Key Points
Question
How does the penetrance and association of myocilin (MYOC) gene p.Gln368Ter variant with glaucoma and ocular hypertension differ across studies ascertained in different ways?
Findings
This cross-sectional study found that 48% of p.Gln368Ter carriers older than 65 years had glaucoma or ocular hypertension, with an odds ratio for primary open-angle glaucoma of 6.76 (95% CI, 4.05-11.29) compared with noncarriers. In registry-based studies, penetrance was very high, with the odds ratio being markedly increased in individuals with advanced glaucoma (12.16 [95% CI, 6.34-24.97]) compared with individuals with nonadvanced glaucoma (odds ratio, 3.97 [95% CI, 1.55-9.75]).
Meaning
Our results suggest the penetrance in population-based studies is higher than previously shown and support the need for early screening of p.Gln368Ter carriers.
This cross-sectional study of population-based and registry-based studies examines the penetrance and association of myocilin (MYOC) gene p.Gln368Ter variant with glaucoma and ocular hypertension.
Abstract
Importance
The p.Gln368Ter (rs74315329) risk allele in the myocilin gene (MYOC) was initially reported to have high penetrance in glaucoma registry-based studies, but much lower estimates were recently obtained from population-based studies. We investigated this disparity using data from Australia and the United Kingdom.
Objectives
To examine the penetrance and effect size of the MYOC p.Gln368Ter variant with glaucoma and ocular hypertension (OHT).
Design, Setting, and Participants
This cross-sectional study within the UK Biobank (UKBB) included participants of white British ancestry. Glaucoma cases were defined by International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10) diagnoses and self-reported questionnaires. Carriers of the MYOC p.Gln368Ter variant were identified using genotype imputation from arrays. In contrast, 2 Australian registry-based studies, the Australian and New Zealand Registry of Advanced Glaucoma and the Glaucoma Inheritance Study in Tasmania, ascertained glaucoma cases referred by eye care clinicians, with historic control participants recruited from other Australian studies. Samples were either directly sequenced or had genotypes determined by imputation (for the Australian registry and historic control participants). Recruitment to the UKBB occurred between 2006 and 2010, and data analysis occurred from September 2017 to July 2018.
Main Outcomes and Measures
The penetrance and odds ratio (OR) were estimated for the MYOC p.Gln368Ter variants in participants with glaucoma and OHT.
Results
A total of 411 337 UKBB participants of white British ancestry (mean [SD] age, 56.6 [8.0] years) were included, plus 3071 Australian registry and 6750 historic control participants. In the UKBB, the minor allele frequency of the MYOC p.Gln368Ter variant was 1 in 786 individuals (0.13%). The odds ratio of p.Gln368Ter in patients with primary open-angle glaucoma (POAG) was 6.76 (95% CI, 4.05-11.29); glaucoma (POAG, self-reported glaucoma, and unspecified glaucoma), 4.40 (95% CI, 3.38-5.71); OHT, 3.56 (95% CI, 2.53-4.92); and OHT and glaucoma combined, 4.18 (95% CI, 3.05-5.67). The penetrance of the MYOC p.Gln368Ter variant was 7.6% in patients with glaucoma, 24.3% in patients with OHT, and 30.8% in patients with OHT and glaucoma combined. In the Australian registry studies, the odds of MYOC p.Gln368Ter variant were 12.16 (95% CI, 6.34-24.97) in patients with advanced glaucoma and 3.97 (95% CI, 1.55-9.75) in those with nonadvanced glaucoma; the penetrance of glaucoma was 56.1%, and penetrance in those considered to have glaucoma or be glaucoma suspects was 69.5%.
Conclusions and Relevance
The MYOC p.Gln368Ter variant confers a very high-risk effect size for advanced glaucoma; the risk is lower in nonadvanced glaucoma and OHT. In the general population sample, approximately 50% of MYOC p.Gln368Ter carriers 65 years and older had glaucoma or OHT, with higher prevalence in the Australian registry studies.
Introduction
Glaucoma is the leading cause of irreversible blindness globally. The most common forms of glaucoma are primary open-angle glaucoma (POAG) and primary angle-closure glaucoma (PACG). For the population older than 40 years, the worldwide age-standardized prevalence of glaucoma is 3.54%; for POAG and PACG, it is 3.05% and 0.50%, respectively.1 It is estimated there were 60.5 million patients with POAG and PACG worldwide in 2010, and that number will be 112 million by 2040.1,2 Elevated intraocular pressure (IOP) is the major modifiable risk factor for POAG. Progression of POAG is arrested or reduced if the IOP is lowered by 30% to 50% from baseline levels.3
Genetic factors play an important role in glaucoma.4,5,6 Having a first-degree relative with glaucoma raises the likelihood of developing glaucoma by 9.4-fold relative to the general population.7 A recent large-scale study estimated the heritability of glaucoma to be 70% using reconstructed family data.8 The myocilin gene (MYOC) at the GLC1A locus was the first gene discovered to be associated with POAG.9,10 Pathogenic variants in MYOC have been found in 2% to 4% of individuals with POAG.11,12 The exact pathogenic mechanisms by which disease-causing variants in MYOC might cause glaucoma have not been elucidated completely, but evidence supports a dominant-negative mechanism.13,14
The p.Gln368Ter variant (rs74315329) is the most common MYOC variant among populations of European ancestry.11,12,15 The association between p.Gln368Ter and POAG has an odds ratio (OR) greater than 10, with the p.Gln368Ter variant associated with younger age at onset and greater severity of IOP elevation.16,17 The estimated penetrance of p.Gln368Ter in glaucoma and ocular hypertension (OHT) has been inconsistent between family studies and general population-based studies.11,12,15,18,19,20 There are several potential explanations for this inconsistency. Estimates from family studies may be inflated because of ascertainment bias, aggregation of other genetic factors, and/or confounds by common environmental risk factors. Conversely, estimates from general population-based designs are likely to be low because of undersampling of cases (especially more severely affected cases) among volunteer-based studies.20 Additionally, for both family studies and general population-based studies, statistical power is typically low because of the relatively low numbers of p.Gln368Ter carriers.
In this study, we explore the penetrance and association of MYOC p.Gln368Ter variant with glaucoma and OHT in white European individuals enrolled in the UK Biobank (UKBB) study and compare the results with registry-based studies.
Methods
The UKBB project is a large-scale prospective cohort study of approximately 500 000 individuals across the United Kingdom, aged between 40 and 69 years at the time of recruitment (2006-2010). Detailed information on the UKBB study is available online (http://www.ukbiobank.ac.uk/resources/), and the genotype curation process is described in Bycroft et al.21
The study was approved by the National Research Ethics Service Committee North West–Haydock, in accordance with the Declaration of Helsinki. Approval was also obtained from QIMR Berghofer Institute of Medical Research, the Southern Adelaide Clinical Human Research Ethics Committee of Flinders University, the University of Tasmania, and the Royal Victorian Eye and Ear Hospital, also in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
In the UKBB study, the genotypic data were imputed with IMPUTE4 (Oxford University Innovation), using the Haplotype Reference Consortium, UK10K, and 1000 Genomes Phase 3 reference panels. Among 487 409 participants passing genotyping quality control, 409 694 had white British ancestry based on self-reported race/ethnicity data. We identified 438 870 individuals who fell within the same genetic principal components-based clustering as those who self-reported white British ancestry, based on the first 2 genetic principal components (eFigure 1 in the Supplement).
Our previous study showed that the p.Gln368Ter variant can be imputed with high accuracy from genotyping arrays.17 In this study, the imputation posterior probability for each of the 3 genotypes (GG [homozygous for the non-risk allele], AG, and AA [homozygous for the risk allele]) was used to identify p.Gln368Ter carriers. We calculated the genotype dosages based on imputation posterior probability. Because the dosage only ranged from 0 to 1.1, there were no p.Gln368Ter homozygous carriers in our study. For downstream analyses requiring best-guess genotypes, we set the dosage threshold of heterozygous AG at 0.8.
A subset of 112 690 UKBB participants underwent IOP measurements at the UKBB Assessment Centre using Reichert Ocular Response Analyzer (Reichert Technologies)22; the detailed procedure is available online.23 The mean corneal-compensated IOP (IOPcc; UKBB data field 5254 and 5262) and mean Goldmann-correlated IOP (IOPg; UKBB data fields 5255 and 5263) for each participant was calculated at the initial assessment visit, with measurements less than 5 mm Hg or greater than 60 mm Hg set as missing. Ocular hypertension or high IOP was defined as mean IOPcc of more than 21 mm Hg (n = 6827 of 84 481 [8.1%]).
Among the 438 870 participants with genetic data, we removed participants who withdrew consent (n = 10 [<0.01%]). From the remaining 438 860 participants, individuals with glaucoma were identified via the following criteria: they (1) had an International Classification of Diseases, Ninth Revision (ICD-9) or Tenth Revision (ICD-10) diagnosis of primary open-angle glaucoma, other glaucoma, or glaucoma, unspecified; (2) reported glaucoma in a survey item inquiring about eye problems or disorders (UKBB data field 6148); or (3) reported glaucoma in a survey item on self-reported noncancer illness (UKBB data field 20002). Individuals with POAG were identified by an ICD-9 or ICD-10 diagnosis of POAG. Among participants with IOP measurements, those with IOPcc measurements greater than 21 mm Hg were defined as having OHT or glaucoma, as were those individuals identified as having glaucoma. The information for age at glaucoma onset was gathered from UKBB data fields 4689 and 20009; field 21022 was regarded as age at recruitment.
Finally, individuals were selected as healthy control participants for glaucoma, POAG, and OHT if they (1) did not have other serious eye diseases (UKBB data field 6148; 26 576 individuals excluded) and (2) did not have other kinds of glaucoma diagnosed by ICD-9 or ICD-10 (diagnosed as a glaucoma suspect or diagnosed with PACG or secondary glaucoma; 947 individuals excluded). For control participants without POAG, 6886 individuals with other types of glaucoma were regarded as having not-available status. In total, 411 337 UKBB participants were included in this study. The flowchart illustrating selection criteria is shown in eFigure 2 in the Supplement.
In addition to examining population-based data, we considered participants from 2 registry-based studies: the Australian and New Zealand Registry of Advanced Glaucoma (ANZRAG) and the Glaucoma Inheritance Study in Tasmania (GIST). Recruitment has been previously described.24,25 In brief, patients with glaucoma from Australia and New Zealand had been referred to the ANZRAG by their ophthalmologists. Participants in the GIST were recruited from surveys distributed to ophthalmology clinics and advertisements around Tasmania. Clinical information was collected by the patient’s treating ophthalmologist. Participants from ANZRAG and GIST were considered to have glaucoma if they had glaucomatous visual field defects on standard automated perimetry and neuroretinal rim thinning (cup-to-disc ratio [CDR] ≥0.7 or CDR asymmetry ≥0.2). Individuals considered to be glaucoma suspects had OHT defined by an IOP greater than 21 mm Hg or had preperimetric glaucoma based on glaucomatous appearance of the optic disc or thinning or the retinal nerve fiber layer with no glaucomatous field changes.
There were 2 arms to the ANZRAG and GIST component. First, there was a sequencing-based study within the ANZRAG and GIST data sets alone, to estimate the penetrance of p.Gln368Ter variants. Second, an array-based genome-wide association study allowed the estimation of the odds of glaucoma in a large sample of individuals with cases and control participants sourced from outside ANZRAG and GIST.
In the sequencing-based study, all participants with glaucoma and their relatives in the ANZRAG and GIST registries underwent Sanger sequencing for MYOC exon 3, as previously described.26 Participants who were found to be MYOC p.Gln368Ter carriers were considered Sanger validated, and age at diagnosis was ascertained.
In the array-based study, we selected a total of 3071 unrelated participants with glaucoma from the ANZRAG and GIST registries and 6750 unscreened control participants from the Brisbane Adolescent Twin Study, the Australian Cancer Study, a study of inflammatory bowel diseases, and a study of endometriosis.27,28,29 The samples were genotyped on Omni 1M (Illumina), OmniExpress (Illumina), or HumanCoreExome (Illumina) arrays; approximately two-thirds of cases were genotyped on HumanCoreExome arrays, with the remainder typed on arrays with higher single-nucleotide polymorphism density (Omni 1M and OmniExpress), with a similar proportion among the control participants.30 Genotype imputation was performed using Minimac3 through the Michigan Imputation Server, with the Haplotype Reference Consortium release 1.1 as the reference panel.
We investigated the effect size of p.Gln368Ter for individuals with advanced glaucoma (n = 1753 of 3071 [57.1%]) and nonadvanced glaucoma (n = 1318 [42.9%]) separately. Individuals with advanced and nonadvanced glaucoma were defined as previously described.31
Statistical Analysis
Descriptive statistics are presented as means (SDs) for continuous variables or as numbers (percentages) for categorical variables. Continuous variables were compared between groups using analysis of variance, whereas Pearson χ2 or Fisher exact tests were used for categorical variables. We explored the prevalence of glaucoma and OHT in 4 age groups (younger than 50 years, 50-59 years, 60-65 years, and older than 65 years) of MYOC p.Gln368Ter carriers. We also investigated the cumulative risk of glaucoma by age 50 years, 60 years, and 65 years using a Cox model (adjusted for sex and the first 6 genetic principal components of the UKBB) or the Kaplan-Meier method (ANZRAG and GIST). The association between p.Gln368Ter dosage and disease status was estimated using logistic regression adjusted for sex, age, and the first 6 genetic principal components. To control bias from familial relationships in association analysis, we used a relationship-based pruning strategy in plink to exclude 1 member of each pair of samples if the genomic relatedness was greater than 0.2.32 We used the basic packages and survival package in analyses in R (version 3.4.1; http://www.r-project.org). We used 2-tailed P values and an α level of .05. Analysis was completed from September 2017 to July 2018.
Results
Table 1 shows the baseline characteristics of the 411 337 UKBB participants included in this study. Of these, 188 725 participants (45.9%) were male. The mean (SD) age of participants was 56.6 (8.0) years, with a mean (SD) IOPcc of 16.1 (3.5) mm Hg and a mean (SD) IOPg of 15.9 (3.6) mm Hg. We observed a trend that the mean level of IOP increased with age.
Table 1. Characteristics of 411 337 UK Biobank Study Participants.
| Characteristics | Mean (SD) | |||
|---|---|---|---|---|
| Age <50 y (n = 94 164) | Age 50-59 y (n = 138 395) | Age 60-65 y (n = 101 322) | Age >65 y (n = 77 456) | |
| Age, y | 45.0 (2.7) | 54.8 (2.9) | 61.9 (1.4) | 66.9 (1.5) |
| Sex, No. (%) | ||||
| Male | 42 529 (45.2) | 60 806 (43.9) | 46 539 (45.9) | 38 851 (50.2) |
| Female | 51 635 (54.8) | 77 589 (56.1) | 54 783 (54.1) | 38 605 (49.8) |
| Corneal-compensated intraocular pressure, mm Hg | 15.20 (3.2) | 15.78 (3.4) | 16.5 (3.6) | 16.9 (3.7) |
| Goldmann-correlated intraocular pressure, mm Hg | 15.38 (3.4) | 15.76 (3.6) | 16.23 (3.6) | 16.38 (3.8) |
Among the 411 337 UKBB participants, 7997 individuals with glaucoma (1.9%) were identified. A total of 1111 individuals with POAG were identified by an ICD-9 or ICD-10 diagnosis of primary open-angle glaucoma.
From 411 337 UKBB participants, it was estimated that 1046 carried the p.Gln368Ter AG genotype. The minor allele frequency (MAF) of risk allele A of p.Gln368Ter was 1 of 786 participants (0.13%), and the observed MAFs were roughly the same across different age groups. As expected, given the frequency, no AA homozygotes were observed.
The MYOC p.Gln368Ter penetrance and its association with glaucoma and OHT are summarized in Table 2. The penetrance of p.Gln368Ter in glaucoma was estimated to be 7.6% (n = 79 of 1046); in POAG, it was 1.6% (n = 16 of 1046); in OHT, 24.3% (n = 52 of 214); and in OHT or glaucoma combined, it was estimated to be 30.8% (n = 66 of 214). The odds ratio of p.Gln368Ter in glaucoma was 4.40 (95% CI, 3.38-5.71); in POAG, it was 6.76 (95% CI, 4.05-11.29); in OHT, it was 3.56 (95% CI, 2.53-4.92); and in OHT and glaucoma combined, it was 4.18 (95% CI, 3.05-5.67). For p.Gln368Ter carriers, their IOPcc was 2.04 (95% CI, 1.44-2.64) mm Hg higher than in individuals of GG genotype.
Table 2. Disease Prevalence, Penetrance, and Risk Effect of Myocilin (MYOC) Gene p.Gln368Ter Variant in the UK Biobank.
| Phenotype | No. (%) | |||
|---|---|---|---|---|
| rs74315329 (AG) | rs74315329 (GG) | Odds Ratio (95% CI)a | P Valueb | |
| Individuals included | ||||
| All | 1046 (0.3) | 410 291 (99.7) | NA | NA |
| With intraocular pressure measurements | 214 (0.3) | 84 267 (99.7) | NA | NA |
| Disease | ||||
| Glaucoma (n = 1046) | 79 (7.6) | 7918 (1.9) | 4.40 (3.38-5.7) | <.001 |
| Primary open-angle glaucoma (n = 1046) | 16 (1.6) | 1095 (0.3) | 6.76 (4.05-11.3) | <.001 |
| Ocular hypertension (n = 214) | 52 (24.3) | 6775 (8.0) | 3.56 (2.53-4.9) | <.001 |
| Ocular hypertension or glaucoma (n = 214) | 66 (30.8) | 8015 (9.5) | 4.18 (3.05-5.7) | <.001 |
| Intraocular pressure | ||||
| Corneal-compensated intraocular pressure, mean (SD), mm Hg | 18.10 (4.5) | 16.06 (3.5) | NA | <.001 |
| Goldmann-correlated intraocular pressure, mean (SD), mm Hg | 17.74 (4.3) | 15.92 (3.6) | NA | <.001 |
Abbreviation: NA, not applicable.
In association analysis, relatives are removed if the genomic relatedness was greater than 0.2; approximately 7% of individuals are removed because of this prior to the statistical test being applied.
P values are the association between p.Gln368Ter dosage and disease status using logistic regression adjusted for sex, age, and the first 6 genetic principal components; a general linear model was used for intraocular pressure levels.
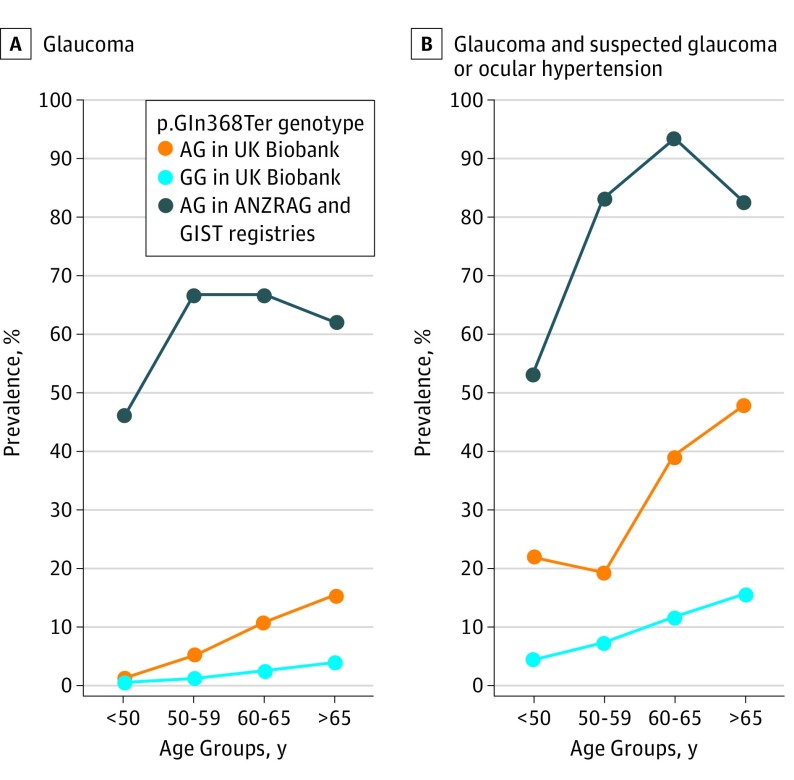
In the UKBB, the age-associated prevalence of glaucoma, POAG, OHT, and OHT or glaucoma is summarized in Table 3 and the Figure. In p.Gln368Ter carriers older than 65 years, the prevalence of glaucoma was 15.5% (n = 30); in POAG, it was 4.1% (n = 7); in OHT, 40.0% (n = 20); and in OHT and glaucoma combined, 48.0% (n = 24).
Table 3. Age-Related Prevalence of Glaucoma and Ocular Hypertension in the UK Biobanka.
| Age Group, y | No. (%) | P Value | |
|---|---|---|---|
| rs74315329 (AG) | rs74315329 (GG) | ||
| Glaucoma | |||
| <50 | 3 (1.3)b | 469 (0.5) | .12 |
| 50-59 | 19 (5.2) | 1817 (1.3) | <.001 |
| 60-65 | 27 (10.8) | 2555 (2.5) | <.001 |
| >65 | 30 (15.5) | 3077 (4.0) | <.001 |
| Primary open-angle glaucoma | |||
| <50 | 1 (0.4) | 36 (0.0) | .09 |
| 50-59 | 3 (0.9) | 216 (0.2) | .02 |
| 60-65 | 5 (2.2) | 326 (0.3) | <.001 |
| >65 | 7 (4.1) | 517 (0.7) | <.001 |
| Ocular hypertension | |||
| <50 | 9 (22.0) | 715 (4.0) | <.001 |
| 50-59 | 12 (15.6) | 1730 (6.4) | <.001 |
| 60-65 | 11 (23.9) | 2172 (9.8) | <.001 |
| >65 | 20 (40.0) | 2158 (12.7) | <.001 |
| Ocular hypertension or glaucoma | |||
| <50 | 9 (22.0) | 791 (4.4) | <.001 |
| 50-59 | 15 (19.5) | 1985 (7.3) | <.001 |
| 60-65 | 18 (39.1) | 2583 (11.7) | <.001 |
| >65 | 24 (48.0) | 2656 (15.6) | <.001 |
Approximately 5% of the individuals are removed because of relatedness prior to determination of significance; P values are from χ2 test or Fisher exact test comparing disease prevalence based on p.Gln368Ter genotypes in different age groups.
Number of cases (prevalence).
Figure. Age-Associated Prevalence of Glaucoma, Suspected Glaucoma, and Ocular Hypertension in p.Gln368Ter Risk Allele Carriers.
ANZRAG indicates the Australian and New Zealand Registry of Advanced Glaucoma; GIST, the Glaucoma Inheritance Study in Tasmania; UKBB, the UK Biobank.
We gathered information on age at glaucoma onset for 4915 individuals; the mean (SD) age at diagnosis was 53.5 (10.8) years. The cumulative risk of glaucoma at 50 years was 2.3% (95% CI, 1.3%-3.2%). At 60 years, it was 8.1% (95% CI, 6.0%-10.3%), and at 65 years, it was 15.6% (95% CI, 11.7%-19.3%; eTable 1 in the Supplement).
In the sequencing-based study, 174 participants in the ANZRAG and GIST registries were found to be Sanger-validated MYOC p.Gln368Ter carriers, including 164 with a known age at diagnosis. Among these 164 individuals (77 male and 87 female), 92 (56.1%) had glaucoma,24 22 (13.4%) were considered to be glaucoma suspects, and 50 (30.5%) were unaffected. The mean (SD) age at glaucoma diagnosis was 53.2 (12.9) years, and their mean (SD) IOP at diagnosis was 32.5 (9.5) mm Hg. The penetrance of p.Gln368Ter with respect to glaucoma was 56.1% (n = 92); for a wider definition, including both patients with glaucoma and patients who were glaucoma suspects, the penetrance was 69.5% (n = 114; Table 4).
Table 4. Penetrance of p.Gln368Ter in the Australian and New Zealand Registry of Advanced Glaucoma and the Glaucoma Inheritance Study in Tasmania Registry-Based Studies.
| Patient Status | rs74315329 (AG), No. (%) | Age at Last Examination, Mean (SD), y | P Value | Max Recorded IOP, Mean (SD), mm Hg | P Value |
|---|---|---|---|---|---|
| Patients with glaucoma | |||||
| No | 72 (43.9) | 48.11 (16.7) | <.001 | 19.32 (6.07) | <.001 |
| Yes | 92 (56.1) | 69.13 (12.7) | 31.26 (9.55) | ||
| Patients with glaucoma and patients considered glaucoma suspects | |||||
| No | 50 (30.5) | 42.50 (16.3) | <.001 | 16.48 (2.87) | <.001 |
| Yes | 114 (69.5) | 67.14 (13.1) | 30.01 (9.43) | ||
The Figure and eTable 2 in the Supplement present the age-associated prevalence of glaucoma and suspected glaucoma among p.Gln368Ter carriers in the ANZRAG and GIST registry-based studies. The cumulative risk of glaucoma in MYOC p.Gln368Ter carriers at 50 years was 55.9% (95% CI, 44.1%-67.7%); at age 60 years, it was 80.5% (95% CI, 71.9%-89.1%), and at 65 years, it was 87.1% (95% CI, 79.9%-94.2%). The cumulative risk of patients being considered to have glaucoma or be glaucoma suspects was 77.6% (95% CI, 66.9%-88.3%) at age 50 years, 94.4% (95% CI, 89.6%-99.2%) at age 60 years, and 96.0% (95% CI, 92.1%-99.8%) at age 65 years (eTable 3 in the Supplement). Based on imputed p.Gln368Ter status, the odds of p.Gln368Ter among individuals with advanced glaucoma were 12.2 (95% CI, 6.3-25.0), and for those with nonadvanced glaucoma, they were 3.97 (95% CI, 1.6-9.8).
Discussion
To our knowledge, this is the largest study to examine the penetrance and association of the MYOC p.Gln368Ter variant with glaucoma and OHT in a cohort of individuals of European ancestry and compare it with data from 2 large registry-based studies. We found that p.Gln368Ter was robustly associated with glaucoma, POAG, and OHT and that its penetrance increased with age.
The p.Gln368Ter variant was well imputed (with an imputation quality score of 93.8%), and the MAF was 0.13% in the UKBB data. In this study, the MAF was similar to those reported from exome sequencing databases (ie, 192 of 126 640 [0.15%]) in non-Finnish European individuals in the Genome Aggregation Database (http://gnomad.broadinstitute.org/) but much higher than that recently reported in the TwinsUK cohort (MAF, 8 of 12 184 [0.07%]).20 The lower MAF seen in the TwinsUK cohort suggests that the set of volunteers ascertained was biased toward healthy individuals.
In the UKBB study, individuals with POAG or glaucoma were identified by ICD-9 or ICD-10 diagnosis or self-reported questionnaires; the prevalence of POAG was estimated at 0.27%, and the prevalence of glaucoma was estimated at 1.94%. A previous study estimated the prevalence in Europe of POAG at 2.51% and glaucoma at 2.93%.1 Previous studies also showed that 50% of glaucoma cases are undiagnosed.33,34 Because of the lack of a comprehensive eye examination in the UKBB, the proportion of individuals with glaucoma or POAG found here was lower than expected. However, IOP is a key risk factor for POAG, and the main mechanism of p.Gln368Ter is via elevation of IOP.10,31,35 The penetrance and risk effect of p.Gln368Ter in OHT serves as a proxy for POAG.20 The prevalence of OHT (defined as IOPcc >21 mm Hg) reported in this study was 8.08%, which is similar to an earlier study.36
Family studies have shown that p.Gln368Ter had a high penetrance in POAG and OHT.12,15,18 For instance, Craig et al15 reported the age-related penetrance of p.Gln368Ter for OHT or POAG as 28 of 39 individuals (72%) at age 40 years and 14 of 17 individuals (82%) at age 65 years. Another study by Allingham et al18 observed that 9 of 9 people (100%) with the p.Gln368Ter variant had elevated IOP, and 7 of 9 individuals (78%) had POAG by age 70 years.18 The current analysis of ANZRAG and GIST registry data indicated that the cumulative risk at 65 years old was 87.1% for glaucoma and 96.0% for a combination of glaucoma and suspected glaucoma, which was consistent with findings from previous family-based studies.12,15,18
From their population-based study, Nag et al20 reported that the penetrance of p.Gln368Ter in relation to OHT was 1 of 8 people (12.5%) in the TwinsUK study and 6 of 31 (19.4%) in the Rotterdam Study. The penetrance of p.Gln368Ter for POAG was 1 of 8 (12.5%) in the TwinsUK study and 3 of 31 (9.7%) in the Rotterdam Study. For OHT, our study showed that the penetrance of p.Gln368Ter (n = 52 of 214 [24.3%]) was lower than in the previous family studies but higher than in the population-based study.
With approximately 100 000 participants with IOP measurements, our study provided a more robust estimation of p.Gln368Ter penetrance in OHT in population-based studies. However, the number of individuals with POAG was much lower than expected, given the typical prevalence of POAG in Europe (2.51%).1 Hence, the true penetrance of p.Gln368Ter in POAG is likely to be larger than estimated in the UKBB samples in this analysis. This again may reflect the bias of a volunteer-based study design.37
We also proposed a method to calculate the penetrance of p.Gln368Ter based on its odds ratio, MAF, and disease prevalence (eMethods in the Supplement). According to the proposed method, if the prevalence of glaucoma was 2.93% and that of POAG is 2.51% in populations older than 40 years, then using the odds ratios and MAF of p.Gln368Ter from the UKBB, the estimated overall penetrance of p.Gln368Ter for glaucoma was derived to be 10.7% and that of POAG was derived to be 15.1%.
The penetrance of p.Gln368Ter with respect to OHT and glaucoma combined is a more comprehensive indicator. This study showed that in p.Gln368Ter carriers, the cumulative risk of OHT or glaucoma was 38.69% at age 65 years in the population-based study and 95.96% in glaucoma-based registries; p.Gln368Ter genotyping has great potential for early identification of individuals at risk for developing these eye diseases19,31,38
The penetrance of p.Gln368Ter with respect to glaucoma in the UKBB data was lower than in the family studies. There are several potential reasons for this. On the one hand, estimates from family studies may have been inflated by ascertainment bias. On the other hand, the penetrance in general population-based studies may be underestimated because of undersampling of affected individuals. Furthermore, it remains possible that aggregated genetic and environmental risk factors in family studies may have led to increased penetrance in p.Gln368Ter carriers. Recruitment based on families with multiple affected individuals is likely to lead to an increase in the number of common variants of individually small effect (polygenes) in a family, potentially increasing the penetrance of variants such as p.Gln368Ter. This supports the use of cascade testing for close relatives who share the same genetic background.
Limitations
This study has some limitations. The genotypes of p.Gln368Ter in the UKBB study are based on best-guess imputed genotypes. Reassuringly, our previous study presented evidence that the p.Gln368Ter variant could be imputed with high accuracy.17 Thus, the imputed genotype is unlikely to make a meaningful difference in our results.
Another limitation of the UKBB study is that some individuals with glaucoma were defined by self-reported questionnaires, which could lead to recall bias. However, our study is one of the largest studies to investigate the penetrance and risk effect of p.Gln368Ter in OHT, which could serve as a proxy for glaucoma or POAG.20 Furthermore, individuals with glaucoma who also have other eye disorders may be less likely to participate the UKBB project, compared with healthy individuals,37 which could lead to a lower estimated penetrance of p.Gln368Ter in glaucoma. Moreover, in the ANZRAG and GIST registries, the controls were genotyped on different platforms. As a sensitivity analysis, we substituted the Australian control participants for controls from the UKBB data; our results were essentially unchanged.
Finally, some individuals with high IOP present in the UKBB cohort may be taking medications or have undergone ophthalmic surgery to reduce their IOP levels. In our sensitivity analysis to adjust for the change in IOP postmedication, when we added 25% to the measured IOP levels for individuals taking IOP-lowering medications,39,40 the resultant odds ratios and penetrance with respect to OHT increased only slightly.
Conclusions
Our study suggests that the MYOC p.Gln368Ter variant has a high penetrance in OHT and glaucoma. Genetic testing for p.Gln368Ter could help identify individuals who at greater risk of developing glaucoma and direct them to early screening and appropriate management.
eFigure 1. Ancestral background in the UK Biobank study
eFigure 2. Flowchart of the UK Biobank study overview
eTable 1. Cumulative Risk (95% CI) of p.Gln368Ter in UK Biobank
eTable 2. Age-related prevalence of glaucoma and glaucoma suspects in ANZRAG and GIST registry-based studies in p.Gln368Ter
eTable 3. Cumulative risk (95% CI) of p.Gln368Ter in ANZRAG and GIST registry-based studies
eMethods. Penetrance of p.Gln368Ter based on OR, prevalence and MAF
References
- 1.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-2090. doi: 10.1016/j.ophtha.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 2.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262-267. doi: 10.1136/bjo.2005.081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390(10108):2183-2193. doi: 10.1016/S0140-6736(17)31469-1 [DOI] [PubMed] [Google Scholar]
- 4.Hewitt AW, Craig JE, Mackey DA. Complex genetics of complex traits: the case of primary open-angle glaucoma. Clin Exp Ophthalmol. 2006;34(5):472-484. doi: 10.1111/j.1442-9071.2006.01268.x [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Allingham RR. Major review: Molecular genetics of primary open-angle glaucoma. Exp Eye Res. 2017;160:62-84. doi: 10.1016/j.exer.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiggs JL, Pasquale LR. Genetics of glaucoma. Hum Mol Genet. 2017;26(R1):R21-R27. doi: 10.1093/hmg/ddx184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolfs RC, Klaver CC, Ramrattan RS, van Duijn CM, Hofman A, de Jong PT. Genetic risk of primary open-angle glaucoma: population-based familial aggregation study. Arch Ophthalmol. 1998;116(12):1640-1645. doi: 10.1001/archopht.116.12.1640 [DOI] [PubMed] [Google Scholar]
- 8.Wang K, Gaitsch H, Poon H, Cox NJ, Rzhetsky A. Classification of common human diseases derived from shared genetic and environmental determinants. Nat Genet. 2017;49(9):1319-1325. doi: 10.1038/ng.3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheffield VC, Stone EM, Alward WL, et al. Genetic linkage of familial open angle glaucoma to chromosome 1q21-q31. Nat Genet. 1993;4(1):47-50. doi: 10.1038/ng0593-47 [DOI] [PubMed] [Google Scholar]
- 10.Stone EM, Fingert JH, Alward WL, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275(5300):668-670. doi: 10.1126/science.275.5300.668 [DOI] [PubMed] [Google Scholar]
- 11.Alward WL, Fingert JH, Coote MA, et al. Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A). N Engl J Med. 1998;338(15):1022-1027. doi: 10.1056/NEJM199804093381503 [DOI] [PubMed] [Google Scholar]
- 12.Fingert JH, Héon E, Liebmann JM, et al. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999;8(5):899-905. doi: 10.1093/hmg/8.5.899 [DOI] [PubMed] [Google Scholar]
- 13.Gobeil S, Rodrigue MA, Moisan S, et al. Intracellular sequestration of hetero-oligomers formed by wild-type and glaucoma-causing myocilin mutants. Invest Ophthalmol Vis Sci. 2004;45(10):3560-3567. doi: 10.1167/iovs.04-0300 [DOI] [PubMed] [Google Scholar]
- 14.Jain A, Zode G, Kasetti RB, et al. CRISPR-Cas9-based treatment of myocilin-associated glaucoma. Proc Natl Acad Sci U S A. 2017;114(42):11199-11204. doi: 10.1073/pnas.1706193114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craig JE, Baird PN, Healey DL, et al. Evidence for genetic heterogeneity within eight glaucoma families, with the GLC1A Gln368STOP mutation being an important phenotypic modifier. Ophthalmology. 2001;108(9):1607-1620. doi: 10.1016/S0161-6420(01)00654-6 [DOI] [PubMed] [Google Scholar]
- 16.Fingert JH, Stone EM, Sheffield VC, Alward WL. Myocilin glaucoma. Surv Ophthalmol. 2002;47(6):547-561. doi: 10.1016/S0039-6257(02)00353-3 [DOI] [PubMed] [Google Scholar]
- 17.Gharahkhani P, Burdon KP, Hewitt AW, et al. Accurate imputation-based screening of gln368ter myocilin variant in primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2015;56(9):5087-5093. doi: 10.1167/iovs.15-17305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allingham RR, Wiggs JL, De La Paz MA, et al. Gln368STOP myocilin mutation in families with late-onset primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 1998;39(12):2288-2295. [PubMed] [Google Scholar]
- 19.Hewitt AW, Mackey DA, Craig JE. Myocilin allele-specific glaucoma phenotype database. Hum Mutat. 2008;29(2):207-211. doi: 10.1002/humu.20634 [DOI] [PubMed] [Google Scholar]
- 20.Nag A, Lu H, Arno M, et al. Evaluation of the myocilin mutation Gln368Stop demonstrates reduced penetrance for glaucoma in European populations. Ophthalmology. 2017;124(4):547-553. doi: 10.1016/j.ophtha.2016.11.018 [DOI] [PubMed] [Google Scholar]
- 21.Bycroft C, Freeman C, Petkova D, et al. Genome-wide genetic data on ~500,000 UK Biobank participants. https://www.biorxiv.org/content/early/2017/07/20/166298. Published July 20, 2017. Accessed August 15, 2018.
- 22.Chan MP, Grossi CM, Khawaja AP, et al. ; UK Biobank Eye and Vision Consortium . Associations with intraocular pressure in a large cohort: results from the UK Biobank. Ophthalmology. 2016;123(4):771-782. doi: 10.1016/j.ophtha.2015.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biobank UK. Measurement of intraocular pressure: version 1.0. http://biobank.ctsu.ox.ac.uk/crystal/docs/Intraocularpressure.pdf Published May 3, 2011. Accessed August 15, 2018.
- 24.Souzeau E, Goldberg I, Healey PR, et al. Australian and New Zealand Registry of Advanced Glaucoma: methodology and recruitment. Clin Exp Ophthalmol. 2012;40(6):569-575. doi: 10.1111/j.1442-9071.2011.02742.x [DOI] [PubMed] [Google Scholar]
- 25.Coote MA, McCartney PJ, Wilkinson RM, Mackey DA; Glaucoma Inheritance Study of Tasmania . The ‘GIST’ score: ranking glaucoma for genetic studies. Ophthalmic Genet. 1996;17(4):199-208. doi: 10.3109/13816819609057894 [DOI] [PubMed] [Google Scholar]
- 26.Souzeau E, Burdon KP, Dubowsky A, et al. Higher prevalence of myocilin mutations in advanced glaucoma in comparison with less advanced disease in an Australasian disease registry. Ophthalmology. 2013;120(6):1135-1143. doi: 10.1016/j.ophtha.2012.11.029 [DOI] [PubMed] [Google Scholar]
- 27.Burdon KP, Macgregor S, Hewitt AW, et al. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat Genet. 2011;43(6):574-578. doi: 10.1038/ng.824 [DOI] [PubMed] [Google Scholar]
- 28.Nyholt DR, Low S-K, Anderson CA, et al. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat Genet. 2012;44(12):1355-1359. doi: 10.1038/ng.2445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gharahkhani P, Burdon KP, Fogarty R, et al. ; Wellcome Trust Case Control Consortium 2, NEIGHBORHOOD consortium . Common variants near ABCA1, AFAP1 and GMDS confer risk of primary open-angle glaucoma. Nat Genet. 2014;46(10):1120-1125. doi: 10.1038/ng.3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gharahkhani P, Burdon KP, Cooke Bailey JN, et al. ; NEIGHBORHOOD consortium . Analysis combining correlated glaucoma traits identifies five new risk loci for open-angle glaucoma. Sci Rep. 2018;8(1):3124. doi: 10.1038/s41598-018-20435-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Souzeau E, Tram KH, Witney M, et al. Myocilin predictive genetic testing for primary open-angle glaucoma leads to early identification of at-risk individuals. Ophthalmology. 2017;124(3):303-309. doi: 10.1016/j.ophtha.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 32.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559-575. doi: 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia: the Blue Mountains Eye Study. Ophthalmology. 1996;103(10):1661-1669. doi: 10.1016/S0161-6420(96)30449-1 [DOI] [PubMed] [Google Scholar]
- 34.Wensor MD, McCarty CA, Stanislavsky YL, Livingston PM, Taylor HR. The prevalence of glaucoma in the Melbourne Visual Impairment Project. Ophthalmology. 1998;105(4):733-739. doi: 10.1016/S0161-6420(98)94031-3 [DOI] [PubMed] [Google Scholar]
- 35.Shepard AR, Jacobson N, Millar JC, et al. Glaucoma-causing myocilin mutants require the peroxisomal targeting signal-1 receptor (PTS1R) to elevate intraocular pressure. Hum Mol Genet. 2007;16(6):609-617. doi: 10.1093/hmg/ddm001 [DOI] [PubMed] [Google Scholar]
- 36.Chan MPY, Broadway DC, Khawaja AP, et al. Glaucoma and intraocular pressure in EPIC-Norfolk Eye Study: cross sectional study. BMJ. 2017;358:j3889. doi: 10.1136/bmj.j3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026-1034. doi: 10.1093/aje/kwx246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Souzeau E, Glading J, Ridge B, et al. Predictive genetic testing in minors for myocilin juvenile onset open angle glaucoma. Clin Genet. 2015;88(6):584-588. doi: 10.1111/cge.12558 [DOI] [PubMed] [Google Scholar]
- 39.Hysi PG, Cheng C-Y, Springelkamp H, et al. ; BMES GWAS Group; NEIGHBORHOOD Consortium; Wellcome Trust Case Control Consortium 2 . Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. Nat Genet. 2014;46(10):1126-1130. doi: 10.1038/ng.3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Valk R, Webers CAB, Schouten JS, Zeegers MP, Hendrikse F, Prins MH. Intraocular pressure-lowering effects of all commonly used glaucoma drugs: a meta-analysis of randomized clinical trials. Ophthalmology. 2005;112(7):1177-1185. doi: 10.1016/j.ophtha.2005.01.042 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Ancestral background in the UK Biobank study
eFigure 2. Flowchart of the UK Biobank study overview
eTable 1. Cumulative Risk (95% CI) of p.Gln368Ter in UK Biobank
eTable 2. Age-related prevalence of glaucoma and glaucoma suspects in ANZRAG and GIST registry-based studies in p.Gln368Ter
eTable 3. Cumulative risk (95% CI) of p.Gln368Ter in ANZRAG and GIST registry-based studies
eMethods. Penetrance of p.Gln368Ter based on OR, prevalence and MAF