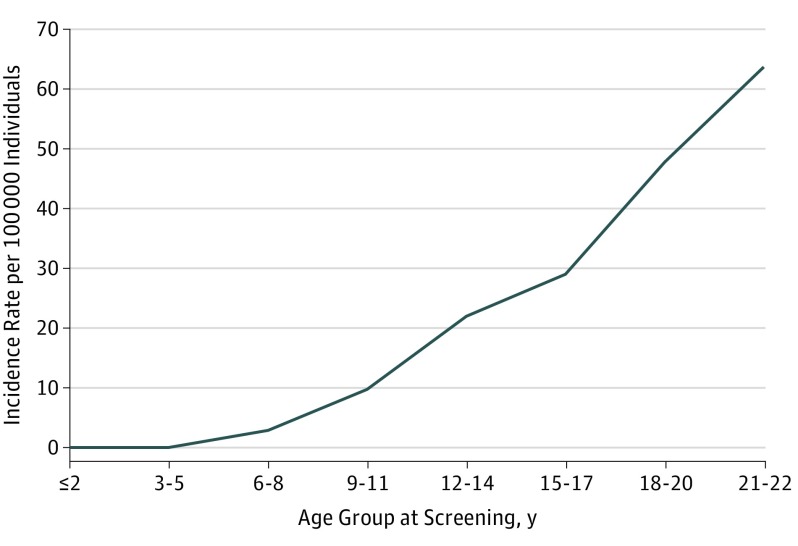Abstract
Importance
Ultrasonographic (US) screening for thyroid cancer was performed in the Fukushima Health Management Survey after the 2011 Fukushima Daiichi nuclear power station accident. Clinical characteristics of thyroid cancers screened by US among children and young adults during the first 5 years after the accident were analyzed.
Objectives
To evaluate the number of detected thyroid cancers by age group within 5 years of the Fukushima Daiichi nuclear power station accident and to compare the basic clinical characteristics and demographic patterns in first- and second-round examinations.
Design, Setting, and Participants
In this observational study, 324 301 individuals 18 years or younger at the time of accident were included. Patients received a cytologic diagnosis of malignant or suspected malignant thyroid cancer during the first (fiscal years 2011-2013) or second round (fiscal years 2014-2015) of screening. Number of detected cases of cancer was evaluated, correcting for the number of examinees by age group at the time of the accident and for the incidence of detected cancers according to age group at the time of the screening (age groups were divided into 3-year intervals). Results were compared using the age-specific incidence of unscreened cancers from a national cancer registry.
Main Outcomes and Measures
Clinical baseline characteristics of the patients and the age-specific number and incidence of thyroid cancers detected during the second round.
Results
Among 299 905 individuals screened in the first round (50.5% male; mean [SD] age at screening, 14.9 [2.6] years), malignant or suspected thyroid cancer was diagnosed in 116. Among 271 083 individuals screened in the second round (50.4% male; age at screening, 12.6 [3.2] years), malignant or suspected thyroid cancer was diagnosed in 71. The most common pathologic diagnosis in surgical cases was papillary thyroid cancer (149 of 152 [98.0%]). The distribution pattern by age group at the time of the accident, where the number of detected thyroid cancer cases was corrected by the number of examinees, increased with older age in both screening rounds. This demographic pattern was similar between the first and second examinations. The distribution pattern of the incidence rate by age group at the time of screening in the second round also increased with older age. The incidence rate detected by screening was 29 cases per 100 000 person-years for those aged 15 to 17 years, 48 cases per 100 000 person-years for those aged 18 to 20 years, and 64 cases per 100 000 person-years for those aged 21 to 22 years.
Conclusions and Relevance
Large-scale mass US screening of young people resulted in the diagnosis of a number of thyroid cancers, with no major changes in overall characteristics within 5 years of the 2011 Fukushima nuclear power station accident. These results suggest that US screening can identify many detectable cancers from a large pool of nonclinical and subclinical thyroid cancers among individuals of a relatively young age, in an age-dependent manner.
This cohort study of 324 301 children and young adults in Fukushima, Japan, evaluates the number of detected thyroid cancers by age group within 5 years of the 2011 Fukushima Daiichi nuclear power station accident and compares basic clinical characteristics and demographic patterns in first-round and second-round examinations.
Key Points
Question
What is the pattern by age group of cancer detection via ultrasonographic screening of the thyroid among children and young adults within 5 years of the 2011 Fukushima Daiichi nuclear power station accident?
Findings
In this cohort study of 324 301 children and young adults, thyroid cancer was diagnosed in 187 individuals within 5 years when health effects of radiation were hardly conceivable. The distribution pattern of the incidence rate by age group in second-round examinations increased with older age.
Meaning
Large-scale mass screening resulted in the diagnosis of many thyroid cancers even in young age; to avoid overdiagnosis, an improvement in screening strategy based on the understanding of the natural history of thyroid cancer will be urgently needed.
Introduction
The Great East Japan Earthquake on March 11, 2011, and the subsequent Fukushima Daiichi nuclear power station accident raised grave concerns regarding the dispersion of radioactive material in the environment, as well as the various health effects caused by the emergency evacuation, prolonged displacement, lifestyle change, and mental health problems of the people of Japan.1 The level of radiation exposure in Fukushima immediately after the accident has been deemed to be much lower than the levels reported in Chernobyl immediately after the 1986 nuclear power station accident.2,3 However, there was a divergence in estimations of the thyroid equivalent dose of radiation during the early phase after the accident because there was little direct measurement of individuals. Radiation-induced thyroid cancers have been rated as causing some of the greatest concern after the accident, and the fear of thyroid cancer caused disaster-related anxiety among the general public after the accident. Thus, health surveillance, including thyroid screening, has been thought to be necessary for both scientific and social reasons.4,5 Ultrasonographic examinations of the thyroid were conducted, targeting Fukushima residents 18 years of age or younger at the time of the nuclear accident, as part of the Fukushima Health Management Survey (FHMS) commissioned by Fukushima Prefecture.6,7
The minimum latent period of radiation-induced thyroid cancer is generally 5 to 10 years.8 After the 1986 Chernobyl nuclear power station accident, the frequency of pediatric thyroid cancers reportedly increased relatively soon (within 5 years) after the accident in iodine-deficient areas owing to high-dose thyroid exposure by intake of radioactive iodine-contaminated milk.9 The possibility of a similarly increased risk of childhood thyroid cancer can be discounted in the case of the Fukushima nuclear power station accident because the absorbed doses to the thyroid after the Fukushima accident were substantially lower; however, risk could be theoretically inferred.3 Because a comprehensive evaluation of the characteristics of screened thyroid cancers is important, a first round (in the first 3 years after the accident) and second round (in the following 2 years) of screening examinations were conducted during the first 5 years after the Fukushima nuclear power station accident.
Apart from lifestyle-related changes or environmental carcinogenic factors, the incidence of thyroid cancer has been increasing worldwide, together with the advancement of highly sensitive ultrasonographic technologies.10,11,12 In its recent update of recommendations for thyroid cancer screening, the US Preventive Services Task Force reconfirmed their prior recommendation against screening for thyroid cancer in asymptomatic adults because the harms due to overdiagnosis outweighed any potential benefit.13 There are currently no thyroid cancer screening guidelines for young people. We began the FHMS thyroid screening program in 2011 and applied a Japanese guideline for the diagnosis of clinical thyroid cancer.14 This protocol was considered to be relatively conservative (eg, incorporating the principle of not examining nodules with a tumor diameter of ≤5 mm).15 To avoid potential overdiagnosis and assess the risk of low-dose radiation, it is essential to understand the characteristics of the natural history of detected thyroid cancer using highly sensitive ultrasonography. In this 5-year study, we analyzed the number of detected cases of thyroid cancer, corrected by the number of examinees in each age group, in a comparison between first and second rounds of screening.
Methods
Thyroid Ultrasonographic Examination in the FHMS
We sent information about the FHMS thyroid ultrasonographic examination to all residents of Fukushima 18 years of age or younger at the time of the Fukushima nuclear power station accident, except for those who refused to participate in the data analysis. The examination comprised 2 steps: a screening examination and a confirmatory examination, as described previously.15 Participants with nodules larger than 5.0 mm in diameter were screened by ultrasonography according to Japanese guidelines for the clinical diagnosis of thyroid cancer.14 In the confirmatory examination, fine-needle aspiration cytology was performed as necessary, and malignant or suspected malignant nodules were cytologically diagnosed. First-round screening examinations were conducted in fiscal year 2011 to fiscal year 2013, with a participation rate of 81.7%16 and second-round screening examinations were conducted in fiscal year 2014 to fiscal year 2015, with a participation rate of 71.0%.17 The study was approved by the Fukushima Medical University Ethics Committee (approval No. 1318). All participants or their guardians provided written informed consent.
Study Participants
A total 324 301 individuals who underwent either the first or second round of screening were enrolled, with 299 905 individuals participating in the first-round examination and 271 083 in the second-round examination. A total of 2086 individuals in the first round and a total of 1787 individuals in the second round underwent a confirmatory examination. Of these, 116 received a diagnosis of malignant or suspected malignant thyroid tumors in the first-round examination and 71 received a diagnosis of malignant or suspected malignant thyroid tumors in the second-round examination, and they were subsequently enrolled in this study.
Detected Cases of Thyroid Cancer by Age Group at Time of Accident
The number of detected or suspected cases of thyroid cancer among participants in the first round and second round of screening was calculated and adjusted by the number of individuals in each age group at the time of the accident. The adjusted number of detected cases in the first round of screening corresponds to the prevalence of thyroid cancer during the first 3 years and the adjusted number of detected cases in the second round of screening corresponds to the incidence of thyroid cancer during the next 2 years.
Incidence Rate of Thyroid Cancer Detected by Ultrasonography in the Second Round
In the calculation of incidence rate by age group at the time of screening, we excluded 116 participants with diagnosed or suspected thyroid cancer from the 299 905 participants in the first-round examination. Next, we excluded 54 259 participants who did not participate in the second-round survey (including 69 who died). There were 245 530 participants with sufficient thyroid examination data in the second-round survey. The incidence of patients with cytologically diagnosed or suspected thyroid cancer was calculated as the number of cases divided by the mean observation period between the dates of the first- and second-round screenings, per 100 000 person-years. Of the 245 530 participants, 70 received a diagnosis of malignant or suspected malignant thyroid tumors and were subsequently enrolled in the incidence analysis. The incidence by age group was determined by dividing the age groups at screening into 3-year intervals (age, ≤2, 3-5, 6-8, 9-11, 12-14, 15-17, 18-20, and 21-22 years).
Estimated Age-Conditional Incidence Rate of Thyroid Cancer
We estimated the expected age-conditional incidence rate of developing thyroid cancer using a life-table method and national estimates of the incidence of thyroid cancer during the period from 2001 to 2010.18,19,20 We then compared the differences in the age-conditional incidence rate between the FHMS and national cancer registry by age group.
Statistical Analysis
We calculated the relative risk and 95% CI of thyroid cancer for the age groups of 12 to 14 years, 15 to 17 years, 18 to 20 years, and 21 to 22 years, with the group aged 9 to 11 years as a control reference, using Poisson regression models adjusted for sex. All data analyses were analyzed using JMP, version 12 (SAS Institute), and SAS, version 9.4 (SAS Institute).
Results
Among 299 905 individuals screened in the first round, 50.5% were male and the mean (SD) age at screening was 14.9 (2.6) years. Among 271 083 screened in the second round, 50.4% were male and the age at screening was 12.6 (3.2) years. Data on all malignant or suspected cases of thyroid cancer that were cytologically diagnosed during thyroid examinations conducted from fiscal year 2011 to fiscal year 2015 are summarized in Table 1. The number of cases of thyroid cancer or suspected thyroid cancer was 116 in the first-round screening and 71 in the second-round screening. In first-round examinations, the mean (SD) age at diagnosis was 17.3 (2.7) years, there were 77 female patients and 39 male patients, the mean (SD) tumor diameter at screening was 12.7 (7.4) mm, and the median tumor size at screening was 10.5 mm. In second-round examinations, the mean (SD) age at diagnosis was 16.9 (3.2) years, there were 39 female patients and 32 male patients, the mean (SD) tumor size at screening was 9.7 (5.3) mm, and median tumor size at screening was 8.6 mm. In the first round, 55 tumors (47.4%) were classified as 5.1 to 10.0 mm in diameter at screening, 30 tumors (25.9%) were classified as 10.1 to 15.0 mm, 14 tumors (12.1%) were classified as 15.1 to 20.0 mm, and 17 tumors (14.7%) were classified as 20.1 mm or larger. In the second round, 47 tumors (66.2%) were classified as 5.1 to 10.0 mm in diameter, 15 tumors (21.1%) were classified as 10.1 to 15.0 mm, 6 tumors (8.5%) were classified as 15.1 to 20.0 mm, and 3 tumors (4.2%) were classified as 20.1 mm or larger. The highest proportion of pathologic diagnoses in surgical cases was papillary thyroid cancer (149 of 152 [98.0%]), with the remainder being 1 case each of benign, poorly differentiated, and other type of thyroid cancer. Of 71 patients with diagnosed or suspected thyroid cancer in the second-round examination, 70 had undergone the first-round examination and 12 had a thyroid nodule detected in the first round.
Table 1. Clinical Characteristics of Diagnosed or Suspected Thyroid Cancer Cases Detected in the First Round and Second Round of Screening.
| Characteristic | First Round (Fiscal Years 2011-2013) (n = 299 905) | Second Round (Fiscal Years 2014-2015) (n = 271 083) |
|---|---|---|
| Screening examinees | ||
| Sex, No. (%) | ||
| Male | 151 371 (50.5) | 136 707 (50.4) |
| Female | 148 534 (49.5) | 134 376 (49.6) |
| Age, mean (SD), y | 14.9 (2.6) | 12.6 (3.2) |
| Confirmatory examinees | 2086 | 1787 |
| Diagnosed or suspected cases of thyroid cancer, No. | 116 | 71 |
| Age at diagnosis, mean (SD), y | 17.3 (2.7) | 16.9 (3.2) |
| Sex, No. | ||
| Male | 39 | 32 |
| Female | 77 | 39 |
| Tumor diameter at screening, mm | ||
| Mean (SD) | 12.7 (7.4) | 9.7 (5.3) |
| Median | 10.5 | 8.6 |
| Cases by tumor size at screening, No. (%) | ||
| 5.1-10.0 mm | 55 (47.4) | 47 (66.2) |
| 10.1-15.0 mm | 30 (25.9) | 15 (21.1) |
| 15.1-20.0 mm | 14 (12.1) | 6 (8.5) |
| ≥20.1 mm | 17 (14.7) | 3 (4.2) |
| Pathologic diagnosis in surgical cases, No. | ||
| Benign nodule | 1 | 0 |
| Papillary thyroid carcinoma | 100 | 49 |
| Poorly differentiated thyroid carcinoma | 1 | 0 |
| Other | 0 | 1 |
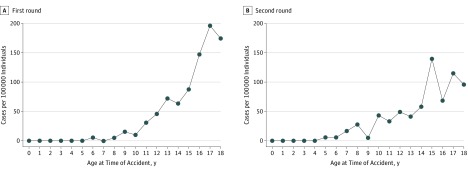
The distribution patterns by age group at the time of the accident, in which the number of detected cases of thyroid cancer was corrected by the number of individuals, increased with older age and were similar in first-round (Figure 1A), and second-round examinations (Figure 1B), with an interval between screenings of about 2.1 years. No individuals younger than 6 years at the time of the accident had thyroid cancer detected in the first-round examination, and none younger than 5 years at the time of the accident had thyroid cancer detected in the second-round examination. The number of detected cases of thyroid cancer per 100 000 persons was estimated to be approximately 175 for those 18 years of age at the time of the accident in the first round of screening and 97 for those 18 years of age at the time of the accident in the second round of screening.
Figure 1. Number of Detected or Suspected Thyroid Cancer Cases Adjusted for Number of Participants in Each Age Group at the Time of the Fukushima Nuclear Accident.
A, Number of cases detected per 100 000 examinees in the first round of screening. B, Number of cases detected per 100 000 examinees in the second round of screening. Numbers of cases increased with older age at the time of the accident.
To compare the results of first- and second-round screenings, we calculated the incidence rate of detected cases of thyroid cancer by age group at the time of screening (Table 2). The incidence rate of thyroid cancer per 100 000 person-years detected in the second-round screening was 0 cases for those 2 years or younger, 0 cases for those 3 to 5 years, 3 cases for those 6 to 8 years, 10 cases for those 9 to 11 years, 22 cases for those 12 to 14 years, 29 cases for those 15 to 17 years, 48 cases for those 18 to 20 years, and 64 cases for those 21 to 22 years. This distribution pattern of the incidence rate by age group at the time of screening in the second-round examination also increased with older age. Compared with the age group of 9 to 11 years, the sex-adjusted relative risk was 2.26 (95% CI, 1.11-1.52) for the age group of 12 to 14 years, 2.95 (95% CI, 1.40-6.26) for the age group of 15 to 17 years, 4.80 (95% CI, 2.11-10.9) for the age group of 18 to 20 years, and 6.28 (95% CI, 0.81-48.7) for the age group of 21 to 22 years. The estimated age-conditional incidence rate of cases per 100 000 person-years was calculated to be approximately 0.5 for those 15 to 17 years of age, 1.0 for those 18 to 20 years of age, and 1.7 for those 21 to 22 years of age. A large excess risk—approximately 63 times in excess for those 15 to 17 years of age, 50 times in excess for those 18 to 20 years of age, and 38 times in excess for those 21 to 22 years of age—was calculated for these age groups, compared with the age-conditional incidence rate estimated using data on people 25 years or younger retrieved from the national cancer registry (2001-2010).20
Table 2. Data on Incidence Rate of Diagnosed or Suspected Thyroid Cancer Cases by Age Group in Second-Round Screening.
| Characteristic | Age Group, y | |||||||
|---|---|---|---|---|---|---|---|---|
| ≤2 | 3-5 | 6-8 | 9-11 | 12-14 | 15-17 | 18-20 | 21-22 | |
| Examinees, No. | 11 270 | 36 868 | 49 591 | 52 798 | 52 679 | 29 849 | 11 569 | 906 |
| Cases of thyroid cancer, No. | 0 | 0 | 3 | 11 | 25 | 18 | 12 | 1 |
| Mean interval between screening rounds, y | 2.3 | 2.1 | 2.1 | 2.1 | 2.2 | 2.1 | 2.2 | 1.7 |
| Person-years | 25 330 | 76 458 | 104 579 | 113 047 | 113 770 | 62 080 | 25 125 | 1571 |
| Incidence rate, No. of cases per 100 000 person-years | 0 | 0 | 3 | 10 | 22 | 29 | 48 | 64 |
Discussion
In this study of the FHMS thyroid screening program, diagnosed or suspected thyroid cancer was detected in 116 individuals in the first round of screening and 71 individuals in the second round. As shown in Table 1 and Figure 1, no large differences were found in the basic characteristics of detected cancers between the first- and second-round examinations. The mean tumor diameter was smaller in the second round than in the first round, and the proportion of tumors with a diameter of 5.1 to 10.0 mm was greater in the second round than in the first round. Because the mean interval between the 2 examinations was 2.1 years, this is considered an initial screening effect of the relatively large-sized tumors discovered in the first-round examination and/or increased sensitivity owing to 2 rounds of screening. The distribution patterns of detected thyroid cancers by age group at the time of screening were also similar, suggesting that the detection rate of thyroid cancer using highly sensitive ultrasonography did not change substantially during the first 5 years after the Fukushima nuclear power station accident. Although a longer observation period is needed, this pattern by age at the time of the accident differs from that of the Chernobyl nuclear power station accident; for example, there was a higher frequency of cases of cancer at younger ages with a relatively short latent period after the Chernobyl nuclear power station accident.21,22 Thus, an association between the large number of thyroid cancers detected and radiation exposure is thought to be very unlikely, in addition to the very low doses of radiation2,3,4 in the Fukushima nuclear power station accident. Although distributions of the radionuclides were affected by the physical state of each nuclide, by geographical features, by wind, and by rainfall,23,24 there was no pattern of association between the geographical dose distribution and the prevalence of thyroid cancer among the participants in the first round of screening.25,26 Furthermore, these findings are supported by pathologic features and the cancer driver mutation profile of these cases,27,28 which are markedly different from those of the cases seen after the Chernobyl nuclear power station accident29,30 but similar to those of low-risk, sporadic cases of adult thyroid cancer.31,32
One question remains in the comparison of data from the first- and second-round examinations. In the present study, the number of cancers detected in the second-round examination was about 61.2% (71 of 116) of that detected in the first round, despite the similar mean age at detection (first round, 17.3 years; second round, 16.9 years) and the relatively short interval between examinations (approximately 2.1 years). If most thyroid cancers in young individuals grow slowly and steadily, the number of thyroid cancers per 100 000 examinees detected in the second-round examination should have decreased from the number detected in the first round owing to the initial screening effect. The reasons why the number of cases detected at young ages did not decrease much can be explained as follows. First, among patients at a relatively young age, a large pool of thyroid cancers that are not routinely diagnosed by screening forms part of the natural disease course. This is consistent with the fact that many more thyroid papillary cancers are observed in autopsies among people who are relatively young than among those in cancer registries.33,34 Second, in a portion of cancers in this large pool, the tumor grows to approximately 5 to 15 mm in diameter, and these tumors are more likely to be detected in an age-dependent manner.35 Third, these tumors initially proliferate and then fall into a nearly arrested growth pattern after a certain period. A recent study reported that most asymptomatic thyroid cancers screened in the FHMS examinations can be observed falling into an arrested growth pattern after the initial proliferation phase, and 6% of cancers or suspected cases of cancer had reduced tumor size.36 Of 70 cases of cancer, 58 detected in the second round were at least newly detected; nodules detected at the first examination in the remaining 12 cases cannot necessarily be diagnosed as cancer. This finding indicates age-dependent increases in detectable cancers from the large pool of nonclinical and subclinical thyroid cancers.
Based on the results of first-round examinations, Katanoda et al18 reported that the observed prevalence of thyroid cancer among residents 20 years of age or younger, without adjusting the number of participants, was 160.1 and that the estimated prevalence of thyroid cancer, without adjusting the number of participants, was 5.2, giving an observed to estimated ratio of 30.8. As seen in a previous report, comparison of FHMS thyroid examination data and National Cancer Center Registry data requires the following 3 points for interpretation: prevalence in the first-round FHMS examination and cancer registry incidence data, mass screening and clinical routine detection data, and sensitivity data.37 The first point is considered comparable according to the incidence of second-round examinations, as shown in Table 2 and Figure 2. The difference in the number of detected cases caused by the latter 2 points is considered to be the outcome of screening effects and/or potential overdiagnosis. A simulation using a cancer progression model based on the National Cancer Registry also indicated that median lead times were 34 years for male patients and 30 years for female patients, under the assumption that all screened cancers will develop into clinical symptomatic cancer.37 If many nonprogressive or regressed thyroid cancers are detected by ultrasonography in children or young adults, frequent examinations may produce a greater potential for overdiagnosis.38
Figure 2. Incidence of Thyroid Cancer in the Second Round of Screening by Age Group at the Time of Screening.
Incidence was expressed per 100 000 person-years by dividing the age groups at screening into 3-year intervals (age groups of ≤2, 3-5, 6-8, 9-11, 12-14, 15-17, 18-20, and 21-22 years).
Like the first-round examination, a large excess risk owing to potential overdiagnosis in the second-round screening was expected compared with the estimated age-conditional probability retrieved from the cancer registry.39 Considering the possibility of overdiagnosis, using conservative criteria is important for thyroid cancer screening. Previous screening studies have addressed the characteristics of low-risk thyroid cancers by raising the level of screening criteria above those of clinical diagnostic criteria. From 1992 to 1997, a survey was conducted using thyroid ultrasonographic screening among 3440 children younger than 17 years of age who were exposed to radioactive iodine released from the Hanford nuclear facility in the United States.40 The fine-needle aspiration biopsy criteria were changed soon after the start of screening, to prevent overdiagnosis, from inclusion of tumors with diameters of 10 mm or more to inclusion of tumors with diameters of 15 mm or more. Takebe et al41 reported that the thyroid tumor ultrasonographic screening criteria for adults were changed from inclusion of tumors with a diameter of 3 mm to those with a diameter of 7 mm, then to 10 mm, and then to 15 mm among female participants from 1990 to 1995 in Japan. The detection rates of thyroid cancer were 3.5% with inclusion of tumors with a diameter of 3 mm, 1.5% with inclusion of tumors with a diameter of 7 mm, 0.9% with inclusion of tumors with a diameter of 10 mm, and 0.28% with inclusion of tumors with a diameter of 15 mm. The American Thyroid Association recently updated its clinical guidelines to recommend that, generally, only nodules larger than 10 mm should be considered for further examination and thyroid cancer screening is not recommended.42 The detection rate of thyroid cancer in the FHMS examinations can be expected to decrease to approximately 44% if screening nodules with a diameter larger than 10 mm, 13% if screening nodules with a diameter larger than 15 mm, and 4% if screening nodules with a diameter larger than 20 mm, compared with screening nodules with a diameter larger than 5 mm.
The natural history of many papillary thyroid cancers is different from some other cancer types, where early diagnosis may improve prognosis.43 To prevent overdiagnosis, screening criteria for asymptomatic individuals need to be higher and their sensitivity should be lower than that of clinical guidelines for symptomatic patients. The EU-OPERRA, SHAMISEN (European Project for the European Radiation Research Area, Nuclear Emergency Situations Improvement of Medical and Health Surveillance) project has indicated that systematic thyroid cancer screening should not be recommended, but it should be made available.44 In this recommendation, thyroid cancer screening programs should be prepared on a voluntary basis and could not be ultrasonography first but based on a clinical examination including thyroid palpation, in which only suspicious cancers are referred to ultrasonography.44 Although there are multiple factors to consider in screening criteria using ultrasonography, we think that increasing the screening size of tumors may be an effective tool for reducing unnecessary diagnoses in a large pool of thyroid cancers.
The fundamental ethical principle of doing more good than harm should be central to accident management, including conducting thyroid screening that is designed to avoid the problems of potential overdiagnosis.13,44 Therefore, we should not be influenced by the negative experiences or psychosocial issues of the examinees under the potential for overdiagnosis.45,46 There are no clinical findings or biomarkers that can distinguish cases in which early diagnosis may be beneficial to patients from a large pool of low-risk thyroid cancers. Thus, a careful follow-up system is required to ensure the merit of early detection and prevent potential overdiagnosis via large-scale screening.
Limitations
This study focused on thyroid cancer screening conducted in accordance with thyroid examinations in the FHMS. Several limitations in this study should be considered. Although participation rates were high in the first- and second-round screenings, selection bias may have occurred despite correcting for age-specific participation rates. For example, the examination rate in second-round screenings was more than 90% among those younger than 14 years but approximately 40% among those 15 years or older. A final pathologic diagnosis was obtained for 152 surgical patients, but not for the remaining 35 patients with cancer or suspected cancer who did not undergo surgery or for whom surgical reports could not be obtained. We did not analyze the detailed clinical or pathologic findings after thyroid cancer surgery or the prognosis for those awaiting surgery. First-round screening was conducted for approximately 2.5 years, and second-round screening was conducted for approximately 2 years. The time until each confirmatory examination varied, ranging from approximately 2 months to approximately 3 years. Because this is an observational study, it is difficult to analyze the effect of low-dose radiation or other confounding factors.
Conclusions
In this observational study, thyroid cancer in young patients as screened by ultrasonography showed age-dependent increases in the number of cases detected in first- and second-round examinations. This study indicates that a large pool of thyroid cancers exist, which are not recognized as clinical cancers without screening, from a relatively young age. Because the natural progression of thyroid cancer based on the possible causes in young patients remains unknown, further studies are required. Data from the FHMS may contribute to understanding how to conduct future screening programs to both limit overdiagnosis and support an accurate evaluation of the effect of low-dose radiation on the thyroid glands of children and adolescents.
References
- 1.Hasegawa A, Tanigawa K, Ohtsuru A, et al. Health effects of radiation and other health problems in the aftermath of nuclear accidents, with an emphasis on Fukushima. Lancet. 2015;386(9992):479-488. doi: 10.1016/S0140-6736(15)61106-0 [DOI] [PubMed] [Google Scholar]
- 2.UN Scientific Committee on the Effects of Atomic Radiation Report to the General Assembly with scientific annex A. Vol I New York, NY: United Nations; 2014. Sources, effects and risks of ionizing radiation: UNSCEAR 2013. [Google Scholar]
- 3.UN Scientific Committee on the Effects of Atomic Radiation Developments since the 2013 UNSCEAR report on the levels and effects of radiation exposure due to the nuclear accident following the Great East-Japan earthquake and tsunami: a 2017 white paper to guide the scientific committee’s future programme of work. New York: United Nations; 2017.
- 4.Yamashita S, Suzuki S. Risk of thyroid cancer after the Fukushima nuclear power plant accident. Respir Investig. 2013;51(3):128-133. doi: 10.1016/j.resinv.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 5.Ohtsuru A, Tanigawa K, Kumagai A, et al. Nuclear disasters and health: lessons learned, challenges, and proposals. Lancet. 2015;386(9992):489-497. doi: 10.1016/S0140-6736(15)60994-1 [DOI] [PubMed] [Google Scholar]
- 6.Yasumura S, Hosoya M, Yamashita S, et al. ; Fukushima Health Management Survey Group . Study protocol for the Fukushima Health Management Survey. J Epidemiol. 2012;22(5):375-383. doi: 10.2188/jea.JE20120105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamashita S. Tenth Warren K. Sinclair keynote address—the Fukushima nuclear power plant accident and comprehensive health risk management. Health Phys. 2014;106(2):166-180. doi: 10.1097/HP.0000000000000007 [DOI] [PubMed] [Google Scholar]
- 8.Ron E, Lubin JH, Shore RE, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 1995;141(3):259-277. doi: 10.2307/3579003 [DOI] [PubMed] [Google Scholar]
- 9.Williams D. Radiation carcinogenesis: lessons from Chernobyl. Oncogene. 2008;27(suppl 2):S9-S18. doi: 10.1038/onc.2009.349 [DOI] [PubMed] [Google Scholar]
- 10.Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic? the increasing impact of overdiagnosis. N Engl J Med. 2016;375(7):614-617. doi: 10.1056/NEJMp1604412 [DOI] [PubMed] [Google Scholar]
- 11.Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer ‘epidemic’—screening and overdiagnosis. N Engl J Med. 2014;371(19):1765-1767. doi: 10.1056/NEJMp1409841 [DOI] [PubMed] [Google Scholar]
- 12.Vaccarella S, Dal Maso L, Laversanne M, Bray F, Plummer M, Franceschi S. The impact of diagnostic changes on the rise in thyroid cancer incidence: a population-based study in selected high-resource countries. Thyroid. 2015;25(10):1127-1136. doi: 10.1089/thy.2015.0116 [DOI] [PubMed] [Google Scholar]
- 13.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. ; US Preventive Services Task Force . Screening for thyroid cancer: US Preventive Services Task Force recommendation statement. JAMA. 2017;317(18):1882-1887. doi: 10.1001/jama.2017.4011 [DOI] [PubMed] [Google Scholar]
- 14.Suzuki S. Diagnosis; thyroid nodules In: Kitanoka M, Suzuki S, eds. Thyroid Ultrasound—A Guidebook for Diagnosis and Management [in Japanese]. Tokyo, Japan: Nankodo; 2012:28-29. [Google Scholar]
- 15.Suzuki S, Yamashita S, Fukushima T, et al. The protocol and preliminary baseline survey results of the thyroid ultrasound examination in Fukushima [rapid communication]. Endocr J. 2016;63(3):315-321. doi: 10.1507/endocrj.EJ15-0726 [DOI] [PubMed] [Google Scholar]
- 16.Fukushima Medical University Thyroid ultrasound examination (preliminary baseline screening): supplemental report of the FY 2016 survey. http://fmu-global.jp/download/thyroid-ultrasound-examination-supplemental-report-of-the-fy-2016-surveypreliminary-baseline-screening/?wpdmdl=2690. Accessed October 18, 2018.
- 17.Fukushima Medical University Report of second-round thyroid ultrasound examinations (first full-scale thyroid screening program). http://fmu-global.jp/download/thyroid-ultrasound-examinations-first-full-scale-thyroid-screening-program/?wpdmdl=3608. Accessed October 18, 2018.
- 18.Katanoda K, Kamo K, Tsugane S. Quantification of the increase in thyroid cancer prevalence in Fukushima after the nuclear disaster in 2011—a potential overdiagnosis? Jpn J Clin Oncol. 2016;46(3):284-286. doi: 10.1093/jjco/hyv191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fay MP. Estimating age conditional probability of developing disease from surveillance data. Popul Health Metr. 2004;2(1):6. doi: 10.1186/1478-7954-2-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Cancer Center, Epidemiology and Prevention Division Cancer prediction (2015-2039) [in Japanese]. https://ganjoho.jp/data/reg_stat/statistics/dl/cancer_prediction(2015-2039).xlsx. Accessed October 18, 2018.
- 21.Takamura N, Orita M, Saenko V, Yamashita S, Nagataki S, Demidchik Y. Radiation and risk of thyroid cancer: Fukushima and Chernobyl. Lancet Diabetes Endocrinol. 2016;4(8):647. doi: 10.1016/S2213-8587(16)30112-7 [DOI] [PubMed] [Google Scholar]
- 22.Ohtsuru A, Midorikawa S, Suzuki S, et al. Five-year interim report of thyroid ultrasound examination in the Fukushima Health Management Survey In: Yamashita S, Thomas G, eds. Thyroid Cancer and Nuclear Accidents: Long-term Aftereffects of Chernobyl and Fukushima. Amsterdam, the Netherlands: Academic Press/Elsevier; 2017:145-153. doi: 10.1016/B978-0-12-812768-1.00014-9 [DOI] [Google Scholar]
- 23.Kinoshita N, Sueki K, Sasa K, et al. Assessment of individual radionuclide distributions from the Fukushima nuclear accident covering central-east Japan. Proc Natl Acad Sci U S A. 2011;108(49):19526-19529. doi: 10.1073/pnas.1111724108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishikawa T, Yasumura S, Ozasa K, et al. The Fukushima Health Management Survey: estimation of external doses to residents in Fukushima Prefecture. Sci Rep. 2015;5:12712. doi: 10.1038/srep12712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohira T, Takahashi H, Yasumura S, et al. ; Fukushima Health Management Survey Group . Comparison of childhood thyroid cancer prevalence among 3 areas based on external radiation dose after the Fukushima Daiichi nuclear power plant accident: the Fukushima Health Management Survey. Medicine (Baltimore). 2016;95(35):e4472. doi: 10.1097/MD.0000000000004472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohira T, Takahashi H, Yasumura S, et al. ; Fukushima Health Management Survey Group . Associations between childhood thyroid cancer and external radiation dose after the Fukushima Daiichi Nuclear Power Plant Accident. Epidemiology. 2018;29(4):e32-e34. doi: 10.1097/EDE.0000000000000839 [DOI] [PubMed] [Google Scholar]
- 27.Mitsutake N, Fukushima T, Matsuse M, et al. BRAF(V600E) mutation is highly prevalent in thyroid carcinomas in the young population in Fukushima: a different oncogenic profile from Chernobyl. Sci Rep. 2015;5(5):16976. doi: 10.1038/srep16976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki S, Suzuki S, Fukushima T, et al. Comprehensive survey results of childhood thyroid ultrasound examinations in Fukushima in the first five years after the Fukushima Daiichi nuclear power plant accident. Thyroid. 2016;26(6):843-851. doi: 10.1089/thy.2015.0564 [DOI] [PubMed] [Google Scholar]
- 29.Thomas GA, Bunnell H, Cook HA, et al. High prevalence of RET/PTC rearrangements in Ukrainian and Belarussian post-Chernobyl thyroid papillary carcinomas: a strong correlation between RET/PTC3 and the solid-follicular variant. J Clin Endocrinol Metab. 1999;84(11):4232-4238. [DOI] [PubMed] [Google Scholar]
- 30.Leeman-Neill RJ, Brenner AV, Little MP, et al. RET/PTC and PAX8/PPARγ chromosomal rearrangements in post-Chernobyl thyroid cancer and their association with iodine-131 radiation dose and other characteristics. Cancer. 2013;119(10):1792-1799. doi: 10.1002/cncr.27893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vuong HG, Altibi AM, Abdelhamid AH, et al. The changing characteristics and molecular profiles of papillary thyroid carcinoma over time: a systematic review. Oncotarget. 2017;8(6):10637-10649. doi: 10.18632/oncotarget.12885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuse M, Yabuta T, Saenko V, et al. TERT promoter mutations and Ki-67 labeling index as a prognostic marker of papillary thyroid carcinomas: combination of two independent factors. Sci Rep. 2017;7:41752. doi: 10.1038/srep41752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furuya-Kanamori L, Bell KJL, Clark J, Glasziou P, Doi SAR. Prevalence of differentiated thyroid cancer in autopsy studies over six decades: a meta-analysis. J Clin Oncol. 2016;34(30):3672-3679. doi: 10.1200/JCO.2016.67.7419 [DOI] [PubMed] [Google Scholar]
- 34.Bondeson L, Ljungberg O. Occult papillary thyroid carcinoma in the young and the aged. Cancer. 1984;53(8):1790-1792. doi: [DOI] [PubMed] [Google Scholar]
- 35.Shimura H, Sobue T, Takahashi H, et al. ; Thyroid Examination Unit of the Radiation Medical Center for the Fukushima Health Management Survey Group . Findings of thyroid ultrasound examination within 3 years after the Fukushima Nuclear Power Plant Accident: the Fukushima Health Management Survey. J Clin Endocrinol Metab. 2018;103(3):861-869. doi: 10.1210/jc.2017-01603 [DOI] [PubMed] [Google Scholar]
- 36.Midorikawa S, Ohtsuru A, Murakami M, et al. Comparative analysis of the growth pattern of thyroid cancer in young patients screened by ultrasonography in Japan after a nuclear accident: The Fukushima Health Management Survey [published online November 16, 2017]. JAMA Otolaryngol Head Neck Surg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi H, Takahashi K, Shimura H, et al. Simulation of expected childhood and adolescent thyroid cancer cases in Japan using a cancer-progression model based on the National Cancer Registry: application to the first-round thyroid examination of the Fukushima Health Management Survey. Medicine (Baltimore). 2017;96(48):e8631. doi: 10.1097/MD.0000000000008631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin JS, Bowles EJA, Williams SB, Morrison CC. Screening for thyroid cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317(18):1888-1903. doi: 10.1001/jama.2017.0562 [DOI] [PubMed] [Google Scholar]
- 39.Hori M, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H; Japan Cancer Surveillance Research Group . Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45(9):884-891. doi: 10.1093/jjco/hyv088 [DOI] [PubMed] [Google Scholar]
- 40.Davis S, Kopecky KJ, Hamilton TE, Onstad L; Hanford Thyroid Disease Study Team . Thyroid neoplasia, autoimmune thyroiditis, and hypothyroidism in persons exposed to iodine 131 from the Hanford nuclear site. JAMA. 2004;292(21):2600-2613. doi: 10.1001/jama.292.21.2600 [DOI] [PubMed] [Google Scholar]
- 41.Takebe K, Date M, Yamamoto Y, Ogino T. Minimal thyroid cancer detected in mass screening with ultrasonography [in Japanese]. Endocr Surg. 1997;14(3):181-184. [Google Scholar]
- 42.Haugen BR, Alexander EK, Bible KC, et al. ; The American Thyroid Association Guideline Task Force on Thyroid Nodules and Differentiated Thyroid Cancer . 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi: 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605-613. doi: 10.1093/jnci/djq099 [DOI] [PubMed] [Google Scholar]
- 44.SHAMISEN ST1—lessons learned from dosimetric and health screening, evacuation and health surveillance: executive summary. https://www.irsn.fr/FR/Actualites_presse/Actualites/Documents/IRSN_Shamisen-recommendation-guide_201709.pdf. Accessed October 18, 2018.
- 45.Midorikawa S, Tanigawa K, Suzuki S, Ohtsuru A. Psychosocial issues related to thyroid examination after a radiation disaster. Asia Pac J Public Health. 2017;29(2_suppl):63S-73S. doi: 10.1177/1010539516686164 [DOI] [PubMed] [Google Scholar]
- 46.Midorikawa S, Ohtsuru A, Suzuki S, et al. Psychosocial impact on the thyroid examination of the Fukushima Health Management Survey In: Yamashita S, Thomas G, eds. Thyroid Cancer and Nuclear Accidents: Long-term Aftereffects of Chernobyl and Fukushima. Amsterdam, the Netherlands: Academic Press/Elsevier; 2017:165-173. doi: 10.1016/B978-0-12-812768-1.00016-2 [DOI] [Google Scholar]