Key Points
Question
What is the association between hospital volume and outcomes for laryngectomy surgery?
Findings
Among 45 156 patients at 5516 hospitals in this cross-sectional study, a minimum hospital volume threshold of more than 6 cases per year was associated with reduced odds of postoperative complications, with a greater reduction in the odds of complications with increasing volume. High-volume hospital care (>28 cases per year) was also associated with lower odds of in-hospital mortality, the mean incremental length of hospitalization, and costs.
Meaning
Laryngectomy outcomes appear to be associated with hospital volume, with reduced morbidity associated with a minimum hospital volume threshold and with reduced mortality, morbidity, length of hospitalization, and costs associated with higher hospital volume.
This cross-sectional study of patients with a diagnosis of larynx cancer characterizes the hospital volume-outcome association specifically for laryngectomy surgery and identifies a minimum hospital volume threshold associated with improved outcomes.
Abstract
Importance
A volume-outcome association exists for larynx cancer surgery, but to date it has not been investigated for specific surgical procedures.
Objectives
To characterize the volume-outcome association specifically for laryngectomy surgery and to identify a minimum hospital volume threshold associated with improved outcomes.
Design, Setting, and Participants
In this cross-sectional study, the Nationwide Inpatient Sample was used to identify 45 156 patients who underwent laryngectomy procedures for a malignant laryngeal or hypopharyngeal neoplasm between January 2001 and December 2011. The analysis was performed in 2018. Hospital laryngectomy volume was modeled as a categorical variable.
Main Outcomes and Measures
Associations between hospital volume and in-hospital mortality, complications, length of hospitalization, and costs were examined using multivariate logistic regression analysis.
Results
Among 45 156 patients (mean age, 62.6 years; age range, 20-96 years; 80.2% male) at 5516 hospitals, higher-volume hospitals were more likely to be teaching hospitals in urban locations; were more likely to treat patients who had hypopharyngeal cancer, were of white race/ethnicity, were admitted electively, had no comorbidity, and had private insurance; and were more likely to perform flap reconstruction or concurrent neck dissection. After controlling for all other variables, hospitals treating more than 6 cases per year were associated with lower odds of surgical and medical complications, with a greater reduction in the odds of complications with increasing hospital volume. High-volume hospitals in the top-volume quintile (>28 cases per year) were associated with decreased odds of in-hospital mortality (odds ratio, 0.45; 95% CI, 0.23-0.88), postoperative surgical complications (odds ratio, 0.63; 95% CI, 0.50-0.79), and acute medical complications (odds ratio, 0.63; 95% CI, 0.48-0.81). A statistically meaningful negative association was observed between very high-volume hospital care and the mean incremental length of hospitalization (−3.7 days; 95% CI, −4.9 to −2.4 days) and hospital-related costs (−$4777; 95% CI, −$9463 to −$900).
Conclusions and Relevance
Laryngectomy outcomes appear to be associated with hospital volume, with reduced morbidity associated with a minimum hospital volume threshold and with reduced mortality, morbidity, length of hospitalization, and costs associated with higher hospital volume. These data support the concept of centralization of complex care at centers able to meet minimum volume thresholds to improve patient outcomes.
Introduction
A favorable association exists between hospital volume and patient outcomes for complex surgical procedures. Hospitals performing high volumes of bariatric surgery, cardiovascular surgery, lung cancer surgery, and gastrointestinal cancer surgery have been shown to have better survival outcomes with fewer surgical complications, with similar observations made for high-volume surgeon care.1,2,3,4,5,6,7 Building on this research around the volume-outcome association, the Leapfrog Group,8 a coalition of large Fortune 500 employers and other health care purchasers, adopted a minimum surgical volume standard for selected high-risk surgical procedures. In 2015, in response to a US News & World Report article highlighting the volume-outcome association for surgery,9 leaders at the Johns Hopkins Hospital and Health System, Dartmouth-Hitchcock Medical Center, and the University of Michigan Health System publicly pledged to limit certain complex elective procedures to surgeons and hospitals meeting volume criteria.10 The “volume pledge” requires surgeons and hospitals to meet modest minimum volume standards for 10 procedures that have the strongest link between hospital volume and patient mortality.11 This approach will effectively result in regionalization of high-risk surgical care to high-volume centers within a health care system, and the Johns Hopkins Health System makes these data publicly available.12
A volume-outcome association has been demonstrated for head and neck cancer surgical care, with improved outcomes reported for high-volume hospitals.13,14,15,16,17,18,19,20,21,22,23,24,25,26,27 These reports often include a variety of head and neck primary sites and are limited by low perioperative mortality rates and the inclusion of a range of both low-risk and high-risk surgical procedures, and minimum volume standards for specific head and neck cancer procedures have not been established to date. Procedures that are most sensitive to a volume-outcome association are high-risk, low-volume procedures with outcomes that show wide variation across hospitals and for which hospital volume can serve as a proxy measure for outcomes.28 This association may be most pronounced for laryngectomy surgery, which has decreased in recent years primarily because of a decreased incidence of larynx cancer concomitant with an increased use of primary nonoperative treatment, with a growing proportion of laryngectomy procedures performed after prior radiotherapy associated with an increase in postoperative complications.22,29,30,31,32,33,34 Flap reconstruction is increasingly used in this setting to improve postoperative outcomes, which further increases the complexity of surgery. Because of this trend, centralization of laryngectomy procedures appears to be occurring at high-volume and academic centers,22,29,31 yet laryngectomy was removed as a key indicator procedure representative of resident training by the American Board of Otolaryngology–Head and Neck Surgery in 2011 because of decreasing numbers even at such institutions.35
We hypothesized that laryngectomy—as a high-risk, increasingly low-volume surgery—would have a strong link between hospital volume and patient morbidity and mortality. We sought to characterize the volume-outcome association for laryngectomy surgery by examining associations between volume and in-hospital mortality, complications, length of hospitalization, and costs and aimed to identify a possible minimum hospital volume threshold for high-volume care.
Methods
A cross-sectional study of patients with a diagnosis of larynx cancer was performed using discharge data from the Nationwide Inpatient Sample (NIS),36 the largest all-payer inpatient care database in the United States, containing data from approximately 8 million hospital stays each year from a stratified sample of 20% of nonfederal US hospitals from participating states. The NIS database provides information regarding the index hospital admission and includes patient demographic data, primary and secondary diagnoses, primary and secondary procedures, hospital characteristics, and inpatient and discharge mortality rates. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes were used to identify adult patients (≥18 years) who specifically underwent laryngectomy for a malignant laryngeal or hypopharyngeal neoplasm between January 2001 and December 2011 (eTable 1 in the Supplement). The analysis was performed in 2018. Additional procedures, such as neck dissection or pedicled or free flap reconstruction, were recorded. Prior radiotherapy was derived from codes for previous exposure to therapeutic or other ionizing radiation (ICD-9-CM code V15.3). This protocol was reviewed and approved by the Johns Hopkins Medical Institutions Institutional Review Board. Informed consent was inapplicable because the data are from a national deidentified database.
The primary clinical end points (dependent variables) were in-hospital mortality, complications, length of hospitalization, and costs. Codes for specific comorbid illnesses were used to create categories for surgical and acute medical complications. Acute medical complications were derived from codes for acute cardiac events, acute pulmonary edema or failure, acute renal failure, acute hepatic failure, acute cerebrovascular events, sepsis, pneumonia, gastrointestinal bleeding, deep vein thrombosis/pulmonary embolism, and urinary tract infection assigned at the time of hospital discharge (eTable 2 in the Supplement). Surgical complications were derived from codes for complications directly resulting from surgical procedures assigned at the time of hospital discharge. Hospital-related charges for each index admission were converted to the organizational cost of providing care using cost-to-charge ratios for individual hospitals. Cost-to-charge ratios were calculated using information from the detailed reports by hospitals to the Centers for Medicare & Medicaid Services, providing an estimate of the all-payer inpatient cost-to-charge ratio by hospital, and were multiplied by each patient’s charge to obtain the cost per admission.37 All costs were adjusted for inflation based on US Bureau of Labor Statistics indexes,38 with the results converted to 2018 US dollars. To obtain national cost estimates, all discharges were reweighted to account for cases in which cost estimates were missing.
Hospital volume was examined as an independent variable. The average annual number of laryngectomy cases performed per year of surgical activity was obtained by calculating the mean of the number of cases performed each year for each individual hospital for the years in which that hospital performed at least 1 laryngectomy. Hospital laryngectomy volume was modeled as a categorical variable. Annual volumes were divided into quintiles, with high volume defined as hospitals above the 80th percentile. Based on the quintile distribution of the annual number of cases per hospital, hospitals were categorized as very low volume (≤3 cases per year), low volume (4-6 cases per year), medium volume (7-15 cases per year), high volume (16-28 cases per year), or very high volume (>28 cases per year). Secondary independent variables included were the following: age, race/ethnicity, sex, nature of admission (elective or urgent/emergent), comorbidity, payer (commercial, private/health maintenance organization, Medicare, Medicaid, self-pay, or other), hospital teaching status, hospital ownership/control (not-for-profit or other), hospital location (rural or urban), geographic region, hospital bed size, prior radiotherapy, and procedure. The American Joint Commission on Cancer tumor stage, tumor grade, histological subtype, and outcome after discharge were not available from the NIS database. Comorbidity was graded using the Romano adaptation of the Charlson Comorbidity Index, excluding ICD-9-CM codes for the index cancer diagnosis from the solid tumor category, as previously described.22 Because cancer staging information is not available in the NIS, ICD-9-CM codes for metastases were excluded because these are not a reliable surrogate for disease stage.
Data were analyzed using statistical software (Stata, version 12; StataCorp LP). Associations between variables were analyzed using cross-tabulations, multivariate logistic regression analysis, and generalized linear regression modeling. Data were weighted, and modified hospital and discharge weights to correct for changes in sampling over time were applied. Variance estimation was performed using procedures for survey data analysis with replacement. Strata with 1 sampling unit were centered at the population mean. Variables with missing data for more than 10% of the population were coded with a dummy variable to represent the missing data in regression analysis. The primary clinical end points were evaluated using multiple logistic regression analysis. Generalized linear regression modeling with a log link was used to analyze length of hospitalization and hospital-related costs because these variables were not normally distributed.
Results
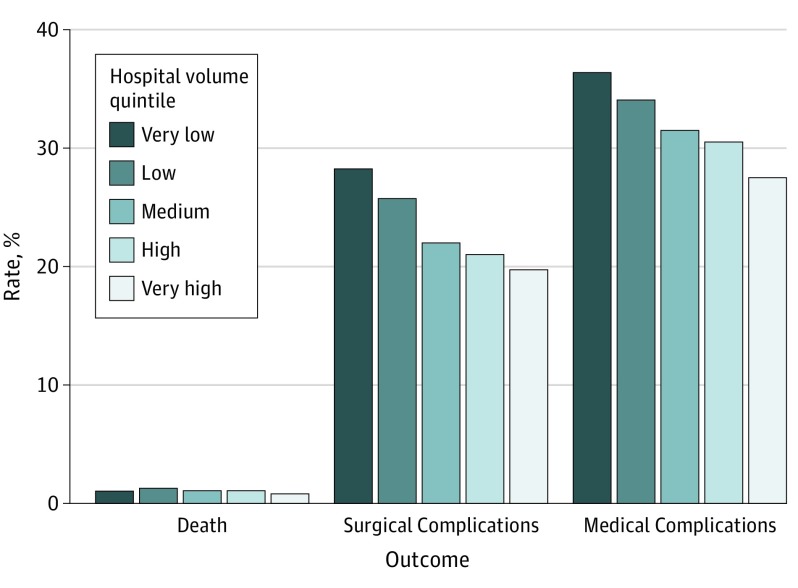
There were 45 156 laryngectomy cases between 2001 and 2011 performed at 5516 hospitals (Table 1). The mean patient age was 62.6 years (age range, 20-96 years). The majority of patients were of white race/ethnicity and male, were admitted electively, had no associated comorbidity, and received care at teaching hospitals located in urban locations. A history of prior radiotherapy was documented in 7.6% of cases. Flap reconstruction was performed in 10.1% of patients, and concurrent neck dissection was performed in 15.1% of patients. Medical complications occurred in 23.6% of patients, and surgical complications occurred in 32.2% of patients. In-hospital mortality occurred in 1.0% of patients.
Table 1. Population Characteristics.
| Variable | % | |||||
|---|---|---|---|---|---|---|
| All Patients (N = 45 156) | Hospital Volume Quintilea | |||||
| Very Low (n = 9132) | Low (n = 9208) | Medium (n = 9074) | High (n = 8721) | Very High (n = 9021) | ||
| Primary site | ||||||
| Larynx | 89.7 | 94.4 | 90.8 | 90.6 | 85.7 | 86.7 |
| Hypopharynx | 10.3 | 5.6 | 9.2 | 9.4 | 14.3 | 13.4 |
| Age group, y | ||||||
| ≤40 | 1.5 | 1.0 | 1.3 | 2.0 | 1.6 | 1.7 |
| 40-64 | 55.0 | 48.9 | 55.4 | 57.3 | 57.1 | 56.5 |
| 65-80 | 38.4 | 43.5 | 39.1 | 35.3 | 36.7 | 37.3 |
| >80 | 5.1 | 6.6 | 4.2 | 5.4 | 4.6 | 4.5 |
| Race/ethnicity | ||||||
| White | 81.8 | 81.4 | 81.3 | 77.0 | 79.1 | 90.1 |
| Black | 10.6 | 10.2 | 11.4 | 11.6 | 13.0 | 6.8 |
| Hispanic | 4.6 | 5.4 | 4.3 | 6.2 | 5.3 | 2.0 |
| Asian/Pacific Islander | 0.9 | 0.7 | 0.8 | 2.0 | 0.5 | 0.6 |
| Other | 2.1 | 2.3 | 2.2 | 3.2 | 2.1 | 0.5 |
| Sex | ||||||
| Male | 80.2 | 81.0 | 80.3 | 80.8 | 79.6 | 79.5 |
| Female | 19.8 | 19.0 | 19.7 | 19.2 | 20.4 | 20.5 |
| Nature of admission | ||||||
| Elective | 79.8 | 74.8 | 81.2 | 76.1 | 81.5 | 85.5 |
| Urgent/emergent | 20.2 | 25.2 | 18.8 | 23.9 | 18.5 | 14.5 |
| Comorbidity score | ||||||
| 0 | 51.3 | 42.1 | 47.3 | 55.6 | 54.8 | 56.7 |
| 1 | 32.2 | 38.9 | 35.1 | 29.8 | 29.9 | 27.3 |
| 2 | 11.4 | 13.6 | 12.5 | 9.5 | 10.5 | 10.9 |
| ≥3 | 5.1 | 5.4 | 5.1 | 5.1 | 4.8 | 5.1 |
| Payer | ||||||
| Private/HMO | 31.8 | 29.9 | 31.9 | 29.2 | 27.9 | 40.1 |
| Medicare | 44.9 | 50.6 | 42.5 | 42.9 | 45.8 | 42.9 |
| Medicaid | 15.4 | 12.5 | 17.6 | 17.9 | 17.2 | 11.7 |
| Other | 7.9 | 7.0 | 8.0 | 10.0 | 9.1 | 5.3 |
| Hospital teaching status | ||||||
| Nonteaching | 19.2 | 60.1 | 28.0 | 5.5 | 1.2 | 0 |
| Teaching | 80.8 | 39.9 | 72.0 | 94.5 | 98.8 | 100 |
| Hospital ownership/control | ||||||
| Not-for-profit | 95.9 | 86.6 | 94.8 | 98.2 | 100 | 100 |
| Other | 4.1 | 13.4 | 5.2 | 1.8 | 0 | 0 |
| Hospital location | ||||||
| Rural | 4.5 | 11.5 | 4.4 | 4.5 | 2.3 | 0 |
| Urban | 95.5 | 88.5 | 95.6 | 95.5 | 97.7 | 100 |
| Hospital bed size | ||||||
| Small | 10.1 | 12.2 | 7.9 | 9.3 | 6.2 | 14.5 |
| Medium | 17.0 | 27.3 | 25.0 | 18.8 | 13.7 | 0 |
| Large | 72.9 | 60.5 | 67.1 | 71.9 | 80.1 | 85.5 |
| Prior radiotherapy | ||||||
| No | 92.4 | 94.9 | 94.8 | 89.6 | 89.4 | 93.3 |
| Yes | 7.6 | 5.1 | 5.2 | 10.4 | 10.6 | 6.7 |
| Procedure | ||||||
| Partial laryngectomy | 17.8 | 16.5 | 14.8 | 19.0 | 16.6 | 22.2 |
| Total laryngectomy | 82.2 | 83.5 | 85.2 | 81.0 | 83.4 | 77.8 |
| Flap reconstruction | ||||||
| No | 89.9 | 96.3 | 93.9 | 88.8 | 83.9 | 86.3 |
| Yes | 10.1 | 3.7 | 6.1 | 11.2 | 16.1 | 13.7 |
| Concurrent neck dissection | ||||||
| No | 84.9 | 91.1 | 89.0 | 84.9 | 80.8 | 78.5 |
| Yes | 15.1 | 8.9 | 11.0 | 15.1 | 19.2 | 21.5 |
| Medical complication | ||||||
| No | 76.4 | 71.7 | 74.1 | 77.8 | 78.8 | 80.0 |
| Yes | 23.6 | 28.3 | 25.9 | 22.2 | 21.1 | 20.0 |
| Surgical complication | ||||||
| No | 67.8 | 63.5 | 65.8 | 68.3 | 69.3 | 72.3 |
| Yes | 32.2 | 36.5 | 34.2 | 31.7 | 30.7 | 27.7 |
| In-hospital mortality | ||||||
| Died in hospital | 1.0 | 1.0 | 1.2 | 1.1 | 1.1 | 0.8 |
Abbreviation: HMO, health maintenance organization.
Hospitals were categorized as very low volume (≤3 cases per year), low volume (4-6 cases per year), medium volume (7-15 cases per year), high volume (16-28 cases per year), or very high volume (>28 cases per year).
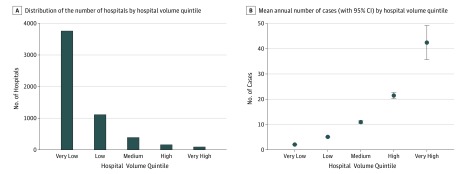
The distribution of the number of hospitals by hospital volume quintile is shown in Figure 1. Very high-volume hospitals comprised 1.7% of all hospitals and performed a mean of 42 cases per year, while very low-volume hospitals comprised 68.2% of all hospitals and performed a mean of 2 cases per year (Figure 1). Higher-volume hospitals were more likely to be not-for-profit teaching hospitals in urban locations; were more likely to treat patients who had hypopharyngeal cancer, were of white race/ethnicity, were admitted electively, had no comorbidity, and had private insurance; and were more likely to perform flap reconstruction or concurrent neck dissection. Rates of mortality and postoperative complications were lowest for hospitals in the very high-volume quintile (Figure 2).
Figure 1. Number of Hospitals and Mean Annual Number of Cases by Hospital Volume Quintile.
A, There were 5516 hospitals represented in the years of this study. Very high-volume hospitals performing more than 28 cases per year (n = 94) accounted for 1.7% of all hospitals, high-volume hospitals performing 16 to 28 cases per year (n = 159) accounted for 2.9% of all hospitals, medium-volume hospitals performing 7 to 15 cases per year (n = 391) accounted for 7.1% of all hospitals, low-volume hospitals performing 4 to 6 cases per year (n = 1111) accounted for 20.1% of all hospitals, and very low-volume hospitals performing 3 or fewer cases per year (n = 3761) accounted for 68.2% of all hospitals. B, Very high-volume hospitals performed a mean of 42.4 (95% CI, 35.7-49.1) cases per year, high-volume hospitals performed a mean of 21.5 (95% CI, 20.2-22.7) cases per year, medium-volume hospitals performed a mean of 10.6 (95% CI, 9.9-11.2) cases per year, low-volume hospitals performed a mean of 4.3 (95% CI, 4.1-4.5) cases per year, and very low-volume hospitals performed a mean of 1.7 (95% CI, 1.6-1.7) cases per year.
Figure 2. In-Hospital Mortality, Postoperative Surgical Complication, and Acute Medical Complication Rates by Hospital Volume Quintile.
Hospitals were categorized as very low volume (≤3 cases per year), low volume (4-6 cases per year), medium volume (7-15 cases per year), high volume (16-28 cases per year), or very high volume (>28 cases per year).
Multiple logistic regression analysis of variables associated with in-hospital mortality, postoperative surgical complications, and acute medical complications, after controlling for all other variables, demonstrated that, compared with hospitals in the bottom quintile, hospitals in the top quintile (>28 cases per year) were associated with lower odds of in-hospital mortality (OR, 0.45; 95% CI, 0.23-0.88) and the lowest odds of postoperative surgical complications (OR, 0.63; 95% CI, 0.50-0.79) and acute medical complications (OR, 0.63; 95% CI, 0.48-0.81) (Table 2). Hospitals treating more than 6 cases per year (medium-volume and high-volume hospitals) also had lower odds of postoperative surgical and acute medical complications but were not associated with differences in in-hospital mortality.
Table 2. Multivariate Logistic Regression Analysis of Variables Associated With Risk of In-Hospital Mortality and Postoperative Complications.
| Variable | Odds Ratio (95% CI) |
|---|---|
| In-Hospital Mortality | |
| Urgent/emergent admission | 1.79 (1.14-2.82) |
| Flap reconstruction | 2.97 (1.73-5.09) |
| Comorbidity score of 2 | 4.42 (2.29-8.52) |
| Comorbidity score of ≥3 | 7.93 (3.87-16.24) |
| Very high-volume hospital | 0.45 (0.23-0.88) |
| Postoperative Surgical Complications | |
| Urgent/emergent admission | 1.91 (1.65-2.20) |
| Age >80 y | 2.33 (1.45-3.76) |
| Hypopharynx primary site | 1.35 (1.16-1.58) |
| Flap reconstruction | 2.04 (1.71-2.44) |
| Medicare | 1.20 (1.03-1.41) |
| Medicaid | 1.19 (1.01-1.41) |
| Comorbidity score of 1 | 1.58 (1.41-1.76) |
| Comorbidity score of 2 | 2.72 (2.35-3.14) |
| Comorbidity score of ≥3 | 5.22 (4.18-6.52) |
| Medium-volume hospital | 0.81 (0.66-0.98) |
| High-volume hospital | 0.72 (0.58-0.88) |
| Very high-volume hospital | 0.63 (0.50-0.79) |
| Acute Medical Complications | |
| Urgent/emergent admission | 2.02 (1.73-2.35) |
| Age 65-80 y | 1.77 (1.00-3.13) |
| Age >80 y | 2.85 (1.57-5.15) |
| Hypopharynx primary site | 1.19 (1.00-1.41) |
| Flap reconstruction | 1.70 (1.40-2.06) |
| Medicare | 1.29 (1.07-1.55) |
| Comorbidity score of 1 | 1.90 (1.67-2.17) |
| Comorbidity score of 2 | 3.92 (3.33-4.60) |
| Comorbidity score of ≥3 | 7.49 (5.92-9.46) |
| Medium-volume hospital | 0.74 (0.59-0.92) |
| High-volume hospital | 0.66 (0.52-0.84) |
| Very high-volume hospital | 0.63 (0.48-0.81) |
Multivariate generalized linear regression analyses of independent variables associated with length of hospitalization and hospital-related costs are summarized in Table 3. After controlling for all other variables, a clinically meaningful negative association was observed between surgery at higher-volume hospitals (>15 cases per year) and length of hospitalization, with a decrease in the mean incremental length of hospitalization at hospitals in the top-volume quintile (−3.7 days; 95% CI, −4.9 to −2.4 days) that exceeded the increase in the mean incremental length of hospitalization associated with patient variables. Surgery at hospitals in the top-volume quintile only was associated with lower mean incremental hospital-related costs (−$4777; 95% CI, −$9463 to −$900).
Table 3. Generalized Linear Regression Analysis of Variables Associated With Length of Hospitalization and Hospital-Related Costs.
| Variable | Estimate (95% CI) | Mean |
|---|---|---|
| Length of Hospitalization, d | ||
| Intercept | 1.8750 (1.6935 to 2.0566) | 11.6 |
| Urgent/emergent admission | 0.3899 (0.3401 to 0.4396) | 4.5 |
| Age 40-64 y | 0.1620 (0.0208 to 0.3031) | 1.9 |
| Age 65-80 y | 0.1674 (0.0261 to 0.3088) | 1.9 |
| Age >80 y | 0.2448 (0.0886 to 0.4010) | 2.9 |
| Hypopharynx primary site | 0.2100 (0.1457 to 0.2744) | 2.5 |
| Flap reconstruction | 0.4000 (0.3291 to 0.4710) | 4.7 |
| Medicare | 0.1292 (0.0812 to 0.1773) | 1.5 |
| Medicaid | 0.2081 (0.1477 to 0.2686) | 2.4 |
| Teaching hospital | 0.1115 (0.0505 to 0.1725) | 1.3 |
| Large hospital bed size | 0.1179 (0.0310 to 0.2048) | 1.4 |
| Comorbidity score of 2 | 0.1810 (0.1306 to 0.2315) | 2.1 |
| Comorbidity score of ≥3 | 0.2625 (0.1698 to 0.3553) | 3.1 |
| High-volume hospital | −0.2193 (−0.3072 to −0.1314) | −2.6 |
| Very high-volume hospital | −0.3140 (−0.4213 to −0.2068) | −3.7 |
| Hospital-Related Costs, 2018 US$ | ||
| Intercept | 9.7837 (9.5197 to 10.0478) | $32 275 |
| Urgent/emergent admission | 0.3039 (0.2394 to 0.3684) | $9810 |
| Age >80 y | 0.2033 (0.0391 to 0.3675) | $6562 |
| Hypopharynx primary site | 0.2551 (0.1922 to 0.3180) | $8234 |
| Hispanic race/ethnicity | 0.2649 (0.1618 to 0.3679) | $9679 |
| Flap reconstruction | 0.5047 (0.4398 to 0.5695) | $16 290 |
| Neck dissection | 0.1027 (0.0503 to 0.1551) | $3317 |
| Medicare | 0.1024 (0.0424 to 0.1624) | $3306 |
| Medicaid | 0.1825 (0.1200 to 0.2350) | $5892 |
| Self-pay | 0.0752 (0.0120 to 0.1383) | $2427 |
| Teaching hospital | 0.1855 (0.0986 to 0.2724) | $5988 |
| Comorbidity score of 1 | 0.0511 (0.0129 to 0.0892) | $1649 |
| Comorbidity score of 2 | 0.2404 (0.1786 to 0.3023) | $7761 |
| Comorbidity score of ≥3 | 0.3196 (0.2253 to 0.4138) | $10 315 |
| Very high-volume hospital | −0.1605 (−0.2952 to −0.0258) | −$5181 |
Discussion
These data demonstrate that there is a significant volume-outcome association for laryngectomy, with improved outcomes seen with increasing hospital volume. A threshold of greater than 6 cases per year was associated with a clinically meaningful lower odds of postoperative surgical complications, acute medical complications, and reduced length of hospitalization, with hospitals in the top-volume quintile that performed more than 28 cases per year also associated with decreased odds of in-hospital mortality and lower costs. These findings are important in an era of health care reform, when increasing attention is focused on health care value, defined as outcomes relative to costs. Because laryngectomy is increasingly becoming a low-volume procedure that is performed more often for salvage and with an increased risk of complications, the use of a minimum hospital volume threshold for laryngectomy may be an appropriate approach to improve value in head and neck cancer care.
To our knowledge, this is the first analysis of minimum hospital volume thresholds for a specific surgical procedure of interest in head and neck cancer. An association between hospital volume and patient outcomes for head and neck cancer surgery has been previously established but is not procedure specific. Studies14,18,19 evaluating surgery for oral cavity, pharynx, larynx, hypopharynx, and salivary gland neoplasms have demonstrated an association between hospital volume and long-term survival, with high-volume hospital care defined as ranging from 60 to 75 cases per year and including a variety of procedures in volume calculations. Using similar cutoffs for volume, high-volume hospital care has been associated with lower in-hospital mortality and decreased failure-to-rescue rates after surgery for oral cavity, pharynx, hypopharynx, and larynx cancer.26,27 Studies evaluating larynx cancer surgery specifically have shown improved long-term survival for high-volume hospital care, defined as 12 or more cases per year,24,25 and lower length of hospitalization and decreased costs at a volume threshold of 18 cases per year,20,22 but the studies combined a variety of surgical procedures exclusive of biopsy to define volumes. In Ontario, Canada, policy changes have been implemented based on such observations. Ontario has a universal single-payer health insurance system and, in 2014, implemented organizational standards for head and neck cancer treatment that include qualitative descriptors of a head and neck cancer center.39 These standards link head and neck cancer center funding to minimum institutional volume cutoffs, effectively regionalizing head and neck cancer surgical care to high-volume hospitals to improve outcomes.
The use of hospital volume as a proxy for quality is controversial in the United States, with opponents arguing that it supports regionalization of care, limits access, and may increase travel burden and care fragmentation, particularly for patients in rural areas.28 Furthermore, it is argued that volume does not accurately reflect the process measures associated with surgical outcomes, including patient selection, preoperative evaluation and preparation, adherence to evidence-based medical guidelines, and postoperative care and does not reflect individual hospital or surgeon performance.40 However, sample size is the greatest limitation to direct process and outcome measurement for procedures that are performed infrequently at individual hospitals because of low case numbers in the denominator of process or outcome measures. For high-risk, low-volume procedures, a structural measure (eg, volume) is a proxy for outcomes if the association between structure and process or between structure and outcome is established and sample sizes are small.28,41 For low-risk, high-volume head and neck cancer procedures, such as neck dissection, outcomes have been shown to be associated with process measures, such as lymph node yield, which neutralizes the volume-outcome association.42,43 The use of volume-based standards requires specific volume-based thresholds for specific procedures to avoid generalizations to low-risk procedures that are more influenced by process or outcome measures than volume.
While process measures may underlie some of the observed association between laryngectomy volume and outcomes, volume may be a stronger predictor of outcomes for laryngectomy. Variables reflecting process measures of care, including patient selection, preoperative evaluation and preparation, adherence to stage-specific evidence-based treatment guidelines, and institutional culture, are not available in the NIS, preventing evaluation of what the effect of process is on outcomes and whether this effect modulates the observed volume-outcome association. Eskander et al44 have reported that guideline adherence for head and neck cancer surgical care in Ontario was associated with hospital volume, but adherence rates were only moderate for high-volume hospitals, which suggests that other factors may underlie the observed favorable volume-outcome association they observed in their population.18 Our core group has previously reported a significant association between hospital volume and adherence to evidence-based guidelines for larynx cancer treatment in Surveillance, Epidemiology, and End Results Medicare data, which was associated with improved survival; however, after controlling for quality of care, a survival advantage remained for high-volume larynx cancer surgical care.25 The findings of these studies suggest that, for larynx cancer surgery, hospital volume may have a greater influence on patient outcomes than evidence-based guideline process measures, which may reflect unmeasured perioperative processes that favorably alter outcomes.
Given the significant association between hospital volume and outcomes, a key policy issue for clinicians and policymakers to explore is regionalization of care in this era of health care reform. Regionalization may reduce operative risks, assure access for all patients with complex conditions to the highest-quality care, and provide an optimal educational environment for surgical trainees. These benefits have to be balanced against the desire of many patients to receive care in their home communities, as well as the cost and logistical challenges of having patients travel to regional centers for complex procedures, which is a particular challenge for patients in rural environments and for those with limited financial means. Federal and local leaders need to develop solutions that balance these different goals and meet the needs of their community. Given patient preferences for receiving care locally, when possible, and the strong economic and reputational incentives associated with the performance of surgery, we recognize that regionalizing care will not be easy and will require committed leadership. One possible starting point for regionalization of care might occur in metropolitan areas, where low-volume hospitals could seek to move their volume to geographically proximate medium- and high-volume hospitals, which may reduce preventable deaths and adverse outcomes while still maintaining patient access and satisfaction. This approach will require a cultural shift that may be most implementable within a health care organization in the current climate of increasing hospital consolidation.
Limitations
There are several limitations to the use of hospital discharge data that may have influenced our findings. The NIS database provides no follow-up data beyond the index admission, is limited to a 30-day postoperative window, and contains no information on stage of disease, grade, subtype, or survival, precluding analysis of long-term outcomes. The NIS database does not contain information regarding readmission, previous surgical procedures, or prior chemotherapy, which could potentially have influenced the results with regard to the extent of surgery and outcomes. The ability to adequately control for case mix is limited if discharge diagnoses from administrative databases are used, which has been done in almost all studies investigating volume and outcome associations to date, and the process measures associated with preoperative care and surgical decision making, as well as long-term outcomes, cannot be assessed. Prior radiotherapy, which is known to influence outcomes, may have been undercoded. Postoperative complications may not have been apparent at the time of discharge; as a result, the incidence of complications may have been underreported. Cost analysis was based on hospital-related charges, adjusted for institutional expense-to-revenue ratios, and did not include physician-related costs because these data are not contained in the NIS database. However, the NIS is the largest database of inpatient surgical procedures that includes hospital-level and cost data and, unlike registry data, represents the spectrum of community, small, and large hospitals, providing a more accurate picture of hospital care across the United States.
Conclusions
Our findings demonstrate that there is a meaningful association between hospital volume and laryngectomy surgery outcomes, and we describe minimum hospital volume thresholds that are associated with in-hospital mortality, surgical and medical complications, length of hospitalization, and costs of care. Institutional leaders and policymakers may want to consider opportunities to shift laryngectomy procedures from lower-volume hospitals to higher-volume hospitals to improve outcomes and reduce costs.
eTable 1. ICD-9-CM Diagnosis and Procedure Codes for Included Cases
eTable 2. ICD-9-CM Diagnosis Codes for Medical and Surgical Complications
References
- 1.Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280(20):1747-1751. doi: 10.1001/jama.280.20.1747 [DOI] [PubMed] [Google Scholar]
- 2.Birkmeyer JD, Finlayson EV, Birkmeyer CM. Volume standards for high-risk surgical procedures: potential benefits of the Leapfrog initiative. Surgery. 2001;130(3):415-422. doi: 10.1067/msy.2001.117139 [DOI] [PubMed] [Google Scholar]
- 3.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128-1137. doi: 10.1056/NEJMsa012337 [DOI] [PubMed] [Google Scholar]
- 4.Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg. 2003;138(7):721-725. doi: 10.1001/archsurg.138.7.721 [DOI] [PubMed] [Google Scholar]
- 5.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117-2127. doi: 10.1056/NEJMsa035205 [DOI] [PubMed] [Google Scholar]
- 6.Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care. 2011;49(12):1076-1081. doi: 10.1097/MLR.0b013e3182329b97 [DOI] [PubMed] [Google Scholar]
- 7.Reames BN, Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and operative mortality in the modern era. Ann Surg. 2014;260(2):244-251. doi: 10.1097/SLA.0000000000000375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Leapfrog Group Surgical volume. http://www.leapfroggroup.org/ratings-reports/surgical-volume. Accessed June 22, 2018.
- 9.Sternberg S, Dougherty G Risks are high at low-volume hospitals. US News & World Report May 18, 2015. https://www.usnews.com/news/articles/2015/05/18/risks-are-high-at-low-volume-hospitals. Accessed June 22, 2018. [Google Scholar]
- 10.Sternberg S. Hospitals move to limit low-volume surgeries. US News & World Report May 19, 2015. https://www.usnews.com/news/articles/2015/05/19/hospitals-move-to-limit-low-volume-surgeries. Accessed June 22, 2018. [Google Scholar]
- 11.Johns Hopkins Medicine. Patient safety and quality: surgical volumes. http://www.hopkinsmedicine.org/patient_safety/surgical_volumes.html. Accessed June 22, 2018.
- 12.Pronovost P. Why surgical volumes should be public. Johns Hopkins Medicine. Voices for safer care: insights from the Armstrong Institute. https://armstronginstitute.blogs.hopkinsmedicine.org/2016/12/06/why-surgical-volumes-should-be-public/. Published December 6, 2016. Accessed June 22, 2018.
- 13.Chen AY, Pavluck A, Halpern M, Ward E. Impact of treating facilities’ volume on survival for early-stage laryngeal cancer. Head Neck. 2009;31(9):1137-1143. doi: 10.1002/hed.21072 [DOI] [PubMed] [Google Scholar]
- 14.Cheung MC, Koniaris LG, Perez EA, Molina MA, Goodwin WJ, Salloum RM. Impact of hospital volume on surgical outcome for head and neck cancer. Ann Surg Oncol. 2009;16(4):1001-1009. doi: 10.1245/s10434-008-0191-9 [DOI] [PubMed] [Google Scholar]
- 15.Chen AY, Fedewa S, Pavluck A, Ward EM. Improved survival is associated with treatment at high-volume teaching facilities for patients with advanced stage laryngeal cancer. Cancer. 2010;116(20):4744-4752. doi: 10.1002/cncr.25364 [DOI] [PubMed] [Google Scholar]
- 16.Morton RP, Gray L, Tandon DA, Izzard M, McIvor NP. Efficacy of neck dissection: are surgical volumes important? Laryngoscope. 2009;119(6):1147-1152. doi: 10.1002/lary.20167 [DOI] [PubMed] [Google Scholar]
- 17.Kim EY, Eisele DW, Goldberg AN, Maselli J, Kezirian EJ. Neck dissections in the United States from 2000 to 2006: volume, indications, and regionalization. Head Neck. 2011;33(6):768-773. doi: 10.1002/hed.21536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eskander A, Irish J, Groome PA, et al. Volume-outcome relationships for head and neck cancer surgery in a universal health care system. Laryngoscope. 2014;124(9):2081-2088. doi: 10.1002/lary.24704 [DOI] [PubMed] [Google Scholar]
- 19.Eskander A, Merdad M, Irish JC, et al. Volume-outcome associations in head and neck cancer treatment: a systematic review and meta-analysis. Head Neck. 2014;36(12):1820-1834. doi: 10.1002/hed.23498 [DOI] [PubMed] [Google Scholar]
- 20.Gourin CG, Forastiere AA, Sanguineti G, Koch WM, Marur S, Bristow RE. Impact of surgeon and hospital volume on short-term outcomes and cost of laryngeal cancer surgical care. Laryngoscope. 2011;121(1):85-90. doi: 10.1002/lary.21348 [DOI] [PubMed] [Google Scholar]
- 21.Gourin CG, Forastiere AA, Sanguineti G, Marur S, Koch WM, Bristow RE. Impact of surgeon and hospital volume on short-term outcomes and cost of oropharyngeal cancer surgical care. Laryngoscope. 2011;121(4):746-752. doi: 10.1002/lary.21456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gourin CG, Frick KD. National trends in laryngeal cancer surgery and the effect of surgeon and hospital volume on short-term outcomes and cost of care. Laryngoscope. 2012;122(1):88-94. doi: 10.1002/lary.22409 [DOI] [PubMed] [Google Scholar]
- 23.Gourin CG, Frick KD. National trends in oropharyngeal cancer surgery and the effect of surgeon and hospital volume on short-term outcomes and cost of care. Laryngoscope. 2012;122(3):543-551. doi: 10.1002/lary.22447 [DOI] [PubMed] [Google Scholar]
- 24.Gourin CG, Dy SM, Herbert RJ, et al. Treatment, survival, and costs of laryngeal cancer care in the elderly. Laryngoscope. 2014;124(8):1827-1835. doi: 10.1002/lary.24574 [DOI] [PubMed] [Google Scholar]
- 25.Gourin CG, Frick KD, Blackford AL, et al. Quality indicators of laryngeal cancer care in the elderly. Laryngoscope. 2014;124(9):2049-2056. doi: 10.1002/lary.24593 [DOI] [PubMed] [Google Scholar]
- 26.Mulvey CL, Pronovost PJ, Gourin CG. Hospital volume and failure to rescue after head and neck cancer surgery. Otolaryngol Head Neck Surg. 2015;152(5):783-789. doi: 10.1177/0194599815570026 [DOI] [PubMed] [Google Scholar]
- 27.Nieman CL, Stewart CM, Eisele DW, Pronovost PJ, Gourin CG. Frailty, hospital volume, and failure to rescue after head and neck cancer surgery. Laryngoscope. 2018;128(6):1365-1370. doi: 10.1002/lary.26952 [DOI] [PubMed] [Google Scholar]
- 28.Chhabra KR, Dimick JB. Strategies for improving surgical care: when is regionalization the right choice? JAMA Surg. 2016;151(11):1001-1002. doi: 10.1001/jamasurg.2016.1059 [DOI] [PubMed] [Google Scholar]
- 29.Gourin CG, Forastiere AA, Sanguineti G, Marur S, Koch WM, Bristow RE. Volume-based trends in laryngeal cancer surgery. Laryngoscope. 2011;121(1):77-84. doi: 10.1002/lary.21393 [DOI] [PubMed] [Google Scholar]
- 30.Chen AY, Fedewa S, Zhu J. Temporal trends in the treatment of early- and advanced-stage laryngeal cancer in the United States, 1985-2007. Arch Otolaryngol Head Neck Surg. 2011;137(10):1017-1024. doi: 10.1001/archoto.2011.171 [DOI] [PubMed] [Google Scholar]
- 31.Orosco RK, Weisman RA, Chang DC, Brumund KT. Total laryngectomy: national and regional case volume trends 1998-2008. Otolaryngol Head Neck Surg. 2013;148(2):243-248. doi: 10.1177/0194599812466645 [DOI] [PubMed] [Google Scholar]
- 32.Verma SP, Mahboubi H. The changing landscape of total laryngectomy surgery. Otolaryngol Head Neck Surg. 2014;150(3):413-418. doi: 10.1177/0194599813514515 [DOI] [PubMed] [Google Scholar]
- 33.Maddox PT, Davies L. Trends in total laryngectomy in the era of organ preservation: a population-based study. Otolaryngol Head Neck Surg. 2012;147(1):85-90. doi: 10.1177/0194599812438170 [DOI] [PubMed] [Google Scholar]
- 34.Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116(9, pt 2)(suppl 111):1-13. doi: 10.1097/01.mlg.0000236095.97947.26 [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg TL, Franzese CB. Extremes in otolaryngology resident surgical case numbers. Otolaryngol Head Neck Surg. 2012;147(2):261-270. doi: 10.1177/0194599812444533 [DOI] [PubMed] [Google Scholar]
- 36.Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality. Overview of the National (Nationwide) Inpatient Sample (NIS) http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed May 19, 2018.
- 37.Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality. Cost-to-Charge Ratio Files http://www.hcup-us.ahrq.gov/db/state/costtocharge.jsp. Accessed May 19, 2018.
- 38.Bureau of Labor Statistics, United States Department of Labor Consumer Price Index (CPI) inflation calculator. http://www.bls.gov/bls/inflation.htm. Accessed May 19, 2018.
- 39.Eskander A, Goldstein DP, Irish JC. Health services research and regionalization of care—from policy to practice: the Ontario experience in head and neck cancer. Curr Oncol Rep. 2016;18(3):19. doi: 10.1007/s11912-016-0500-6 [DOI] [PubMed] [Google Scholar]
- 40.Russell TR. Invited commentary: volume standards for high-risk operations: an American College of Surgeons’ view. Surgery. 2001;130(3):423-424. doi: 10.1067/msy.2001.117137 [DOI] [PubMed] [Google Scholar]
- 41.Donabedian A. Evaluating the quality of medical care: 1966. Milbank Q. 2005;83(4):691-729. doi: 10.1111/j.1468-0009.2005.00397.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schoppy DW, Rhoads KF, Ma Y, et al. Measuring institutional quality in head and neck surgery using hospital-level data: negative margin rates and neck dissection yield. JAMA Otolaryngol Head Neck Surg. 2017;143(11):1111-1116. doi: 10.1001/jamaoto.2017.1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Divi V, Chen MM, Nussenbaum B, et al. Lymph node count from neck dissection predicts mortality in head and neck cancer. J Clin Oncol. 2016;34(32):3892-3897. doi: 10.1200/JCO.2016.67.3863 [DOI] [PubMed] [Google Scholar]
- 44.Eskander A, Monteiro E, Irish J, et al. Adherence to guideline-recommended process measures for squamous cell carcinoma of the head and neck in Ontario: impact of surgeon and hospital volume. Head Neck. 2016;38(suppl 1):E1987-E1992. doi: 10.1002/hed.24364 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. ICD-9-CM Diagnosis and Procedure Codes for Included Cases
eTable 2. ICD-9-CM Diagnosis Codes for Medical and Surgical Complications