Abstract
This study characterizes the extent and cost of the use of corticosteroid-eluting sinus stents between 2012 and 2017.
More than 250 000 individuals undergo ambulatory endoscopic sinus surgery for chronic rhinosinusitis each year. Although estimates vary, up to one-fifth of these patients may undergo revision surgery for symptoms secondary to formation of adhesions, recurrent polyposis, and persistent inflammation.1 In an effort to limit the use of revision surgery, otolaryngologists have used postoperative adjunct treatments such as packing materials and oral corticosteroid therapy.
In August 2011, the US Food and Drug Administration (FDA) approved the first corticosteroid-eluting sinus stent (Intersect ENT) for use in adults undergoing surgery of the ethmoid sinus.2 This bioabsorbable device releases mometasone furoate for a period of 30 days and is intended to stabilize the middle turbinate and reduce mucosal edema and contact, thereby preventing postoperative obstruction by adhesions. After initial approval, the manufacturer subsequently received FDA authorization to market device models designed for placement in the frontal sinuses (2016) and maxillary sinuses (2017).2 To our knowledge, no other manufacturer has received FDA approval for a drug-eluting sinus stent to date.2
The cost-effectiveness of this technology has been debated.1,3,4 However, published data on real-world device use and expenditures are lacking. We therefore sought to characterize the extent and cost of the use of corticosteroid-eluting sinus stents.
Methods
As is the case for most surgical devices, placement of a corticosteroid-eluting sinus stent is not associated with unique procedure codes and cannot be reliably evaluated using administrative claims data. We therefore reviewed publicly available annual 10-K filings submitted by the manufacturer to the US Securities and Exchange Commission to assess device use and expenditures.5 For each year between 2012 and 2017, we extracted (as available) the number of units sold, the number of purchasing accounts, and revenue. We used descriptive statistics to characterize trends over time. Institutional review board review was not necessary, as this research does not contain animal or clinical data, but only publicly available financial information provided by a corporation.
Results
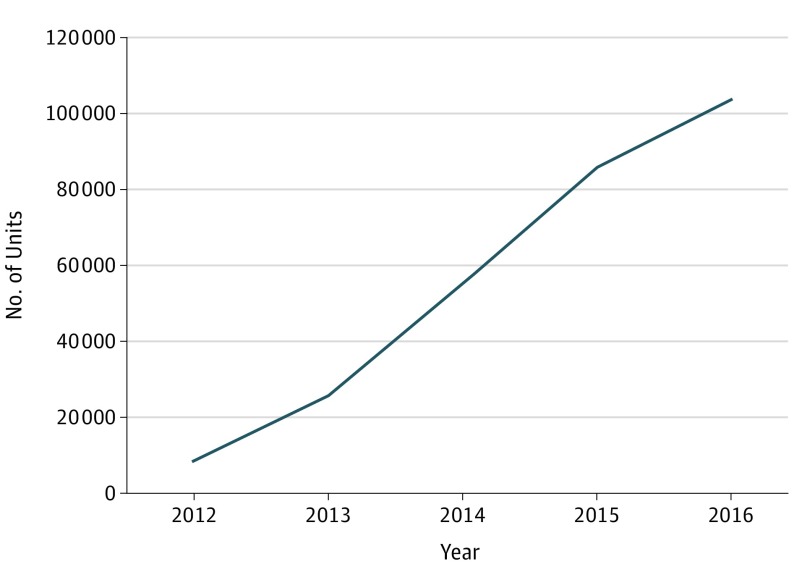
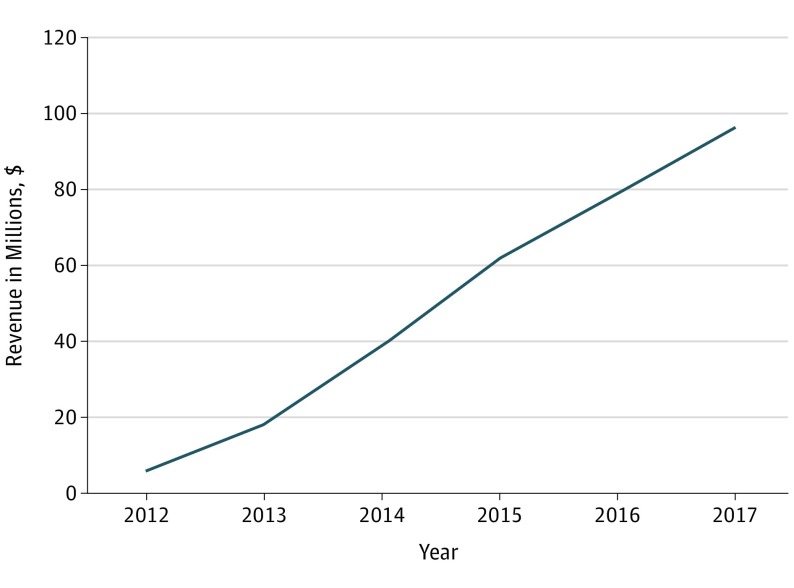
Between 2012 and 2016, annual sales of corticosteroid-eluting sinus stents increased 12.3-fold from 8400 units to 103 400 units (Figure 1); a total of 277 900 units were sold during this period. Between 2014 and 2017, the number of purchasing accounts increased 2.3-fold from 1200 to 2700 accounts. Between 2012 and 2017, annual revenue increased 16.3-fold from $5.9 million to $96.3 million (Figure 2); revenue totaled $299 million during this period.
Figure 1. Corticosteroid-Eluting Sinus Stent Annual Sales, 2012-2016.
Unit sales data for 2017 not publicly available.
Figure 2. Intersect ENT Annual Revenue, 2012-2017.
Discussion
Our findings demonstrate rapid adoption of corticosteroid-eluting sinus stents into clinical practice after initial FDA approval in late 2011, at a cost of nearly $300 million. The manufacturer estimates that more than 200 000 patients received corticosteroid-eluting sinus stents during the study period5; the number of endoscopic sinus surgery procedures performed during this time remained relatively stable.6 This phenomenon reflects successful sales and marketing initiatives to engage new users,5 as evidenced by the increased number of purchasing accounts. As noted by the manufacturer,5 sales growth was also driven by FDA approval expanding device indications for use to include patients undergoing frontal sinus surgery, as well as yearly increases in average selling price (>$750 absolute price per unit).
When deciding whether to adopt these devices in their clinical practices, otolaryngologists should be mindful of notable limitations in the available clinical evidence. Financial modeling studies performed by consultants for the manufacturer suggest that the device may be cost-effective.3,4 However, both the ethmoid and frontal sinus indications were approved based on sham-controlled clinical studies with 30-day endoscopic end point follow-up and without patient-reported outcome measures.2 These investigations provide inadequate insight into device performance vs traditional adjuncts, quality of life, or long-term need for revision surgery. Evidence supporting maxillary sinus placement of corticosteroid-eluting sinus stents is similarly limited; approval was based on a single-arm feasibility study enrolling 15 patients.2 In the absence of more compelling cost-effectiveness data, otolaryngologists should carefully consider device use, particularly as the ongoing implementation of value-based payment models increases provider accountability for the cost of care (eg, through bundled payments for sinus surgery).5
This study has some limitations. We were unable to ascertain trends in device implantation at the provider level or by anatomic subsite based on information provided within 10-K filings. The ongoing implementation of unique device identifiers may facilitate such investigation in the future.
References
- 1.Gray ST, Sedaghat AR. Frontal sinus drug-eluting implants—effective, but for which patients and at what cost? JAMA Otolaryngol Head Neck Surg. 2018;144(1):35-36. doi: 10.1001/jamaoto.2017.1891 [DOI] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration Premarket approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm. Published July 16, 2018. Accessed July 16, 2018.
- 3.Rudmik L, Smith TL. Economic evaluation of a steroid-eluting sinus implant following endoscopic sinus surgery for chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2014;151(2):359-366. doi: 10.1177/0194599814533779 [DOI] [PubMed] [Google Scholar]
- 4.Rizzo JA, Rudmik L, Mallow PJ, Palli SR. Budget impact analysis of bioabsorbable drug-eluting sinus implants following endoscopic sinus surgery. J Med Econ. 2016;19(9):829-835. doi: 10.1080/13696998.2016.1176577 [DOI] [PubMed] [Google Scholar]
- 5.Intersect ENT. SEC filings. http://ir.intersectent.com/financial-information/sec-filings. Published 2018. Accessed July 15, 2018.
- 6.Svider PF, Darlin S, Bobian M, et al. Evolving trends in sinus surgery: what is the impact of balloon sinus dilation? Laryngoscope. 2018;128(6):1299-1303. doi: 10.1002/lary.26941 [DOI] [PubMed] [Google Scholar]