Key Points
Question
Is there an association between psychological stress and uveitis?
Findings
In this cross-sectional, case-control study of 120 patients with uveitis, having uveitis was associated with a significantly higher score on the 10-question Perceived Stress Scale in comparison with individuals serving as nonuveitic controls.
Meaning
These findings suggest that uveitis is associated with higher levels of stress compared with controls without ocular disease; consequently, patients with uveitis may benefit from increased focus on the psychological effect of this disease, especially if future longitudinal studies show that these findings have a cause-and-effect association.
Abstract
Importance
Uveitis involves dysregulation of the ocular immune system. Stress has been shown to affect immune function, but it is unclear whether there is an association between stress and uveitis.
Objective
To determine whether having uveitis is associated with psychological stress.
Design, Setting, and Participants
A cross-sectional, case-control study including a self-administered survey, medical records review, and diurnal salivary cortisol test was conducted at a university-based uveitis clinic and comprehensive eye clinic. Participants included 146 consecutive adults with noninfectious uveitis and age-matched controls with no eye disease. The study was conducted from December 1, 2017, to March 14, 2018.
Main Outcomes and Measures
Participants completed the self-administered, Cohen 10-item Perceived Stress Scale (PSS-10), a demographics questionnaire. Responses to each question were categorized on a 5-point Likert scale, with total scores ranging from 0 (no stress) to 40 (high stress). In addition, participants submitted 3 salivary cortisol samples. Those with uveitis were classified as having recently active or controlled disease through medical records review. The prespecified primary analysis was a linear regression of PSS-10 score and uveitis correcting for age, sex, educational level, employment, and median income. Secondary analyses included comparing PSS-10 scores in patients with recently active and controlled uveitis, determining predictors of stress, and comparing diurnal salivary cortisol between uveitis and control groups.
Results
Of 146 eligible patients, 17 declined participation and 9 consented but were excluded because they did not complete both questionnaires, resulting in 120 patients (80 uveitis; 40 controls) in the final analysis. Eighty participants (66.7%) were women, and 70 (58.3%) were white. Median age was 40 years (interquartile range, 29-59 years). Having uveitis was associated with a 4.3-point increase in PSS-10 score (95% CI, 1.8 to 6.9; P = .002). There was no significant difference in PSS-10 scores between patients with recently active and controlled uveitis (1.0 point greater for patients with active uveitis; 95% CI, −2.0 to 3.9; P = .52). Factors associated with increased PSS-10 score in patients with uveitis included female sex (coefficient, 4.0; 95% CI, 1.6 to 6.5; P = .002), current immunomodulatory therapy (coefficient, 2.5; 95% CI, −0.3 to 5.2; P = .08), history of depression (coefficient, 3.8; 95% CI, 0.8 to 6.8; P = .02), and having posterior or panuveitis (coefficient, 2.6; 95% CI, 0.8 to 4.4; P = .006). Of the 70 participants (58.3%) who had testable samples for cortisol analysis, diurnal salivary cortisol levels did not significantly differ between uveitis and nonuveitis groups.
Conclusions and Relevance
These findings suggest that patients with uveitis have higher levels of psychological stress compared with controls, yet no significant difference was identified in the stress of patients with active vs controlled uveitis. Consequently, comprehensive treatment for noninfectious uveitis may be able to address the psychological results of this disease.
This case-control study examines the association between uveitis and psychological stress in patients with active and controlled uveitis.
Introduction
Uveitis is a significant cause of ocular morbidity that accounts for 10% to 15% of blindness in the United States.1 Most uveitis cases worldwide are presumed to be immune-mediated and noninfectious.2 In many instances, uveitis is associated with autoimmune disease.2,3 Animal models and human peripheral blood specimens suggest that uveitis may involve dysregulation of regulatory T lymphocytes in the eye.4,5,6,7,8,9 The negative consequences of uveitis on vision are well known, but the effect on mental health has not been as well elucidated.10,11,12
An abundance of data support a connection between psychological stress and altered immune function. It has been shown that short-term stress can activate the hypothalamic-pituitary-adrenal axis, leading to redistribution of leukocytes to tissues.13,14,15,16,17,18 Chronic stress can eventually lead to attenuated immune response over time.13,16,18,19,20 Hypothalamic-pituitary-adrenal axis-immune system coordination may be mediated by release of the stress hormone cortisol, which can be measured in saliva.18,21 Psychological stress has been associated with autoimmune disease activity, including diseases that may be associated with ocular inflammation.22,23,24,25,26,27 Several studies have evaluated stress in uveitis, predominantly in its anterior subtype.28,29,30,31,32 However, they have been retrospective and underpowered, and have produced inconsistent results.28,29,30,31,32,33,34
This study aimed to investigate the psychological stress levels of patients with noninfectious uveitis compared with a nonuveitic population, as well as whether demographics and clinical characteristics are predictors of psychological stress. The self-administered Cohen 10-item Perceived Stress Scale (PSS-10) questionnaire was used as a subjective, patient-reported measure of stress levels. Diurnal salivary cortisol measurement was used as an objective, biochemical measure of patients' stress levels. Overall, this study explored a psychosocial outcome of uveitis to highlight the nonocular effects of this disease.
Methods
Participants
This study included consecutive patients seen between December 1, 2017, and March 14, 2018, at the Francis I. Proctor Foundation at the University of California, San Francisco (UCSF) and the UCSF Comprehensive Optometry Clinic. Eligible patients were aged 18 years or older, English-speaking, and able to provide written informed consent. Patients were included in the uveitis group if they had a confirmed diagnosis of noninfectious uveitis and had a prior laboratory workup excluding infectious causes. Controls were individuals seen in the optometry clinic for general eye care who did not have any ocular abnormalities requiring prescription treatment. Controls were recruited by matching for 10-year age strata. Ethical approval of the study design was obtained from the UCSF Institutional Review Board. Participants provided written informed consent; there was no financial compensation.
Design and Procedures
Participants completed the Cohen PSS-10, a validated self-administered questionnaire, to assess psychological stress.35,36,37 Responses to each question were categorized on a 5-point Likert scale, with total scores ranging from 0 (no stress) to 40 (high stress). An additional questionnaire was created to record self-reported comorbid conditions, uveitis history, smoking and drinking status, current pain level on a 100-mm visual analog scale, and demographics, such as race/ethnicity, employment, and educational level. Participants were given the option to complete the questionnaires at the clinic or at home and return them by mail with a prepaid, preaddressed envelope.
Participants’ medical records were reviewed for best-corrected visual acuity, diagnosis of comorbidities, and use of psychiatric medication. The comorbid conditions recorded were heart disease, cancer, depression, anxiety, type 2 diabetes, hypertension, and systemic autoimmune diseases. Patients with uveitis were subgrouped as having recently active or controlled disease based on medical records review. The recently active subgroup was defined by any of the following in either eye within the past 90 days: greater than 0.5+ anterior chamber cell according to Standardization of Uveitis Nomenclature Working Group criteria,38 greater than 0.5+ vitreous haze according to the National Eye Institute vitreous haze scale,39 presence of an active retinal or choroidal lesion, or presence of cystoid macular edema as recorded by spectral-domain optical coherence tomography. The controlled subgroup was defined by absence of these criteria. Information on disease cause, anatomic location of inflammation, current treatments, and recent ocular injections was collected.
Anatomic location of inflammation was reported as anterior, intermediate, anterior and intermediate, posterior, and panuveitis using the Standardization of Uveitis Nomenclature classification system.38 Visual acuity using a Snellen eye chart was recorded for each eye, then converted to a logMAR scale.40 Low vision of count fingers, hand motion, light perception, and no light perception were recorded as logMAR 1.7, 1.8, 1.9, and 2.0, respectively. Median household income by zip code served as a proxy for patient socioeconomic status and was determined using the 2010 US census.41
Patients performed an at-home saliva collection for measuring salivary cortisol levels as a biometric indicator of stress. At enrollment, patients were given a collection kit with instructions to collect their saliva 3 times during 1 day within 3 weeks of their appointment and return the samples to UCSF through priority mail. Patients were instructed to collect saliva on wakening, 30 minutes after wakening, and before bed. Patients recorded the exact times of collection and returned the recorded times with the kit. Samples were stored at −20°C on arrival at UCSF. Samples were thawed and analyzed in duplicate on a 96-well plate using an expanded-range, high-sensitivity salivary cortisol enzyme-linked immunoassay kit (Salimetrics). The enzyme-linked immunoassay kit has been tested for the off-target effects of prednisolone, prednisone, and cortisone, which have cross-reactivities of 0.568%, less than 0.004%, and 0.130%, respectively.42 Prior data suggest that topical corticosteroids should not affect systemic cortisol levels.43 Oral corticosteroids should minimally cross-react with the enzyme-linked immunoassay but could affect the hypothalamic-pituitary-adrenal axis.
Statistical Analysis
The sample size was determined for the prespecified primary analysis, which was a linear regression of the PSS-10 score and uveitis correcting for significant covariates from the most-recent normative data set of Americans answering the PSS-10 questionnaire.44 Using an α level of .05, a sample size of 120 patients gave us greater than 90% power to detect a 4-point difference (one-half of an SD in the normative data set) in the PSS-10 score between the uveitis and control groups. Participants were recruited in a ratio of 1 recently active subgroup patient to 1 controlled uveitis subgroup patient to 1 control patient to allow for greater than 90% power to detect a 4-point difference in the PSS-10 score between patients with recently active and controlled disease. Participants were included in the analysis if they provided informed consent and completed both questionnaires within 3 weeks of enrollment.
The Fisher exact test, Mann-Whitney test, and t test were used to compare baseline characteristics. Visualization of the distribution of PSS-10 scores was performed using the Epanechnikov kernel function with a bandwidth of 1.5951. The prespecified primary analysis was a linear regression model predicting the PSS-10 score, with the main predictor being diagnosis of uveitis, adjusting for age, employment, sex, income, and educational level. A subanalysis comparing PSS-10 scores between the recently active uveitis, controlled uveitis, and nonuveitis groups used linear regression with the same covariates as the primary analysis. Predictors of PSS-10 scores in patients with uveitis were assessed in a multivariable model using backward stepwise linear regression retaining variables with a P < .10 (likelihood ratio test). Association between PSS-10 score and logMAR best-corrected visual acuity in the better-seeing eye was assessed using Spearman rank correlation.
Measures of diurnal salivary cortisol levels were compared between the uveitis and control groups using linear regression predicting the diurnal cortisol measure, with the main predictor being uveitis diagnosis, correcting for oral corticosteroid use as a dichotomous variable. Samples were used to calculate the following measures: awakening cortisol response (ACR) (difference between cortisol at wakening and 30 minutes after wakening), a measure of how much cortisol is released from waking to its daily peak; diurnal slope (difference between evening cortisol and 30 minutes after wakening cortisol over time between samples), an estimate of the rate of decline in cortisol release from peak to nadir; and normalized area under the curve with respect to ground (AUCg) (total AUC of all data points divided by total waking hours), which is an estimation of total daily cortisol release.
Statistical tests were 2-sided and a P value of <.05 was considered significant. For the secondary analysis comparing recently active uveitis, inactive uveitis, and nonuveitis controls, a P value of <.02 was considered significant. Statistical analyses were conducted in Stata, version 14 (StataCorp).
Results
Of 146 patients eligible for the study, 17 declined participation and 9 consented but did not complete both questionnaires, resulting in 120 consecutive patients (80 uveitis; 40 controls) included in this analysis. Eighty participants (66.7%) were women, and 70 (58.3%) were white. Median age was 40 years (interquartile range, 29-59 years). Demographic characteristics were predominantly balanced between the uveitis and control groups (Table 1). The uveitis group had worse converted logMAR best-corrected visual acuity in the better-seeing eye (uveitis group: median, 0.1; interquartile range [IQR], 0-0.25]; control group: median, 0; IQR, 0-0; P < .001) and greater mean (SD) pain score on a 100-mm visual analog scale (16.2 [23.5] vs 6.9 [17.0] mm; P = .02). Patients with uveitis were more likely to have an autoimmune condition (37 patients [46.3%]) compared with the controls (3 patients [7.5%]) (P < .001). However, the groups did not differ significantly in other comorbidities, with a median of 1 comorbid condition (IQR, 1-1) in the control group and 1 comorbid condition (IQR, 0-1) in the uveitis group (P = .25).
Table 1. Demographic and Clinical Characteristics of Uveitis and Control Groups.
| Variable | No. (%) | |
|---|---|---|
| Control (n = 40) | Uveitis (n = 80) | |
| Women | 30 (75.0) | 50 (62.5) |
| Age, mean (SD), ya | 44.1 (16.9) | 44.4 (17.3) |
| Race/ethnicity | ||
| White | 26 (65.0) | 44 (55.0) |
| Black | 2 (5.0) | 7 (8.8) |
| Asian | 7 (17.5) | 17 (21.3) |
| Hispanic | 2 (5.0) | 6 (7.5) |
| Other | 1 (2.5) | 1 (1.3) |
| Multiracial | 2 (5.0) | 5 (6.3) |
| Current smoker | 1 (2.5) | 4 (5.0) |
| Household income, median (IQR), $ | 97 090.50 (79 672.00- 122 266.00) |
82 553 (65 121.50- 123 282.00) |
| Marital status | ||
| Single | 22 (55.0) | 31 (39) |
| Married | 18 (45.0) | 43 (54) |
| Other | 0 | 6 (38.8) |
| Living situation | ||
| Alone | 4 (10.0) | 11 (13.8) |
| With others | 36 (90.0) | 69 (86.3) |
| Educational level | ||
| High school or less | 0 | 7 (8.8) |
| Some college | 9 (22.5) | 29 (36.3) |
| Bachelor’s degree | 15 (37.5) | 27 (33.8) |
| Professional or graduate degree | 15 (37.5) | 27 (33.8) |
| Drinks per week, mean (SD) | 2 (3) | 2 (3) |
| Have dependents | 6 (15.0) | 23 (28.8) |
| Comorbid conditions | ||
| Depression | 5 (12.5) | 16 (20.0) |
| Anxiety | 5 (12.5) | 15 (18.8) |
| Systemic autoimmune disease | 3 (7.5) | 37 (46.3) |
| No. of all other comorbid conditions, median (IQR)b | 1 (1-1) | 1 (0-1) |
| Work status | ||
| Unemployed | 3 (7.5) | 6 (7.5) |
| Full-time | 23 (57.5) | 45 (56.3) |
| Parttime | 5 (12.5) | 9 (11.3) |
| Retired | 7 (17.5) | 14 (17.5) |
| Homemaker | 0 | 2 (2.5) |
| Other | 2 (5.0) | 4 (5.0) |
| VAS pain (0-10), mean (SD) | 6.9 (17.0) | 16.2 (23.5) |
| Converted logMAR vision in the better seeing eye, median (IQR) | 0 (0-0) | 0.1 (0-0.25) |
Abbreviations: IQR, interquartile range; VAS, visual analog scale.
Groups were matched for age by 10-year age strata.
Excluding systemic autoimmune diseases.
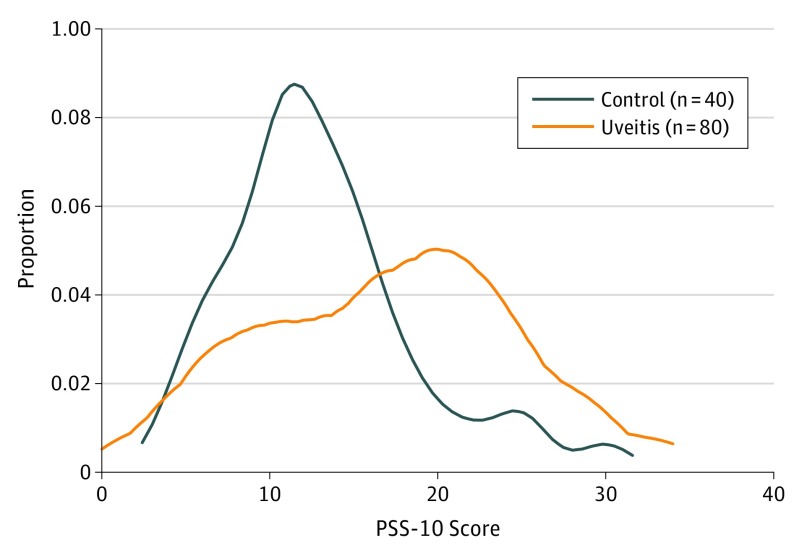
The mean PSS-10 score was 13.1 (5.6) in the control group and 17.0 (7.5) in the uveitis group (Figure 1). The prespecified primary model found that having uveitis was associated with a 4.3-point increase in the PSS-10 score (95% CI, 1.8-6.9; P = .002; 1000 permutations). Additionally controlling for the presence of a comorbid systemic autoimmune disease also found that having uveitis was significantly associated with an increase in the PSS-10 score (coefficient, 3.7; 95% CI, 0.9-6.4; P = .01).
Figure 1. Distribution of Cohen 10-Item Perceived Stress Scale (PSS-10) Scores in Uveitis and Nonuveitis Groups.
Kernel density estimate (Epanechnikov kernel function, bandwidth, 1.5951) of PSS-10 scores in patients with uveitis and nonuveitic controls.
Stress With Recently Active Uveitis, Controlled Uveitis, and Nonuveitis
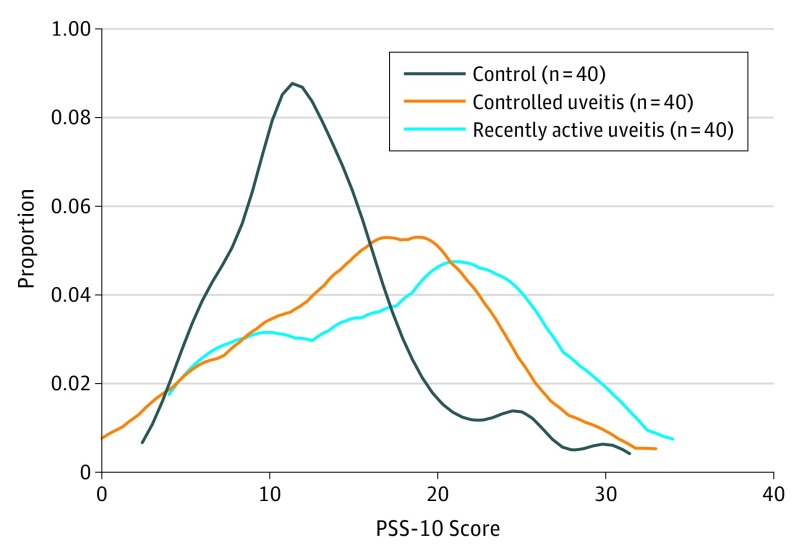
The mean PSS-10 score was 18.0 (7.8) in the recently active uveitis subgroup and 16.0 (7.3) in the controlled uveitis subgroup (Figure 2). In comparison with nonuveitic controls, having recently active uveitis was associated with a 4.8-point increase in the PSS-10 score (95% CI, 1.9 to 7.7; P = .001) and having controlled uveitis was associated with a 3.8-point increase in the PSS-10 score (95% CI, 0.9 to 6.8; P = .01). There was only a 1.0-point, nonsignificant difference in PSS-10 score between recently active and controlled uveitis subgroups (95% CI, −2.0 to 3.9; P = .52).
Figure 2. Distribution of Cohen 10-Item Perceived Stress Scale (PSS-10) Scores in Recently Active Uveitis, Controlled Uveitis, and Nonuveitis Groups.
Kernel density estimate (Epanechnikov kernel function, bandwidth, 1.5951) of PSS-10 scores in patients with recently active uveitis (active inflammation in the past ≤90 days), controlled uveitis, and nonuveitic controls.
Predictors of Stress in Patients With Uveitis
A hypothesis-generating analysis was performed using backward stepwise linear regression of PSS-10 score in patients with uveitis (Table 2) with demographic and clinical predictors (eTable in the Supplement). Backward stepwise linear regression found that the following predictors were associated with significant increases in the PSS-10 score: female sex (coefficient, 4.0; 95% CI, 1.6 to 6.5; P = .002), a history of depression (coefficient, 3.8; 95% CI, 0.8 to 6.8; P = .02), having posterior or panuveitis compared with having anterior or intermediate uveitis (coefficient, 2.6; 95% CI, 0.8 to 4.4; P = .006), and current immunomodulatory therapy (coefficient, 2.5; 95% CI, −0.3 to 5.2; P = .08). A 1-standard drink per week increase in average alcohol consumption was associated with a decrease in the PSS-10 score (coefficient, −0.5; 95% CI, −0.8 to −0.1; P = .01). Spearman correlation between converted logMAR best-corrected visual acuity in the better eye and PSS-10 score found a Spearman r of 0.25 (P = .03).
Table 2. Multivariable Predictors of PSS-10 Score in Patients With Uveitis.
| Variable | Coefficient (95% CI) | P Value |
|---|---|---|
| Female sex | 4.0 (1.6 to 6.5) | .002 |
| Immunomodulatory therapy | 2.5 (−0.3 to 5.2) | .08 |
| Diagnosis of depression | 3.8 (0.8 to 6.8) | .02 |
| No. of standard drinks of alcohol per week | −0.5 (−0.8 to −0.1) | .01 |
| Anatomic location of uveitis (posterior/panuveitis vs anterior/intermediate) | 2.6 (0.8 to 4.4) | .006 |
Abbreviation: PSS-10, Cohen 10-item Perceived Stress Scale.
Measures of Diurnal Salivary Cortisol
Eighty-five patients (70.8%) completed the saliva collection: 56 (70.0%) in the uveitis group and 29 (72.5%) in the control group. Four patient sample sets with inadequate volume for testing were excluded. Six patients’ sample sets were excluded because the first 2 samples were taken less than 15 or greater than 45 minutes apart and would not accurately represent the diurnal cortisol peak. After excluding samples that had a pH outside the testing range (3.5-9.0),42 44 patients (55.0%) in the uveitis group and 26 patients (65.0%) in the control group had complete cortisol test data for analysis, summing to a total of 70 patients (58.3%) who completed a per protocol saliva collection with analyzable samples.
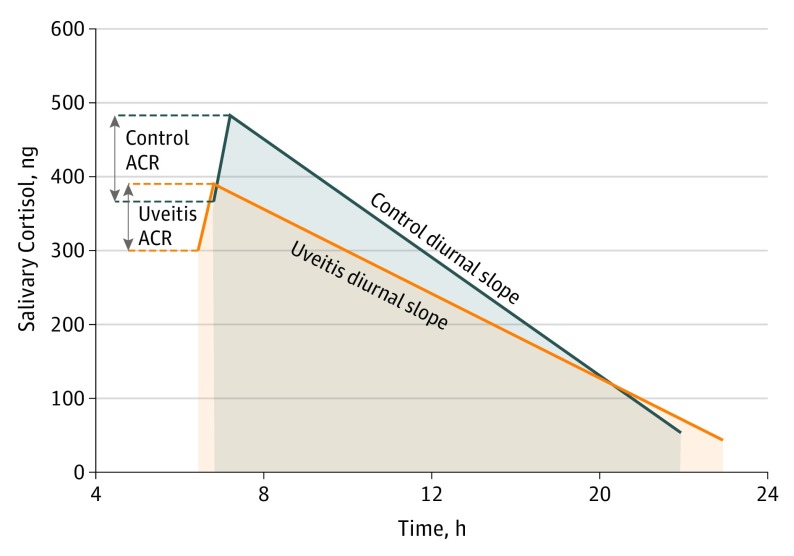
Three measures of diurnal cortisol levels were calculated. Raw cortisol data indicated controls had a mean (SD) awakening cortisol response of 118 (303) ng/dL, diurnal slope of −41.0 (57.0) ng/dL• h, and a normalized AUCg of 275 (154) ng/dL. Patients with uveitis had a mean ACR of 92 (166 ng/dL), diurnal slope of −23.0 (17.0) ng/dL• h, and an AUCg of 220 (135) ng/dL (Figure 3). All comparisons were corrected for oral corticosteroid use. The awakening cortisol response did not differ in patients with and without uveitis, with a mean ACR response 4 ng/dL lower in the uveitis group (95% CI, 117 ng/dL lower, 110 ng/dL higher; P = .95). Patients with uveitis had a mean 15-ng/dL• h higher diurnal slope than patients without uveitis (95% CI, 4 ng/dL• h lower, 34 ng/dL• h higher; P = .11). Mean normalized AUCg was 33 ng/dL lower in patients with uveitis compared with nonuveitic controls (95% CI, 103 ng/dL lower, 36 ng/dL higher; P = .34). As a sensitivity analysis, we included baseline cortisol level in the regression model for each diurnal cortisol level measure, and the difference between the uveitis and control groups remained nonsignificant for all 3 measures.
Figure 3. Measures of Diurnal Cortisol Levels in Uveitis and Nonuveitis Groups.
Summary of diurnal salivary cortisol measures in patients with uveitis and nonuveitic controls. ACR indicates awakening cortisol response.
Discussion
The results of this cross-sectional, case-control study suggest that uveitis is associated with greater psychological stress compared with nonuveitis as measured by the PSS-10 questionnaire, yet there was no statistically significant difference in PSS-10 scores between patients with controlled and those with recently active uveitis. Multivariable analysis found that female sex, a history of depression, posterior or panuveitis, and immunomodulatory therapy were associated with increased PSS-10 scores. No measure of diurnal cortisol levels (awakening cortisol response, diurnal slope, or AUCg) was significantly different between the uveitis and nonuveitis groups.
Although no formal clinically meaningful difference has been proposed for the PSS-10 questionnaire, the difference in PSS-10 scores found in our study is comparable to that noted in 2 randomized trials of psychological interventions where a 5-point change was thought to be meaningful.45,46 Because patients in the uveitis group were being treated in our clinic, it is possible that the association between uveitis and the PSS-10 scores was lowered owing to better control of their disease and that patients with less-optimal control of their inflammation could have worse PSS-10 scores.
Prior studies have found that ocular inflammation is associated with negative outcomes in mental health. Patients with a uveitis score lower on the Medical Outcomes Study 36-Item Short Form Questionnaire and have higher rates of depression.11,12,31,32,47,48 However, the role of psychological stress in noninfectious uveitis is less clear. Most prior studies on this subject focused on recurrent acute anterior uveitis to determine if high stress is a risk factor for disease flares. One study compared symptoms of distress in 60 patients with idiopathic, recurrent acute anterior uveitis and a group of patients without ocular disease.28 If any recurrent acute anterior uveitis relapsed in any patient within 12 months, they were reinterviewed to assess for recent changes in distress. Similar to our study, that study found no significant difference in distress between patients in remission and those experiencing acute recurrence of uveitis, but noted a significant difference between the patients with uveitis and controls. The authors postulated that the increase in distress among patients with uveitis was not related to disease activity, but rather represented a response to the general experience of having a chronic disease. However, only 15 patients in this study experienced a recurrence, so the sample of patients with active uveitis was small.
Two other studies of patients with recurrent acute anterior uveitis suggested that patients with uveitis have higher levels of psychological distress, lower disease-specific quality of life, and less variety in coping strategies.30,33 While all of these studies were limited by small size and low generalizability, overall, they support the idea that psychological stress may play a role in uveitis. Still, it is unclear whether stress is a trigger for uveitis activity or a result of the additional anxieties that come with having a chronic, vision-compromising disease. Our study cannot determine whether stress is a trigger or a result of uveitis. However, the findings contribute to the growing body of evidence suggesting that patients with uveitis appear to have higher levels of stress compared with nonuveitic controls and would benefit from additional attention to their psychological well-being.
We also identified predictors of greater stress. Traditional demographics, such as age, race/ethnicity, educational level, work status, and income did not explain as much of the variability in PSS-10 scores compared with prior studies using the questionnaire.44 However, median household income by zip code is a weak proxy for socioeconomic status, and this variable may have not fully captured the association with a key confounder that is a known contributor to psychological stress.44 The predictors of the PSS-10 score that we found are consistent with reported predictors of health- and vision-related quality of life in patients with uveitis.49,50 Prior analyses suggest that visual acuity is associated with poorer quality of life, but consistent with our study, it is a weak to moderate predictor.49,50,51,52 The low number of predictors associated with PSS-10 scores illustrates the complexity of the concept being evaluated. Psychological stress is a subjective and heterogeneous experience that can be affected by many factors, not all of which can be captured by standardized survey data.
Limitations and Strengths
The strengths of this study include the use of a control group, collection of an objective biometric measure of stress, and a relatively large and adequately powered sample size. These findings should be validated by additional assessments to better understand the connection between stress, immune dysregulation, and the eye.
This study has limitations. The design is cross-sectional and the questionnaire is subject to recall bias. Our primary analysis is associative—not causative—and therefore we cannot declare that stress is a risk factor for the development or recurrence of uveitis. A longitudinal study would help to better characterize the association between stress and uveitis. In addition, it is challenging to find an appropriate control for this kind of investigation. It is possible that patients could have been receiving other systemic medications, which could be a confounding factor for stress.
In addition, our study found no significant differences in measures of diurnal salivary cortisol between the uveitis and control groups. This aim required patient participation in an at-home saliva collection. Despite frequent reminders, adherence was low, with 70.8% of all patients mailing their samples and only 70 (58.3%) completing the saliva collection per protocol with analyzable samples. Moreover, per-protocol participation was evaluated by patient self-report, which likely overestimates the number of collections completed correctly. There were also mail delays affecting cortisol analysis despite use of priority postage, especially around US holiday times. Further studies are needed to investigate whether cortisol levels are different in patients with uveitis.
Conclusions
The findings of this study suggest that patients with uveitis exhibit higher levels of stress compared with nonuveitic controls; and the findings suggest recommending heightened awareness and attention to psychological stress in this population.
eTable. Demographic and Clinical Characteristics of Uveitis Group
References
- 1.Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: a literature survey. Br J Ophthalmol. 1996;80(9):844-848. doi: 10.1136/bjo.80.9.844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCannel CA, Holland GN, Helm CJ, Cornell PJ, Winston JV, Rimmer TG; UCLA Community-Based Uveitis Study Group . Causes of uveitis in the general practice of ophthalmology. Am J Ophthalmol. 1996;121(1):35-46. doi: 10.1016/S0002-9394(14)70532-X [DOI] [PubMed] [Google Scholar]
- 3.Rothova A, Buitenhuis HJ, Meenken C, et al. Uveitis and systemic disease. Br J Ophthalmol. 1992;76(3):137-141. doi: 10.1136/bjo.76.3.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ke Y, Jiang G, Sun D, Kaplan HJ, Shao H. Ocular regulatory T cells distinguish monophasic from recurrent autoimmune uveitis. Invest Ophthalmol Vis Sci. 2008;49(9):3999-4007. doi: 10.1167/iovs.07-1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerr EC, Raveney BJE, Copland DA, Dick AD, Nicholson LB. Analysis of retinal cellular infiltrate in experimental autoimmune uveoretinitis reveals multiple regulatory cell populations. J Autoimmun. 2008;31(4):354-361. doi: 10.1016/j.jaut.2008.08.006 [DOI] [PubMed] [Google Scholar]
- 6.Mochizuki M. [Intraocular inflammation and homeostasis of the eye] [Japanese]. Nippon Ganka Gakkai Zasshi. 2009;113(3):344-377. [PubMed] [Google Scholar]
- 7.Molins B, Mesquida M, Lee RW, Llorenç V, Pelegrín L, Adán A. Regulatory T cell levels and cytokine production in active non-infectious uveitis: in-vitro effects of pharmacological treatment. Clin Exp Immunol. 2015;179(3):529-538. doi: 10.1111/cei.12479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou W, Wu Z, Xiang X, Sun S, Zhang J. The expression and significance of T helper cell subsets and regulatory T cells CD4+ CD25+ in peripheral blood of patients with human leukocyte antigen B27-positive acute anterior uveitis. Graefes Arch Clin Exp Ophthalmol. 2014;252(4):665-672. doi: 10.1007/s00417-014-2567-9 [DOI] [PubMed] [Google Scholar]
- 9.Silver PB, Horai R, Chen J, et al. Retina-specific T regulatory cells bring about resolution and maintain remission of autoimmune uveitis. J Immunol. 2015;194(7):3011-3019. doi: 10.4049/jimmunol.1402650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiffman RM, Jacobsen G, Whitcup SM. Visual functioning and general health status in patients with uveitis. Arch Ophthalmol. 2001;119(6):841-849. doi: 10.1001/archopht.119.6.841 [DOI] [PubMed] [Google Scholar]
- 11.Miserocchi E, Modorati G, Mosconi P, Colucci A, Bandello F. Quality of life in patients with uveitis on chronic systemic immunosuppressive treatment. Ocul Immunol Inflamm. 2010;18(4):297-304. doi: 10.3109/09273941003637510 [DOI] [PubMed] [Google Scholar]
- 12.Qian Y, Glaser T, Esterberg E, Acharya NR. Depression and visual functioning in patients with ocular inflammatory disease. Am J Ophthalmol. 2012;153(2):370-378.e2. doi: 10.1016/j.ajo.2011.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhabhar FS. Acute stress enhances while chronic stress suppresses skin immunity. The role of stress hormones and leukocyte trafficking. Ann N Y Acad Sci. 2000;917(1):876-893. doi: 10.1111/j.1749-6632.2000.tb05454.x [DOI] [PubMed] [Google Scholar]
- 14.Dhabhar FS, Malarkey WB, Neri E, McEwen BS. Stress-induced redistribution of immune cells—from barracks to boulevards to battlefields: a tale of three hormones—Curt Richter Award winner. Psychoneuroendocrinology. 2012;37(9):1345-1368. doi: 10.1016/j.psyneuen.2012.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy PJ, Cryan JF, Quigley EMM, Dinan TG, Clarke G. A sustained hypothalamic-pituitary-adrenal axis response to acute psychosocial stress in irritable bowel syndrome. Psychol Med. 2014;44(14):3123-3134. doi: 10.1017/S003329171400052X [DOI] [PubMed] [Google Scholar]
- 16.Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci U S A. 1999;96(3):1059-1064. doi: 10.1073/pnas.96.3.1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morey JN, Boggero IA, Scott AB, Segerstrom SC. Current directions in stress and human immune function. Curr Opin Psychol. 2015;5:13-17. doi: 10.1016/j.copsyc.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11(4):286-306. doi: 10.1006/brbi.1997.0508 [DOI] [PubMed] [Google Scholar]
- 19.Yin D, Tuthill D, Mufson RA, Shi Y. Chronic restraint stress promotes lymphocyte apoptosis by modulating CD95 expression. J Exp Med. 2000;191(8):1423-1428. doi: 10.1084/jem.191.8.1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Chen L, Zhang Y, et al. Chronic stress promotes lymphocyte reduction through TLR2 mediated PI3K signaling in a β-arrestin 2 dependent manner. J Neuroimmunol. 2011;233(1-2):73-79. doi: 10.1016/j.jneuroim.2010.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine A, Zagoory-Sharon O, Feldman R, Lewis JG, Weller A. Measuring cortisol in human psychobiological studies. Physiol Behav. 2007;90(1):43-53. doi: 10.1016/j.physbeh.2006.08.025 [DOI] [PubMed] [Google Scholar]
- 22.O’Donovan A, Cohen BE, Seal KH, et al. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77(4):365-374. doi: 10.1016/j.biopsych.2014.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bricou O, Taïeb O, Baubet T, Gal B, Guillevin L, Moro MR. Stress and coping strategies in systemic lupus erythematosus: a review. Neuroimmunomodulation. 2006;13(5-6):283-293. doi: 10.1159/000104856 [DOI] [PubMed] [Google Scholar]
- 24.Cutolo M, Straub RH. Stress as a risk factor in the pathogenesis of rheumatoid arthritis. Neuroimmunomodulation. 2006;13(5-6):277-282. doi: 10.1159/000104855 [DOI] [PubMed] [Google Scholar]
- 25.Srivastava S, Boyer JL. Psychological stress is associated with relapse in type 1 autoimmune hepatitis. Liver Int. 2010;30(10):1439-1447. doi: 10.1111/j.1478-3231.2010.02333.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herpertz S, Johann B, Lichtblau K, et al. Patients with diabetes mellitus: psychosocial stress and use of psychosocial support: a multicenter study [German]. Med Klin (Munich). 2000;95(7):369-377. doi: 10.1007/s000630050014 [DOI] [PubMed] [Google Scholar]
- 27.Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ. 2004;328(7442):731. doi: 10.1136/bmj.38041.724421.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Secchi AG, Magni G, Tognon MS, et al. A psychosomatic approach to idiopathic recurrences of anterior uveitis. Am J Ophthalmol. 1987;104(2):174-178. doi: 10.1016/0002-9394(87)90011-0 [DOI] [PubMed] [Google Scholar]
- 29.Mulholland B, Marks M, Lightman SL. Anterior uveitis and its relation to stress. Br J Ophthalmol. 2000;84(10):1121-1124. doi: 10.1136/bjo.84.10.1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrim ZI, Ahmed TY, Taguri AH. The relationship between stress and acute anterior uveitis. Acta Ophthalmol Scand. 2006;84(6):795-798. doi: 10.1111/j.1600-0420.2006.00752.x [DOI] [PubMed] [Google Scholar]
- 31.Maca SM, Schiesser AW, Sobala A, et al. Distress, depression and coping in HLA-B27–associated anterior uveitis with focus on gender differences. Br J Ophthalmol. 2011;95(5):699-704. doi: 10.1136/bjo.2009.174839 [DOI] [PubMed] [Google Scholar]
- 32.Maca SM, Wagner J, Weingessel B, Vécsei-Marlovits PV, Gruber K, Schiesser AW. Acute anterior uveitis is associated with depression and reduction of general health. Br J Ophthalmol. 2013;97(3):333-337. doi: 10.1136/bjophthalmol-2012-302304 [DOI] [PubMed] [Google Scholar]
- 33.Franke GH, Schütte E, Heiligenhaus A. [Rehabilitation-psychological aspects of uveitis]. Psychother Psychosom Med Psychol. 2005;55(2):65-71. doi: 10.1055/s-2004-828504 [DOI] [PubMed] [Google Scholar]
- 34.Khanfer R, Wallace G, Keane PA, Phillips AC. Uveitis and psychological stress. Insight. 2012;37(2):11-16. [PubMed] [Google Scholar]
- 35.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396. doi: 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 36.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. Soc Psychol Heal. 1988;13:31-67. doi: 10.1111/j.1559-1816.1983.tb02325.x [DOI] [Google Scholar]
- 37.Lee EH. Review of the psychometric evidence of the perceived stress scale. Asian Nurs Res (Korean Soc Nurs Sci). 2012;6(4):121-127. doi: 10.1016/j.anr.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 38.Jabs DA, Nussenblatt RB, Rosenbaum JT; Standardization of Uveitis Nomenclature (SUN) Working Group . Standardization of uveitis nomenclature for reporting clinical data: results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509-516. doi: 10.1016/j.ajo.2005.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92(4):467-471. doi: 10.1016/S0161-6420(85)34001-0 [DOI] [PubMed] [Google Scholar]
- 40.Rathinam SR, Babu M, Thundikandy R, et al. A randomized clinical trial comparing methotrexate and mycophenolate mofetil for noninfectious uveitis. Ophthalmology. 2014;121(10):1863-1870. doi: 10.1016/j.ophtha.2014.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.United States Census Bureau https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml. Accessed March 21, 2018.
- 42.Salimetrics. Expanded range high sensitivity salivary cortisol enzyme immunoassay kit. Salimetrics.com. https://salimetrics.com/wp-content/uploads/2018/03/salivary-cortisol-elisa-kit.pdf. Accessed December 14, 2017.
- 43.Sandhu SS, Smith JM, Doherty M, James A, Figueiredo FC. Do topical ophthalmic corticosteroids suppress the hypothalamic-pituitary-adrenal axis in post-penetrating keratoplasty patients? Eye (Lond). 2012;26(5):699-702. doi: 10.1038/eye.2012.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen S, Janicki-Deverts D. Who’s stressed? distributions of psychological stress in the United States in probability samples from 1983, 2006, and 2009. J Appl Soc Psychol. 2012;42(6):1320-1334. doi: 10.1111/j.1559-1816.2012.00900.x [DOI] [Google Scholar]
- 45.Sood A, Prasad K, Schroeder D, Varkey P. Stress management and resilience training among department of medicine faculty: a pilot randomized clinical trial. J Gen Intern Med. 2011;26(8):858-861. doi: 10.1007/s11606-011-1640-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willert MV, Thulstrup AM, Hertz J. Changes in stress and coping from a randomized controlled trial of a three-month stress management intervention. Scand J Work Environ Health. 2009;35(2):145-152. doi: 10.5271/sjweh.1313 [DOI] [PubMed] [Google Scholar]
- 47.Maca SM, Amirian A, Prause C, Gruber K, Mejdoubi L, Barisani-Asenbauer T. Understanding the impact of uveitis on health-related quality of life in adolescents. Acta Ophthalmol. 2013;91(3):e219-e224. doi: 10.1111/aos.12016 [DOI] [PubMed] [Google Scholar]
- 48.Onal S, Savar F, Akman M, Kazokoglu H. Vision- and health-related quality of life in patients with Behçet uveitis. Arch Ophthalmol. 2010;128(10):1265-1271. doi: 10.1001/archophthalmol.2010.209 [DOI] [PubMed] [Google Scholar]
- 49.Frick KD, Drye LT, Kempen JH, et al. ; Multicenter Uveitis Steroid Treatment-MUST Trial Research Group . Associations among visual acuity and vision- and health-related quality of life among patients in the multicenter uveitis steroid treatment trial. Invest Ophthalmol Vis Sci. 2012;53(3):1169-1176. doi: 10.1167/iovs.11-8259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niemeyer KM, Gonzales JA, Rathinam SR, et al. Quality-of-life outcomes from a randomized clinical trial comparing antimetabolites for intermediate, posterior, and panuveitis. Am J Ophthalmol. 2017;179:10-17. doi: 10.1016/j.ajo.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jampel HD, Frick KD, Janz NK, et al. ; CIGTS Study Group . Depression and mood indicators in newly diagnosed glaucoma patients. Am J Ophthalmol. 2007;144(2):238-244. doi: 10.1016/j.ajo.2007.04.048 [DOI] [PubMed] [Google Scholar]
- 52.Brody BL, Gamst AC, Williams RA, et al. Depression, visual acuity, comorbidity, and disability associated with age-related macular degeneration. Ophthalmology. 2001;108(10):1893-1900. doi: 10.1016/S0161-6420(01)00754-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Demographic and Clinical Characteristics of Uveitis Group