no drops increased postoperatively (64.7%, 20-24 months, P < .001, χ2). Patients receiving at least 1 topical agent at baseline had mean reduction of 1.01 and 0.61 medications used at 20 to 24 months with bilateral or unilateral surgery, respectively (both P < .001, paired t). Sustained reduction in glaucoma medication use was more likely in patients receiving at least 3 vs 1 medication at baseline (hazard ratio, 1.68; 95% CI, 1.36-2.09). Compared with matched control individuals undergoing CEIOL, patients undergoing bilateral iStent/CEIOL had a greater mean reduction in drops used (0.99 vs 0.49; postoperative month 20-24; P < .001; paired t) and a higher proportion receiving no drops postoperatively (73.5% vs 55.3%, postoperative month 20-24; P < .001; χ2).
Conclusions and Relevance
Implantation of the iStent trabecular micro-bypass stent concurrent with cataract surgery was associated with moderately reduced use of topical ocular antihypertensive medication. Reduction in the use of glaucoma medications may lessen the burden of medication adverse effects and promote better adherence.
This study determines whether implantation of the iStent concurrent with cataract surgery is associated with reduced use of ocular antihypertensive medications in a US health care claims database.
Introduction
In 2012, the US Food and Drug Administration (FDA) approved the first ab interno glaucoma implant, the iStent Trabecular Micro-Bypass (Glaukos Corporation), for use concurrent with cataract surgery (iStent/CEIOL) for the treatment of mild to moderate open-angle glaucoma.1 This 1-mm titanium device is designed to be implanted into the Schlemm canal, creating a bypass through the trabecular meshwork to improve aqueous outflow and reduce intraocular pressure (IOP), potentially allowing patients to reduce dependence on glaucoma medications.
Clinical trials, designed with a washout period when patients undergoing iStent implantation discontinue glaucoma medications preoperatively, have reported IOP reductions ranging from 8% to 17%2,3 and 1.1 to 1.62,3 fewer medications used postoperatively. The largest reported randomized clinical trial of iStent/CEIOL surgery was the investigational drug exemption study of 240 patients conducted by Glaukos that showed that 72% of patients undergoing iStent/CEIOL surgery had washout IOP of 21 or less without glaucoma medications at postoperative month (POM) 12 or last follow-up visit, and a mean decrease of 1.4 medications compared with baseline.2 However, in clinical practice, patients with glaucoma often do not discontinue all ocular antihypertensives prior to surgery, and it is unclear whether in typical clinical practice patients achieve reduction in use of topical medications after iStent/CEIOL surgery or which clinical characteristics may be associated with reduction in medication dependence.
In this study, we used a large US health care claims database to assess whether patients undergoing iStent/CEIOL surgery experience a sustained reduction in number of topical glaucoma medications used and how this group compares with others with glaucoma undergoing cataract surgery (CEIOL) alone.
Methods
Data Source
We accessed Clinformatics DataMart (OptumInsight), which captures insurance claims records of enrollees in a nationwide managed care network from January 1, 2006, to December 31, 2016. Claims from outpatient health care encounters and associated International Classification of Diseases, Ninth Revision (ICD-9) and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) diagnosis codes4 and Current Procedural Terminology (CPT) billing codes5 linked to each encounter were available. Age and sex of each enrollee were available. Pharmacy records of all outpatient medication fills for all enrollees were available. The Stanford University institutional review board approved this study involving deidentified data.
Inclusion/Exclusion Criteria
We included all individuals undergoing iStent/CEIOL surgery in 1 or both eyes from January 1, 2012, to December 31, 2016. iStent implantation was identified by the CPT code 0191T, and concurrent cataract surgery was identified by CPT codes 66982 or 66984. Eligible individuals had at least 3 years of continuous enrollment in the plan, including at least a 1-year lookback period in the plan prior to initial iStent/CEIOL surgery. Glaucoma diagnoses were identified by ICD-9 or ICD-10 codes from the surgical encounter and preoperative outpatient encounters with ophthalmologists during the lookback period. Glaucoma subtype was categorized into secondary glaucomas (including pigmentary and exfoliation) (ICD-9: 365.81-3, 365.13-4, 365.03, and 365.3-6- and ICD-10: H40.81-83-, H40.13-14-, Q150, H40.04, and H40.3-6-), narrow angles (365.2-6,365.02-3, 365.06, H40.2-6-, H40.03-4-, and H40.06-), and primary open-angle glaucoma (ICD-9: 365.10-365.15, 365.89, 365.9; ICD10: H40.10-12-, and H40.15-). Patients with only a glaucoma suspect diagnosis were excluded. When patients had encounters related to more than 1 category of diagnosis, the more specific one was used, favoring narrow angle over secondary glaucoma over primary open-angle glaucoma.
Outcomes
The primary outcome was prescription fills for the following classes of ocular antihypertensive medications: prostaglandin analogues; single-agent topical β-blockers, topical carbonic anhydrase inhibitors, topical α-agonists, and miotics; combination topical medications; and oral carbonic anhydrase inhibitors. The number of different topical ocular antihypertensives each patient used was totaled for the baseline period (0-4 months prior to first iStent/CEIOL surgery) and for postoperative periods (0-4 months, 4-8 months, 8-12 months, 12-16 months, 16-20 months, and 20-24 months after last iStent/CEIOL surgery). Combination agents were counted as 2 agents. If medication changes were made in the middle of a period, the highest number of medications the patient used during that period was counted. A patient would be considered to be receiving no glaucoma medications for a period if there were no pharmacy fill records for any ocular antihypertensive for the entire period.
Secondary outcomes included determination of whether patients underwent subsequent procedural intervention (either laser trabeculoplasty or incisional glaucoma surgery) to further lower IOP after iStent/CEIOL surgery, as identified through CPT codes (laser trabeculoplasty: 65855; other glaucoma surgeries including trabeculectomies, tube shunts, and other minimally invasive glaucoma surgeries: 66170, 66172, 66174, 66175, 66179, 66180, 66183, 66184, 66185, 0474T, 0253T, 0449T, and 65820). If patients underwent any subsequent glaucoma procedural (laser or surgical) intervention, medication use afterwards was not included in analyses because medication reduction subsequent to additional procedures could be attributed to these procedures rather than to the original iStent/CEIOL surgery.
Matched Control Individuals
Persons who underwent bilateral iStent/CEIOL surgery as described in previous paragraphs (cases) were matched to similar control individuals with glaucoma who underwent bilateral CEIOL only. Cases were matched 1:1 to controls based on sex, glaucoma diagnosis category, number of preoperative topical glaucoma medications, and baseline oral CAI use. Age and first surgery date were matched to within 2 years. Controls were required to have follow-up after surgery at least as long as the matching case and to have 3 years of continuous enrollment in the plan. Matching was performed in SAS using the %gmatch macro (SAS Institute Inc),6 resulting in 1486 matched pairs.
Data Analysis
Data analyses were performed using SAS software, version 9.4 (SAS Institute Inc). Characteristics of the study population were summarized using means/standard deviations or frequencies/percentages as appropriate. The proportion of patients undergoing iStent/CEIOL surgery who were using 0, 1, 2, or at least 3 topical agents during each postoperative period was compared with the baseline period and with matched control individuals using χ2 tests. Mean reduction in number of topical glaucoma drugs used during each postoperative period for patients undergoing iStent/CEIOL was compared with baseline and with matched control individuals undergoing CEIOL using paired t tests. Multivariable Cox regression modeling was performed to analyze predictors for sustained reduction in use of at least 1 topical glaucoma medication after surgery through 20 to 24 months after surgery (or throughout follow-up period if available follow-up was shorter) without additional procedural intervention, among patients who underwent iStent/CEIOL and had been receiving at least 1 topical glaucoma medication at baseline. Patients who had a transient reduction in number of topical agents used that did not persist through 20 to 24 months or last available follow-up were considered not to have achieved the outcome. Patients who had a procedural intervention to lower their IOP (such as laser trabeculoplasty or additional incisional glaucoma surgery as described previously) were included in the model until the date of such intervention and were considered not to have achieved the outcome (censored). Predictors included age and calendar year at first surgery, sex, glaucoma diagnosis type, number of topical ocular antihypertensive agents at baseline, and whether the surgery was unilateral or bilateral. A separate univariate Cox regression model was performed comparing the bilateral iStent/CEIOL cohort to the matched control individuals undergoing CEIOL on the previously defined outcome. For all analyses, a 2-sided P value less than .05 was considered statistically significant.
Results
Population Characteristics
Among the 2971 eligible enrollees who underwent iStent/CEIOL surgery, 1462 (49.2%) underwent unilateral surgery and 1509 (50.8%) underwent bilateral surgery (Table 1). The mean (SD) age at the time of first surgery for all patients was 74.3 (7.8) years. Most patients had primary open-angle glaucoma (n = 2329; 78.4%); 381 (12.8%) had narrow angles; and 261 (8.8%) had secondary open-angle glaucoma, including pigmentary and exfoliation glaucoma. More iStent/CEIOL surgeries occurred over time, with more than 60% of surgeries performed in 2015 and 2016. The median follow-up time from most recent surgery until censoring, exit from health plan, or subsequent laser trabeculoplasty or glaucoma surgery was 1.12 years (interquartile range, 0.45-1.91 years), with 678 persons (22.8%) completing at least 2 years of postoperative follow-up. Control patients undergoing cateract surgery had similar characteristics to patients undergoing bilateral iStent/CEIOL owing to matching.
Table 1. Population Characteristics of Patients Who Underwent iStent Concurrent With Cataract Surgery.
| Characteristic | iStent/CEIOL, No. (%) | |
|---|---|---|
| Unilateral (n = 1462) | Bilateral (n = 1509) | |
| Age at first surgery, mean (SD), y | 74.6 (8.05) | 74.0 (7.5) |
| Female | 788 (53.9) | 871 (57.7) |
| Diagnosis | ||
| POAG | 1073 (73.4) | 1256 (83.2) |
| Narrow angle | 221 (15.1) | 160 (10.6) |
| Secondary glaucomas | 168 (11.5) | 93 (6.2) |
| First iStent/CEIOL surgery, y | ||
| 2012 | 10 (0.7) | 10 (0.7) |
| 2013 | 137 (9.4) | 130 (8.6) |
| 2014 | 338 (23.2) | 336 (22.3) |
| 2015 | 450 (30.8) | 459 (30.4) |
| 2016 | 527 (36.1) | 574 (38.0) |
| Baseline topical antihypertensive agents, No. | ||
| 0 | 568 (38.9) | 655 (43.4) |
| 1 | 429 (29.3) | 447 (29.6) |
| 2 | 214 (14.6) | 223 (14.8) |
| ≥3 | 251 (17.2) | 184 (12.2) |
| Baseline oral CAI | 26 (1.8) | 12 (0.8) |
Abbreviations: CAI, carbonic anhydrase inhibitor; CEIOL, cataract extraction with intraocular lens implantation; POAG, primary open-angle glaucoma.
Glaucoma Medication Use Among the iStent/CEIOL Cohort
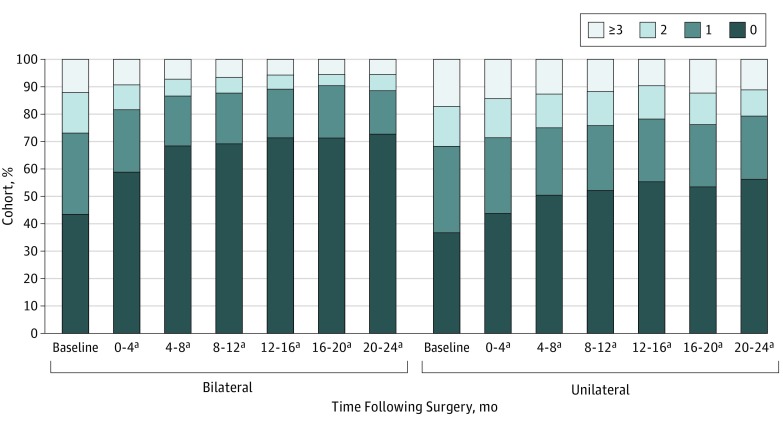
At baseline, 1223 (41.2%) were taking no topical glaucoma agents, 876 (29.5%), 437 (14.7%), and 435 (14.6%) were receiving 1, 2, or at least 3 topical agents, respectively. By POM 20 to 24, the proportion of patients receiving no glaucoma medications rose to 64.7% (n = 409), while the proportion of those receiving 1, 2, or 3 more drops decreased to 19.3% (n = 122), 7.8% (n = 49), and 8.2% (n = 52) respectively. The increase in proportion of patients receiving 0 topical antihypertensives following surgery was more pronounced among patients undergoing bilateral iStent/CEIOL surgery, but there was still an association among patients undergoing unilateral iStent/CEIOL surgery (Figure 1). A sensitivity analysis was performed excluding patients who were receiving no glaucoma medications at baseline, which still showed an increase in proportion of patients receiving zero topical antihypertensives postoperatively; by POM 20 to 24, 32.6% of patients (n = 57) undergoing unilateral surgery were receiving no medications, and 56.5% of patients (n = 96) undergoing bilateral surgery were receiving no medications (see the eFigure in the Supplement for distribution of medication use at all times). Among patients who were receiving at least 1 topical glaucoma agent preoperatively, there was a statistically significant reduction in mean number of medications used at every period postoperatively compared with baseline, with mean reduction of 1.01 (95% CI, 0.85-1.16) and 0.61 (95% CI, 0.43-0.78) at POM 20 to 24 among patients who underwent bilateral and unilateral iStent/CEIOL surgery, respectively (P < .001 for all points compared with baseline, paired t test) (Figure 2). Thirty-eight patients (1.3%) were receiving oral carbonic anhydrase inhibitors preoperatively; none were still using oral carbonic anhydrase inhibitors postoperatively. Procedural escalation of IOP-lowering treatment after iStent/CEIOL surgery was achieved by means of laser trabeculoplasty in 104 patients (3.5%) or additional incisional glaucoma surgery in 79 patients (2.7%). These patients were excluded from analyses of medication use after these additional interventions were performed.
Figure 1. Number of Topical Antihypertensive Medications Used at Baseline and Postoperatively Among Patients Undergoing Bilateral or Unilateral iStent Implantation With Cataract Extraction and Intraocular Lens Implantation.
Proportions and number of patients receiving 0, 1, 2, or 3 or more topical antihypertensive agents at baseline and postoperatively are shown for those who underwent bilateral or unilateral iStent implantation with cataract extraction and intraocular lens implantation.
aStatistically significant difference from baseline time proportions of agents used (P value <.001 for all time points, χ2 tests).
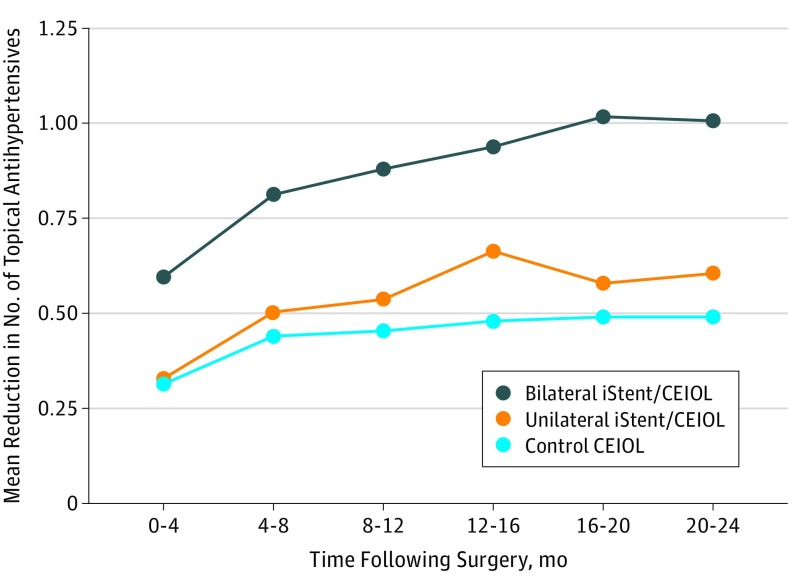
Figure 2. Mean Reduction in Number of Postoperative Topical Antihypertensive Agents for Patients Undergoing Bilateral or Unilateral iStent Implantation With Cataract Extraction and Intraocular Lens Implantation (iStent/CEIOL) and Matched Control Patients.
Mean reduction in number of topical antihypertensive agents used from baseline are shown at each period for patients who underwent iStent/CEIOL as well as matched control individuals with glaucoma who underwent bilateral cataract surgery (CEIOL) only. The 95% confidence interval ranges for mean drop reduction are given in parentheses.
Multivariable Cox regression modeling was performed to investigate predictors for achieving sustained reduction of at least 1 topical glaucoma medication without subsequent laser trabeculoplasty or glaucoma surgery by POM 20 to 24 among patients who were receiving at least 1 topical glaucoma medication at baseline. Type of glaucoma diagnosis was not a significant predictor of the outcome. Patients undergoing bilateral iStent/CEIOL surgery had 97% increased odds of achieving sustained reduction in glaucoma medications used (HR, 1.97; 95% CI, 1.64-2.38). Patients receiving more agents at baseline were more likely to achieve reduction in postoperative agents used (HR, 1.71; 95% CI, 1.37-2.13 for 3 or more medications vs 1 medication at baseline) (Table 2).
Table 2. Multivariable Cox Proportional Hazards Model for Sustained Reduction of at Least 1 Topical Antihypertensive Agent Without Subsequent Laser Trabeculoplasty or Glaucoma Surgery, Among iStent/CEIOL Patients Receiving at Least 1 Agent at Baseline.
| Characteristic | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Age at first surgery (per year increase) | 1.00 (0.99-1.01) | .99 |
| Female (reference: male) | 1.04 (0.86-1.25) | .69 |
| Glaucoma diagnosis (reference: POAG) | ||
| Narrow angle | 0.95 (0.73-1.24) | .72 |
| Secondary glaucomas | 0.92 (0.65-1.31) | .65 |
| Calendar year of surgery, per year increase | 1.04 (0.94-1.16) | .43 |
| Bilateral iStent/CEIOL (reference: unilateral iStent/CEIOL) | 1.97 (1.64-2.37) | <.001 |
| Baseline number of topical antihypertensive agents (reference: 1 agent) | ||
| 2 Agents | 1.23 (0.98-1.54) | .07 |
| ≥3 Agents | 1.71 (1.37-2.13) | <.001 |
Abbreviations: CEIOL, cataract extraction with intraocular lens implantation; POAG, primary open-angle glaucoma.
Glaucoma Medication Use Compared With Matched Control Individuals Undergoing CEIOL Surgery Only
To compare outcomes between patients who underwent concurrent iStent/CEIOL surgery with patients who underwent only CEIOL surgery, control patients with glaucoma who underwent bilateral CEIOL surgery without iStent implantation were matched 1:1 to patients in the bilateral iStent/CEIOL cohort on age, calendar year of first surgery, sex, glaucoma type, and number of baseline ocular antihypertensives used. Successful matches were found for 1486 of 1509 patients (98.5%) undergoing bilateral iStent/CEIOL surgery. Of 1486 patients in the matched bilateral iStent/CEIOL cohort, 40 (2.7%) underwent subsequent laser trabeculoplasty and 18 (1.2%) underwent glaucoma surgery; 68 (4.6%) and 15 (1.0%) of the matched control patients underwent subsequent laser trabeculoplasty or glaucoma surgery, respectively. These patients were excluded from analyses of topical medications used after these additional interventions were performed.
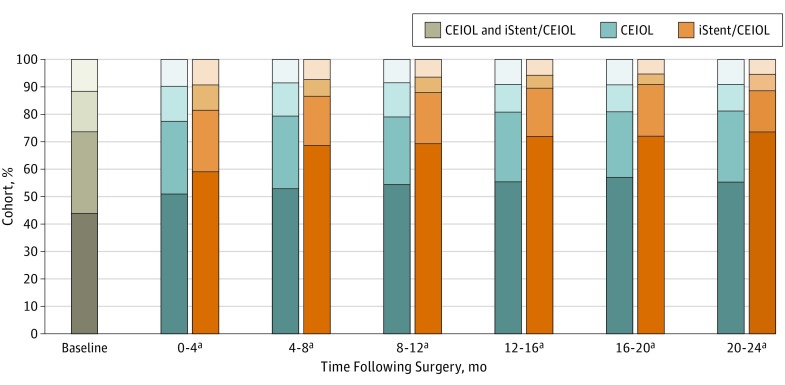
Proportions of patients receiving 0, 1, 2, and at least 3 topical drops at baseline and postoperatively are shown in Figure 3 for cases and controls. At every postoperative period, a higher proportion of the iStent/CEIOL cohort were receiving no topical glaucoma agents compared with CEIOL control individuals. While the proportion of the iStent/CEIOL cohort receiving no glaucoma medications rose from 43.9% (n = 653 of 1486) to 73.5% (n = 233 of 317) at POM 20 to 24, there was a smaller rise in the proportion of control patients undergoing CEIOL receiving no drops (n = 422 of 763; 55.3%) (Figure 3). There was a greater reduction in mean number of drops used at every postoperative period for the iStent/CEIOL cohort compared with the control individuals undergoing CEIOL (P < .001 for all periods, paired t tests) (Figure 2). At POM 20 to 24, the mean reduction in number of glaucoma drops for control patients undergoing CEIOL was 0.49 (95% CI, 0.38-0.60) compared with 0.99 (95% CI, 0.84-1.15) for matched iStent/CEIOL cases. Cox regression modeling showed that patients in the iStent/CEIOL cohort were 160% more likely to achieve reduction in at least 1 topical glaucoma medication postoperatively without subsequent escalating intervention compared with the CEIOL control patients (HR, 2.60; 95% CI 2.18-3.12), for those who were receiving at least 1 glaucoma medication at baseline.
Figure 3. Number of Topical Antihypertensive Medications Used at Baseline and Postoperatively Among Patients Undergoing Bilateral iStent Implantation With Cataract Extraction and Intraocular Lens Implantation (iStent/CEIOL) Compared With Matched CEIOL Control Patients.
Proportions and number of patients receiving 0, 1, 2, or 3 or more topical antihypertensive agents at baseline and postoperatively are shown for patients who underwent bilateral iStent/CEIOL and their matched control patients who underwent CEIOL only. The CEIOL control patients are shown in blue bars and iStent/CEIOL cases are shown in orange bars. Baseline medications used are shown with 1 bar representing both controls and cases because they were identically matched on this characteristic at baseline.
aDenotes statistically significant difference between cases and controls in the proportions of agents used (P value <.001 for all postoperative points, χ2 tests).
Discussion
In this study of nearly 3000 patients enrolled in a large nationwide US managed care insurance plan who underwent iStent/CEIOL surgery, we found that by POM 20 to 24 postoperatively, almost three-quarters of patients who underwent bilateral surgery were taking no topical glaucoma medications compared with just more than 40% who were receiving no drops at baseline; we also observed a mean reduction of approximately 1 medication used among those who were taking at least 1 medication prior to their surgery. In contrast, matched control individuals with glaucoma who underwent bilateral CEIOL surgery only achieved a mean reduction of 0.49 agents.
Our results are consistent with previous studies of patients undergoing iStent/CEIOL surgery, which have reported a mean decrease of approximately 1 ocular antihypertensive agent at 12 to 24 months postoperatively (range, 1.2-1.6) among patients who were receiving at least 1 medication at baseline,2,3,7,8,9,10 similar to our bilateral iStent/CEIOL cohort. The proportion of patients reported to be glaucoma medication–free after surgery has varied, ranging from 50% to 85% at 12 to 24 months2,3,7,10 among previous studies that required perioperative discontinuation of glaucoma drops, and from 41% to 63%8,11 at 12 to 24 months in retrospective case series where patients did not discontinue glaucoma medications perioperatively and may or may not have trialed reduced medications postoperatively. In our study, 72.8% of the bilateral iStent/CEIOL cohort were medication-free at 20 to 24 months postoperatively compared with 43.4% at baseline. We found a higher percentage of patients receiving no medications preoperatively than in previous case series (9.4%-28%),8,11 which may reflect a higher proportion of patients with milder glaucoma undergoing iStent/CEIOL surgery.
In a typical clinical setting, some surgeons may have patients stop taking glaucoma medications after iStent/CEIOL while others may not. We note that the proportion of patients taking no glaucoma drops postoperatively steadily increased from the immediate postoperative period of months 0 to 4, through months 12 to 16, and thereafter stayed at a similar level through months 20 to 24, which may reflect the progressive discontinuation of medications after surgery as patients stop taking medications as their IOP decreases. This is consistent with previous studies that have suggested that the IOP-lowering effect after iStent/CEIOL surgery was achieved by 3 months and persisted through at least 6 to 12 months postoperatively.2,12 We also found that patients who were receiving 3 or more glaucoma medications preoperatively were more likely than those receiving only 1 medication at baseline to achieve postoperative glaucoma medication reduction. Several studies have reported that patients receiving more glaucoma medications or more complex medication regimens may have greater difficulty with medication adherence.13 Glaucoma medications also have many adverse effects, including an adverse effect on the success of future glaucoma surgeries.14 Thus, patients receiving more numerous medications at baseline and their eye care professionals may be especially motivated to pursue medication reduction trial after iStent/CEIOL.
Strengths of this study include our large sample of patients who underwent iStent/CEIOL surgery, with nearly 3000 patients in the cohort, which, to our knowledge, far exceeds previous studies. Patients were drawn from a large and diverse population, which may be well representative of the US insured population. Patients were treated according to their physicians’ usual practice; thus, results in reduction of medication use may be more reflective of what is realistically achievable in clinical practice compared with results from randomized clinical trials. Pharmacy medication fill records were available to determine medication use, which was aggregated in 4-month periods postoperatively; therefore, reduction in medications used postoperatively would not be expected to be greatly affected by patients who may occasionally have poor adherence as long as medications were refilled at least once in a 4-month period. Control patients who underwent CEIOL surgery alone could be matched to those undergoing iStent/CEIOL, which is especially important because CEIOL alone has been shown to reduce IOP by 1 to 2 mm Hg and glaucoma medication use by 0.6 to 1.1 agents.2,3
Limitations
There are several limitations to this study. Patients’ history of glaucoma procedures and the severity of glaucoma could not be reliably determined; however, our control patients with glaucoma were matched by number of medications used preoperatively to approximate disease severity. Only 22.8% of patients in this cohort had 2-year follow-up data; additional research is required to study longer-term outcomes. Some eyes may have been implanted with more than 1 iStent, an off-label choice, likely uncommon because patients would need to pay out of pocket for the additional device. Clinical information, such as IOP or visual acuity, was not available, so postoperative medication use could not be correlated with IOP changes. Some surgeons may choose not to remove any medications after iStent/CEIOL surgery to achieve greater IOP lowering for patients with more severe glaucoma, thus apparently decreasing the medication reduction effect of surgery. Alternatively, some surgeons may automatically reduce glaucoma medications after iStent surgery regardless of whether IOP is reduced to align with patients’ expectations. Laterality could not be determined for iStent/CEIOL surgery, glaucoma medication use, or subsequent laser trabeculoplasty or glaucoma surgeries, which affected analyses for the unilateral iStent/CEIOL cohort. Patients in the unilateral iStent/CEIOL cohort had lower mean reduction in glaucoma medication fills likely because medication usage could not be studied at the individual eye level; patient-level estimations in the reduction of topical ocular antihypertensive use are likely to be conservative for this group because medications needed for the contralateral eye would continue to be filled at the pharmacy.
Conclusions
In this observational study of nearly 3000 patients who underwent unilateral or bilateral iStent/CEIOL surgery, patients were able to achieve a moderate and sustained reduction in the use of topical antihypertensive agents postoperatively. Although only 23% completed at least 2 years of postoperative follow-up, at 2 years after iStent/CEIOL surgery, almost three-quarters of patients who underwent bilateral implantation were taking no glaucoma medications, with a mean reduction of approximately 1 medication used among those who were receiving at least 1 medication at baseline. Patients who were receiving more glaucoma medications at baseline were more likely to achieve a sustained reduction of at least 1 topical glaucoma agent. These findings support many smaller-scale studies and offer evidence that iStents are associated with reduced medication dependence in patients with glaucoma. Further studies would be needed to determine to test whether this association has a cause and effect relationship.
eFigure. Number of Topical Antihyperintensive Medications Used at Baseline and Postoperatively Among iStent/CEIOL Cohort.
References
- 1.Food and Drug Administration Premarket approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm?id=P080030. Published December 11, 2017. Accessed December 12, 2017.
- 2.Samuelson TW, Katz LJ, Wells JM, Duh Y-J, Giamporcaro JE; US iStent Study Group . Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459-467. doi: 10.1016/j.ophtha.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 3.Fea AM. Phacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma: randomized double-masked clinical trial. J Cataract Refract Surg. 2010;36(3):407-412. doi: 10.1016/j.jcrs.2009.10.031 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization International Classification of Diseases. http://www.who.int/classifications/icd/en/. Published November 29, 2016. Accessed December 14, 2017.
- 5.American Medical Association CPT (Current Procedural Terminology) https://www.ama-assn.org/practice-management/cpt-current-procedural-terminology. Published December 14, 2017. Accessed December 14, 2017.
- 6.Bergstralh E, Kosanke J Division of biomedical statistics and informatics: locally written SAS macros: %gmatch. http://www.mayo.edu/research/departments-divisions/department-health-sciences-research/division-biomedical-statistics-informatics/software/locally-written-sas-macros. Accessed December 12, 2017.
- 7.Tan SZ, Au L. Manchester iStent study: 3-year results and cost analysis. Eye (Lond). 2016;30(10):1365-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seibold LK, Gamett KM, Kennedy JB, et al. Outcomes after combined phacoemulsification and trabecular microbypass stent implantation in controlled open-angle glaucoma. J Cataract Refract Surg. 2016;42(9):1332-1338. doi: 10.1016/j.jcrs.2016.07.023 [DOI] [PubMed] [Google Scholar]
- 9.Spiegel D, Wetzel W, Neuhann T, et al. Coexistent primary open-angle glaucoma and cataract: interim analysis of a trabecular micro-bypass stent and concurrent cataract surgery. Eur J Ophthalmol. 2009;19(3):393-399. [DOI] [PubMed] [Google Scholar]
- 10.Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle D, Morales-Fernandez L, Fernandez-Perez C, Garcia-Feijoo J. Glaukos iStent inject trabecular micro-bypass implantation associated with cataract surgery in patients with coexisting cataract and open-angle glaucoma or ocular hypertension: a long-term study. J Ophthalmol. 2016;2016:1056573. doi: 10.1155/2016/1056573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson TJ, Berdahl JP, Schweitzer JA, Sudhagoni RG. Clinical evaluation of a trabecular microbypass stent with phacoemulsification in patients with open-angle glaucoma and cataract. Clin Ophthalmol. 2016;10:1767-1773. doi: 10.2147/OPTH.S114306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel I, de Klerk TA, Au L. Manchester iStent study: early results from a prospective UK case series. Clin Exp Ophthalmol. 2013;41(7):648-652. doi: 10.1111/ceo.12098 [DOI] [PubMed] [Google Scholar]
- 13.Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;53(suppl 1):S57-S68. doi: 10.1016/j.survophthal.2008.08.002 [DOI] [PubMed] [Google Scholar]
- 14.Broadway D, Grierson I, Hitchings R. Adverse effects of topical antiglaucomatous medications on the conjunctiva. Br J Ophthalmol. 1993;77(9):590-596. doi: 10.1136/bjo.77.9.590 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Number of Topical Antihyperintensive Medications Used at Baseline and Postoperatively Among iStent/CEIOL Cohort.