Abstract
Importance
Current treatment options for progressive ovarian cancer provide limited benefit, particularly in patients whose disease has become resistant to platinum-based chemotherapy.
Objective
To assess the efficacy and safety of avelumab, an anti–programmed death-ligand 1 agent, in a cohort of patients with previously treated recurrent or refractory ovarian cancer.
Design, Setting, and Participants
In an expansion cohort of a phase 1b, open-label study (JAVELIN Solid Tumor), 125 patients with advanced ovarian cancer who had received chemotherapy including a platinum agent were enrolled between November 6, 2013, and August 27, 2015. Statistical analysis was performed from December 31, 2016, to October 9, 2018.
Intervention
Patients received avelumab, 10 mg/kg, every 2 weeks until disease progression, unacceptable toxic effects, or withdrawal from the study.
Main Outcomes and Measures
Prespecified end points in this cohort included confirmed best overall response (per Response Evaluation Criteria In Solid Tumors, version 1.1), immune-related best overall response, duration of response, progression-free survival, overall survival, results of programmed death-ligand 1 expression–based analyses, and safety.
Results
A total of 125 women (median age, 62.0 years [range, 27-84 years]) who had received a median of 3 prior lines of treatment (range, 0-10) for advanced disease were enrolled in the study. Patients received avelumab for a median of 2.8 months (range, 0.5-27.4 months), with a median follow-up of 26.6 months (range, 16-38 months). A confirmed objective response occurred in 12 patients (9.6%; 95% CI, 5.1%-16.2%), including a complete response in 1 patient (0.8%) and a partial response in 11 patients (8.8%). The 1-year progression-free survival rate was 10.2% (95% CI, 5.4%-16.7%) and median overall survival was 11.2 months (95% CI, 8.7-15.4 months). Infusion-related reactions occurred in 25 patients (20.0%). Other frequent treatment-related adverse events (any grade event occurring in ≥10% of patients) were fatigue (17 [13.6%]), diarrhea (15 [12.0%]), and nausea (14 [11.2%]). Grade 3 or higher treatment-related adverse events occurred in 9 patients (7.2%), of which only the level of lipase increased (3 [2.4%]) occurred in more than 1 patient. Twenty-one patients (16.8%) had an immune-related adverse event of any grade. No treatment-related deaths occurred.
Conclusions and Relevance
Avelumab demonstrated antitumor activity and acceptable safety in heavily pretreated patients with recurrent or refractory ovarian cancer.
Trial Registration
ClinicalTrials.gov identifier: NCT01772004
This phase 1b open-label study assesses the efficacy and safety of avelumab, an anti–programmed death ligand 1 agent, in a cohort of patients with previously treated recurrent or refractory ovarian cancer.
Key Points
Question
Does avelumab have clinical activity in the treatment of recurrent or refractory ovarian cancer?
Findings
In this phase 1b cohort study, 125 patients with heavily pretreated ovarian cancer (median, 3 prior lines) received avelumab, 10 mg/kg, every 2 weeks. The objective response rate was 9.6%, complete response occurred in 1 patient (0.8%), the 1-year progression-free survival rate was 10.2%, median overall survival was 11.2 months, grade 3 or 4 treatment-related adverse events occurred in 7.2% of patients, and immune-related adverse events occurred in 16.8% of patients.
Meaning
Avelumab demonstrated antitumor activity and an acceptable safety profile in heavily pretreated patients with recurrent or refractory ovarian cancer.
Introduction
Ovarian cancer is the most common cause of death among gynecologic malignant neoplasms in the United States and is responsible for 5% of cancer-related deaths in women.1 Approximately 239 000 new cases are diagnosed worldwide annually2 and more than 70% of US patients are diagnosed with late-stage disease, largely owing to the absence of effective screening.3 Ovarian cancer is a heterogeneous disease with various subtypes that have varying histologic characteristics, molecular characteristics, and prognosis.4 Platinum-based chemotherapy, with or without bevacizumab, is the standard-of-care first-line treatment,5 although rates of relapse are high (approximately 70% within 3 years).6 Clear cell carcinoma, in particular, responds poorly to chemotherapy and has a poorer prognosis than cancers with more common histologic types.7,8 Standard therapies for platinum-resistant or refractory ovarian cancer, including pegylated liposomal doxorubicin, weekly paclitaxel, and topotecan, provide limited benefits,3,5,6 and overall survival (OS) in patients with relapsed disease who have received multiple lines of prior treatment is particularly short (eg, median, 10.6 months with fourth-line chemotherapy vs 3.3 months without treatment).9 Standard chemotherapy regimens are also associated with a high level of toxic effects. Poly-(ADP [adenosine diphosphate]-ribose) polymerase (PARP) inhibitors have efficacy in patients with BRCA-mutated (BRCA1: OMIM, 113705; and BRCA2: OMIM, 600185) recurrent ovarian cancer10,11 or with high-grade serous and endometrioid tumors, irrespective of BRCA status, when used as switch-maintenance treatment after platinum-sensitive recurrence.12,13,14 Additional treatment options are needed to prolong OS and improve quality of life in patients with advanced ovarian cancer regardless of their treatment history.
Increasing evidence indicates that immune responses may influence patient outcomes in ovarian cancer.15 In particular, the presence of tumor-infiltrating lymphocytes, especially CD8+ tumor-infiltrating lymphocytes, is associated with a better prognosis.15,16,17,18 Furthermore, ovarian tumor cells often express the immune checkpoint protein programmed death-ligand 1 (PD-L1) and tumor-infiltrating lymphocytes often express its receptor (programmed cell death 1 [PD-1]).19,20 The interaction between PD-1 and PD-L1 is a key therapeutic target for reactivating immune responses against multiple cancers21,22,23; thus, agents targeting this interaction could provide therapeutic benefit in ovarian cancer.
Avelumab is a human immunoglobulin G1 monoclonal antibody with a wild-type Fc region that blocks PD-L1.24 In addition to activating adaptive immune responses by inhibiting interactions between PD-L1 and PD-1, preclinical models suggest that avelumab may also activate innate immune effector cells.25 Avelumab has been well tolerated and associated with durable clinical activity in various types of tumors, including advanced non–small cell lung cancer and urothelial carcinoma that progressed after platinum-based chemotherapy.26,27,28 Avelumab has been approved in various countries for the treatment of metastatic Merkel cell carcinoma and for locally advanced or metastatic urothelial carcinoma that has progressed during or after platinum-containing chemotherapy.29,30
We report phase 1b data from the JAVELIN Solid Tumor trial in patients with recurrent or refractory ovarian cancer and disease progression after platinum-based chemotherapy.
Methods
Study Design and Patients
The JAVELIN Solid Tumor trial (protocol in Supplement 1) is an ongoing phase 1, global, open-label trial. In the dose-expansion cohort reported here, eligible patients enrolled between November 6, 2013, and August 27, 2015, had stage III to IV epithelial ovarian, fallopian tube, or peritoneal cancer (according to American Joint Committee on Cancer [Cancer Staging Manual, 7th edition]/Union for International Cancer Control TNM [TNM Classification of Malignant Tumours, 6th edition]31 and International Federation of Gynecology and Obstetrics Staging System, seventh edition)32 and recurrent or refractory disease (defined as progression within 6 months of platinum-based chemotherapy [ie, platinum-resistant disease] or progression after subsequent therapy in patients whose disease had previously relapsed). Patients who had progressed after adjuvant therapy or therapy for metastatic disease were eligible. Other eligibility criteria included age 18 years or older, Eastern Cooperative Oncology Group performance status of 0 or 1, estimated life expectancy more than 3 months, and adequate hematologic, hepatic, and renal function values. All patients were required to have a fresh or archival tumor specimen for assessment of PD-L1 expression, but eligibility was independent of tumor PD-L1 status. Exclusion criteria included prior treatment with a T-cell–targeting immune checkpoint inhibitor, other cancer diagnosis within 5 years, rapidly progressive disease, central nervous system metastases, and known autoimmune disease.27
The trial was conducted in accordance with the ethics principles of the Declaration of Helsinki33 and the International Council on Harmonisation Guidelines on Good Clinical Practice. The protocol was approved by the institutional review board or independent ethics committee of each center (eTable 1 in Supplement 2). All patients provided written informed consent before enrollment.
Procedures and Assessments
All patients received avelumab, 10 mg/kg, via 1-hour intravenous infusion every 2 weeks until disease progression, unacceptable toxic effects, or any other protocol-based criteria for withdrawal from the study occurred.27 Dose modifications were not permitted. Premedication with an antihistamine (eg, diphenhydramine hydrochloride, 25 to 50 mg) and acetaminophen, 500 to 650 mg (modified per local standards), was administered 30 to 60 minutes before all infusions of avelumab. Grade 2 adverse events (AEs) were managed by treatment delays; events that did not resolve to grade 1 or lower by the end of the next treatment cycle or that recurred led to permanent discontinuation of avelumab therapy (except for hormone insufficiencies that could be managed by replacement therapy).
Safety was assessed at each biweekly trial visit. Safety assessments included assessment of AEs, physical examination, clinical laboratory tests (hematology, hepatic panels, and serum chemistry), and documentation of concurrent medications. Adverse events and laboratory abnormalities were classified and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.34 A serious AE was defined as any untoward event that was life threatening, required hospitalization, resulted in disability, was a congenital anomaly, or resulted in death. Immune-related AEs were identified using a prespecified list of Medical Dictionary for Regulatory Activities35 terms followed by comprehensive medical review.
Clinical activity was assessed by investigators using Response Evaluation Criteria In Solid Tumors (RECIST), version 1.1, and modified immune-related response criteria to determine the best overall response.36 Radiographic tumor assessments were performed at baseline and then every 6 weeks. For patients who had a partial response (PR) or complete response (CR), a confirmatory computed tomographic scan or magnetic resonance imaging scan was performed no sooner than 28 days later and preferably at the scheduled 6-week interval.
Expression of PD-L1 was assessed using a proprietary immunohistochemistry assay (Dako PD-L1 IHC 73-10 pharmDx) based on an anti–PD-L1 rabbit monoclonal antibody clone (73-10) under license to Merck KGaA.26,37 Collection of blood samples for cancer antigen 125 (CA125) testing was mandatory and performed before the first administration of avelumab, at week 7, and then once every 6 weeks. Germline BRCA1/2 mutational status was collected where available, but was not mandatory for this study.
Outcomes
Prespecified end points in this expansion cohort of the JAVELIN Solid Tumor trial included best overall response per investigator assessment (defined as best response per RECIST, version 1.1, obtained among all tumor assessments after the start of treatment with avelumab until documented progression of disease), immune-related best overall response, duration of response (defined as the time from first documented PR or CR until disease progression or death, whichever occurred first), progression-free survival (PFS), OS, evaluation of PD-L1 expression in tumors, and safety (including incidence and severity of AEs).
Statistical Analysis
Statistical analysis was performed from December 31, 2016, to October 9, 2018. The sample size of this expansion cohort was based on planned enrollment of at least 120 patients to provide 95% Clopper-Pearson CIs for potential objective response rates (ORRs; proportion of patients with a PR or CR; eg, 10% [95% CI, 5.3%-16.8%] or 20% [95% CI, 13.3%-28.3%]). Safety and activity were analyzed in all patients who received 1 or more doses of avelumab. Follow-up duration was defined as time from the start of treatment to data cutoff. Change in the sum of target lesion diameters from baseline over time was evaluated in patients with baseline tumor assessments and 1 or more assessments after baseline. Time-to-event end points were estimated using the Kaplan-Meier method, and 95% CIs for the median were calculated using the Brookmeyer-Crowley method. P values for associations between categorical variables were determined using the Fisher exact test. All P values were from 2-sided tests and results were deemed statistically significant at P < .05. Change in CA125 level was based on the lowest value while receiving treatment compared with the baseline value.
Results
Patients
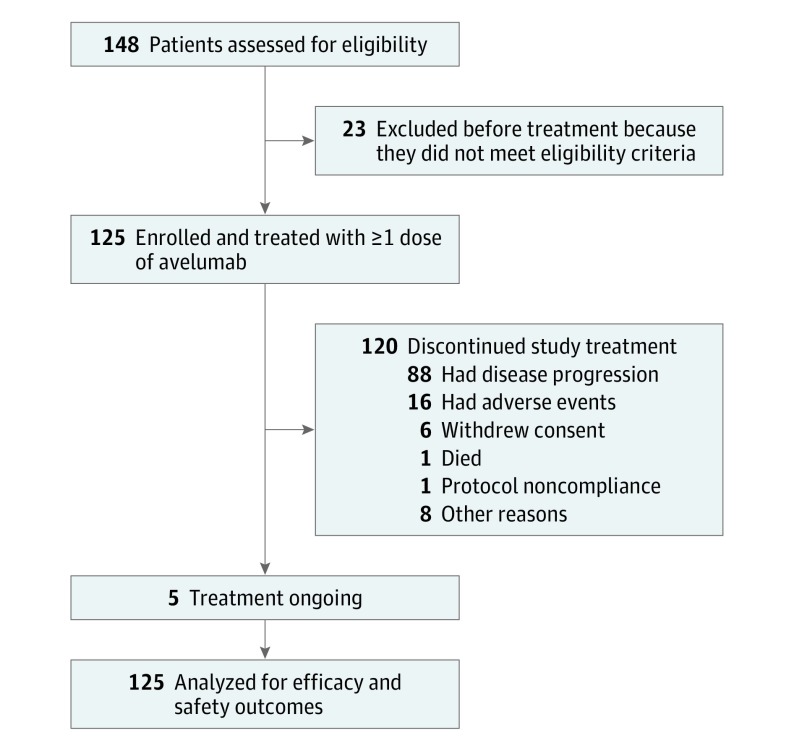
Between November 6, 2013, and August 27, 2015, a total of 125 women were enrolled and received avelumab. At the data cutoff date of December 31, 2016, median follow-up was 26.6 months (range, 16-38 months) (Figure 1). Median patient age was 62.0 years (range, 27-84 years) and the most common tumor histologic type was serous of any grade (93 [74.4%]; Table 1). Prior to initiating avelumab, all patients had received standard chemotherapy including a platinum agent. The median number of prior lines of treatment for metastatic or locally advanced disease was 3 (range, 0-10); 5 patients (4.0%) had received prior treatment in the adjuvant setting only; 81 patients (64.8%) had received 3 or more prior lines of treatment for locally advanced or metastatic disease; and 53 patients (42.4%) had received 4 or more prior lines of treatment. Of 46 patients in whom germline BRCA1/2 status was available, tumors were BRCA mutated in 8 patients (17.4%) and BRCA wildtype in 38 (82.6%). Based on a PD-L1 cutoff of 1% or more in tumor cells, 76 patients (60.8%) had a PD-L1–positive tumor. Based on a PD-L1 cutoff of 5% or more in tumor cells, 32 patients (25.6%) had a PD-L1–positive tumor. Based on a PD-L1 cutoff of 10% or more in tumor-infiltrating immune cells, 16 patients (12.8%) had a PD-L1–positive tumor. The PD-L1 status was not evaluable in 11 patients (8.8%). The median duration of avelumab treatment was 2.8 months (range, 0.5-27.4 months) and patients received a median of 6 administrations of avelumab (range, 1-57). Five patients (4.0%) continued to receive avelumab treatment after the data cutoff date. The most common reason for treatment discontinuation was disease progression (88 [70.4%]); other reasons were AEs (16 [12.8%]), death (1 [0.8%]), withdrawal of consent (6 [4.8%]), nonadherence to the protocol (1 [0.8%]), and other (8 [6.4%]).
Figure 1. Study Flowchart.
Table 1. Demographic and Baseline Characteristics of Patients.
| Characteristic | Valuea (N = 125) |
|---|---|
| Age, median (range), y | 62.0 (27-84) |
| <65 | 74 (59.2) |
| ≥65 | 51 (40.8) |
| ECOG performance status | |
| 0 | 60 (48.0) |
| 1 | 65 (52.0) |
| Time since first diagnosis, median (range), y | 4.0 (0.8-24.3) |
| Time since diagnosis of metastatic disease, median (range), y | 2.4 (0.03-17.2) |
| No. of prior anticancer therapy lines for metastatic or locally advanced disease | |
| 0 | 5 (4.0) |
| 1 | 14 (11.2) |
| 2 | 25 (20.0) |
| 3 | 28 (22.4) |
| 4 | 22 (17.6) |
| ≥5 | 31 (24.8) |
| Median (range) | 3 (0-10) |
| Histologic type | |
| Serous | 93 (74.4) |
| Mucinous | 4 (3.2) |
| Endometrioid | 3 (2.4) |
| Clear cell | 2 (1.6) |
| Transitional cell | 1 (0.8) |
| Other | 3 (2.4) |
| Uncoded or missing | 19 (15.2) |
| CA125 level, IU/mL | |
| <35 | 8 (6.4) |
| 35-70 | 13 (10.4) |
| >70 | 52 (41.6) |
| Unavailable | 52 (41.6) |
| BRCA mutation status | |
| Negative | 38 (30.4) |
| Positiveb | 8 (6.4) |
| Unknown | 79 (63.2) |
Abbreviations: CA125, cancer antigen 125; ECOG, Eastern Cooperative Oncology Group.
SI conversion factor: To convert CA125 to kilounits per liter, multiply by 1.0.
Data are presented as number (percentage) of patients unless otherwise indicated.
Deleterious mutation or mutation of uncertain significance.
Antitumor Activity
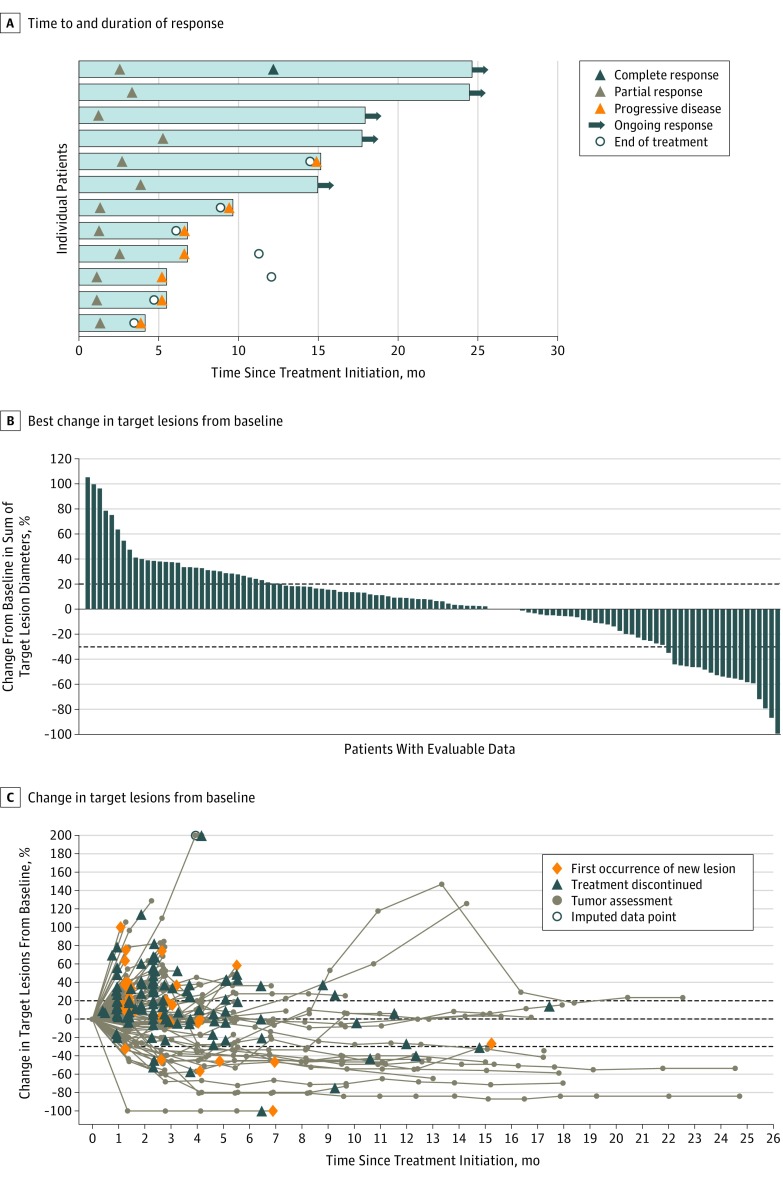
Of 125 patients, 12 had a confirmed objective response (ORR, 9.6%; 95% CI, 5.1%-16.2%), including CR in 1 patient (0.8%) and PR in 11 patients (8.8%) (Table 2). The median time to response was 8.9 weeks (range, 5.3-23.6 weeks) and median duration of response was 10.4 months (95% CI, 4.2-not estimable); 5 patients had an ongoing response at the data cutoff date (Figure 2A). In addition, 53 patients (42.4%) had stable disease of any duration as the best response, resulting in a disease control rate of 52.0%. The immune-related ORR was 12.8% (n = 16), consisting of 1 immune-related CR and 15 immune-related PRs. The median time to immune-related response was 8.9 weeks (range, 5.3-23.6 weeks). Of 114 patients evaluable for change in tumor size, 43 (37.7%) had a reduction of any level vs baseline and 19 (16.7%) had a reduction of 30% or more (Figure 2B and C), including 7 patients who did not meet the criteria for confirmed objective response because of lack of confirmation (n = 2), or because of new lesions, progressive disease in nontarget lesions, or increase in target lesions prior to maximum shrinkage (n = 7), which is suggestive of potential pseudoprogression.
Table 2. Confirmed Objective Response.
| Response | Patients, No. (%) (N = 125) |
|---|---|
| Best overall response | |
| Complete response | 1 (0.8) |
| Partial response | 11 (8.8) |
| Stable disease | 53 (42.4) |
| Progressive disease | 51 (40.8) |
| Not evaluable | 9 (7.2) |
| ORR (95% CI), % | 9.6 (5.1-16.2) |
| Disease control rate | 65 (52.0) |
| Immune-related best overall response | |
| Complete response | 1 (0.8) |
| Partial response | 15 (12.0) |
| Stable disease | 61 (48.8) |
| Progressive disease | 27 (21.6) |
| Not evaluable | 21 (16.8) |
| Immune-related ORR (95% CI), % | 12.8 (7.5-20.0) |
Abbreviation: ORR, objective response rate.
Figure 2. Antitumor Activity of Avelumab.
A, Time to and duration of response (n = 12). B, Best change in target lesions from baseline in patients with evaluable data (n = 114). Each bar represents a patient. Dotted lines indicate changes in target lesion size of −30% and +20%. C, Change in target lesions from baseline over time in patients with evaluable data (n = 114). Dotted lines indicate the changes in target lesion size of −30%, 0%, and +20%. Imputed data point is 205.0%.
The median PFS was 2.6 months (95% CI, 1.4-2.8 months), the 6-month PFS rate was 16.1% (95% CI, 10.1%-23.4%), and the 12-month PFS rate was 10.2% (95% CI, 5.4%-16.7%), with the Kaplan-Meier curve appearing to plateau up to 24 months (eFigure, A, in Supplement 2). The median OS was 11.2 months (95% CI, 8.7-15.4 months) and the 12-month OS rate was 47.0% (95% CI, 37.6%-55.7%) (eFigure, B, in Supplement 2).
Of 2 patients enrolled with clear cell tumors, 1 had a PR and the other had an immune-related PR. Of the other 10 patients who had an objective response, the tumor histologic type was serous in 7, endometrioid in 2, and uncoded in 1. In patients with 1 or fewer prior lines of therapy for locally advanced or metastatic disease (n = 19), the ORR was 21.1% (n = 4), the 12-month PFS rate was 15.8% (95% CI, 3.9%-34.9%), and the median OS was 16.1 months (95% CI, 8.6-18.7 months). In patients with 2 prior lines of therapy for locally advanced or metastatic disease (n = 25), the ORR was 8.0% (n = 2), the 12-month PFS rate was 9.0% (95% CI, 1.6%-24.7%), and the median OS was 11.1 months (5.7-19.8 months). In patients with 3 or more prior lines of therapy for locally advanced or metastatic disease (n = 81), the ORR was 7.4% (n = 6), the 12-month PFS rate was 9.5% (95% CI, 4.0%-17.8%), and the median OS was 10.2 months (6.1-15.3 months). In a post hoc analysis of efficacy based on the best response to last prior platinum-based chemotherapy regimen (in patients for whom this information was available), the ORR was 5.3% (4 of 75 patients; 95% CI, 1.5%-13.1%) in the subgroup with refractory disease (stable disease or disease progression on last prior platinum-based chemotherapy regimen) and 13.6% (3 of 22 patients; 95% CI, 2.9%-34.9%) in the subgroup with resistant disease (CR or PR on last prior platinum-based chemotherapy regimen).
Biomarker Analyses
Responses occurred in patients irrespective of tumor PD-L1 status, with no notable trends (eTable 2 in Supplement 2). Based on a PD-L1 expression cutoff of 1% or more for tumor cells, the ORR for patients with PD-L1–positive tumors was 11.8% (9 of 76 patients), median PFS was 2.7 months, and median OS was 13.8 months. Based on a PD-L1 expression cutoff of 1% or more for tumor cells, the ORR for patients with PD-L1–negative tumors was 7.9% (3 of 38 patients), median PFS was 1.4 months, and median OS was 7.0 months. Based on a PD-L1 expression cutoff of 5% or more for tumor cells, the ORR for patients with PD-L1–positive tumors was 12.5% (4 of 32 patients), median PFS was 2.7 months, and median OS was 10.6 months. Based on a PD-L1 expression cutoff of 5% or more for tumor cells, the ORR for patients with PD-L1–negative tumors was 9.8% (8 of 82 patients), median PFS was 2.2 months, and median OS was 11.9 months. Analyses of higher cutoffs for PD-L1 expression in tumor cells were not informative because few patients had tumors with high-level PD-L1 expression (≥25% in 3 patients and ≥50% in 2 patients). In analyses of PD-L1 expression in tumor-infiltrating immune cells (≥10% cutoff; ie, those with <10% PD-L1 expression in immune cells were included in the PD-L1–negative subgroup), patients in the PD-L1–positive subgroup had an ORR of 0% (0 of 16 patients), median PFS of 1.5 months, and median OS of 11.1 months, and patients in the PD-L1–negative subgroup had an ORR of 12.2% (12 of 98 patients), median PFS of 2.6 months, and median OS of 11.9 months.
Among the 73 patients evaluable for CA125 levels, the concentration increased in 58 patients (79.5%) and decreased in 15 patients (20.5%). Among 12 patients with an objective response, the CA125 concentration increased in 0 patients, decreased in 7 patients (58.3%), and was not obtained in 5 patients (41.7%). For patients with a BRCA-mutated tumor and evaluable data (n = 8), the ORR was 12.5% (n = 1), and for patients with BRCA-wildtype tumors (n = 38), the ORR was 7.9% (n = 3).
Safety
Most patients (122 [97.6%]) had an AE of any grade, including 86 patients (68.8%) who had a treatment-related AE of any grade (Table 3). Infusion-related reactions and related symptoms occurred in 25 patients (20.0%). Other frequent treatment-related AEs of any grade (≥10%) were fatigue (17 [13.6%]), diarrhea (15 [12.0%]), and nausea (14 [11.2%]). Nine patients (7.2%) had a grade 3 or 4 treatment-related AE, of which only a lipase-level increase (3 [2.4%]) occurred in more than 1 patient; no patient had a grade 3 or higher infusion-related reaction. Twenty-one patients (16.8%) had an immune-related AE, which was grade 3 in 3 patients (2.4%); no patient had a grade 4 or 5 immune-related AE (Table 3). Forty-two patients (33.6%) had a serious AE, which was related to treatment in 3 patients (2.4%; 1 patient had type 2 diabetes, 1 patient had peripheral and localized edema, and 1 patient had noncardiac chest pain, pyrexia, flushing, and dyspnea). Fourteen patients (11.2%) had an AE that led to death; none of the deaths were treatment related.
Table 3. Treatment-Related Adverse Events, Infusion-Related Reactions, and Immune-Related Adverse Eventsa,b.
| Adverse Event | Patients, No. (%) (N = 125) | |
|---|---|---|
| Any Grade | Grade 3 or 4 | |
| Any treatment-relatedc | 86 (68.8) | 9 (7.2) |
| Fatigue | 17 (13.6) | 0 |
| Diarrhea | 15 (12.0) | 0 |
| Nausea | 14 (11.2) | 1 (0.8) |
| Rash | 9 (7.2) | 1 (0.8) |
| Lipase-level increase | 3 (2.4) | 3 (2.4) |
| Amylase-level increase | 2 (1.6) | 1 (0.8) |
| Peripheral edema | 2 (1.6) | 1 (0.8) |
| Localized edema | 2 (1.6) | 1 (0.8) |
| Colitis | 1 (0.8) | 1 (0.8) |
| Type 2 diabetes | 1 (0.8) | 1 (0.8) |
| Myositis | 1 (0.8) | 1 (0.8) |
| Any infusion-related reactiond | 25 (20.0) | 0 |
| Any immune-related adverse event | 21 (16.8) | 3 (2.4) |
| Colitis | 1 (0.8) | 1 (0.8) |
| Type 2 diabetes | 1 (0.8) | 1 (0.8) |
| Myositis | 1 (0.8) | 1 (0.8) |
| Hypothyroidism | 8 (6.4) | 0 |
| Maculopapular rash | 3 (2.4) | 0 |
| Rash | 2 (1.6) | 0 |
| Autoimmune hypothyroidism | 1 (0.8) | 0 |
| Pruritus | 1 (0.8) | 0 |
| Diarrhea | 1 (0.8) | 0 |
| Erythema | 1 (0.8) | 0 |
| Pneumonitis | 1 (0.8) | 0 |
| Psoriasis | 1 (0.8) | 0 |
| Thyroiditis | 1 (0.8) | 0 |
Any grade in 10% or more of patients or grade 3 or higher in any patient.
Any grade in any patient.
The incidence of treatment-related infusion-related reaction based on the single Medical Dictionary for Regulatory Activities preferred term is not listed.
Composite term, which includes adverse events categorized as infusion-related reaction, drug hypersensitivity, or hypersensitivity reaction that occurred on the day of infusion or day after infusion, in addition to signs and symptoms of infusion-related reaction that occurred on the same day of infusion and resolved within 2 days (including adverse events classified by investigators as related or unrelated to treatment).
Discussion
In this large phase 1b study, avelumab showed antitumor activity in patients with heavily pretreated recurrent or refractory ovarian cancer that progressed after platinum-based chemotherapy (28 patients [22.4%] received avelumab as fourth-line treatment and 22 [17.6%] patients received avelumab as fifth-line treatment). The ORR was 9.6% and responses were durable (median, 10.4 months). Results of biomarker studies suggested that neither PD-L1 status nor BRCA status was associated with response, which is a novel finding. Very few patients had tumors with high-level PD-L1 expression, which is associated with an increased probability of clinical benefit with anti–PD-1 or anti–PD-L1 treatment of non–small cell lung cancer.38,39,40 Of 2 patients who had clear cell carcinoma, which is known to be chemoresistant, 1 patient had a PR and the other had an immune-related PR. The overall disease control rate was 52.0%, the 1-year PFS rate was 10.2%, and PFS appeared to plateau out to 2 years, consistent with prolonged response or disease control in a subset of patients. Median OS was 11.2 months (12-month OS rate, 47.0%). In subgroup analyses, patients with less pretreatment appeared to have greater clinical benefit: in 19 patients with 1 or fewer prior lines of treatment for locally advanced or metastatic disease, the ORR was 21.1%, the 12-month PFS rate was 15.8%, and the median OS was 16.1 months. Avelumab also showed an acceptable safety profile, including a low rate of grade 3 or higher treatment-related AEs (7.2%) and immune-related AEs of any grade (16.8%). We were unable to draw conclusions about whether response was more likely in patients with immune-related AEs because of the small number of patients and the confounding effects of treatment duration, although this concept has been investigated in a larger cohort with various tumors using a dedicated statistical method.41 Overall, our data provide the rationale for further studies of avelumab in ovarian cancer.
The data reported here for avelumab are generally consistent with those of previously reported studies of anti–PD-1 or anti–PD-L1 agents in advanced ovarian cancer, although direct comparisons are hindered by differences in patient characteristics and study sizes. In a phase 1b study of pembrolizumab in 26 patients with PD-L1–positive ovarian cancer and prior treatment failure, of whom 39% had received 5 or more prior lines of treatment for recurrent or metastatic disease, the ORR was 11.5%, median PFS was 1.9 months, and median OS was 13.1 months.42 In a recently reported phase 2 study of pembrolizumab monotherapy in 376 patients with recurrent advanced ovarian cancer (13.0% had received ≥5 prior lines for recurrent or metastatic disease) who were not selected for PD-L1 expression, the ORR was 8.0%.43 In a single-center study of nivolumab in 20 Japanese patients with platinum-resistant ovarian cancer, of whom 55% had received 4 or more prior chemotherapy regimens, the ORR was 15.0%, median PFS was 3.5 months, and median OS was 20.0 months.44 In 9 patients with ovarian cancer treated with atezolizumab in a phase 1a dose-escalation study, of whom 92% had received 2 or more prior lines of therapy, the ORR was 22%, median PFS was 2.9 months, and median OS was 11.3 months.45
Limitations
This study had some limitations. Interpretation of the findings is limited by its early-phase, single-arm design. Assessment of BRCA status was not mandatory and data were available in only a minority of patients. In addition, the limited number of responding patients hampers any analysis of patient or tumor characteristics associated with response.
Conclusions
Although response and survival findings with avelumab monotherapy in this study are encouraging, it would be of interest to determine whether efficacy can be increased through combination or sequential regimens involving chemotherapy or PARP inhibitors. Two global phase 3 trials of avelumab in combination with chemotherapy have been initiated in patients with ovarian cancer. JAVELIN Ovarian 100 (NCT02718417) is a 3-arm trial comparing first-line carboplatin and paclitaxel chemotherapy given in combination with avelumab or given alone with or without avelumab maintenance therapy. In addition, JAVELIN Ovarian 200 (NCT02580058) is a 3-arm trial comparing avelumab or pegylated liposomal doxorubicin chemotherapy given alone or in combination in patients with platinum-resistant or platinum-refractory disease who have received 3 or fewer prior lines of therapy for platinum-sensitive disease and no prior systemic therapy for platinum-resistant disease.46 Several early-phase studies have been initiated combining anti–PD-1 and anti–PD-L1 antibodies with PARP inhibitors, including a phase 1b/2 trial, JAVELIN PARP Medley, which is enrolling a cohort of patients with recurrent platinum-sensitive ovarian cancer who will be treated with avelumab plus talazoparib (NCT03330405). Results from these ongoing studies will help to define an appropriate role for checkpoint inhibitors within the treatment of ovarian cancer.
Trial Protocol
eTable 1. Sites of Enrollment
eTable 2. Clinical Activity Based on PD-L1 Status in Evaluable Patients (N = 114)
eFigure. Kaplan-Meier Estimates of (A) Progression-Free Survival and (B) Overall Survival (N = 125)
Data Sharing Statement
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 3.Herzog TJ, Monk BJ. Bringing new medicines to women with epithelial ovarian cancer: what is the unmet medical need? Gynecol Oncol Res Pract. 2017;4:13. doi: 10.1186/s40661-017-0050-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurman RJ, Shih IeM. The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am J Pathol. 2016;186(4):733-747. doi: 10.1016/j.ajpath.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology: ovarian cancer. V2.2018. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed September 20, 2018.
- 6.Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C; ESMO Guidelines Working Group . Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi24-vi32. doi: 10.1093/annonc/mdt333 [DOI] [PubMed] [Google Scholar]
- 7.Crotzer DR, Sun CC, Coleman RL, Wolf JK, Levenback CF, Gershenson DM. Lack of effective systemic therapy for recurrent clear cell carcinoma of the ovary. Gynecol Oncol. 2007;105(2):404-408. doi: 10.1016/j.ygyno.2006.12.024 [DOI] [PubMed] [Google Scholar]
- 8.Takano M, Tsuda H, Sugiyama T. Clear cell carcinoma of the ovary: is there a role of histology-specific treatment? J Exp Clin Cancer Res. 2012;31:53. doi: 10.1186/1756-9966-31-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanker LC, Loibl S, Burchardi N, et al. ; AGO and GINECO study group . The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol. 2012;23(10):2605-2612. doi: 10.1093/annonc/mds203 [DOI] [PubMed] [Google Scholar]
- 10.Oza AM, Tinker AV, Oaknin A, et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: integrated analysis of data from Study 10 and ARIEL2. Gynecol Oncol. 2017;147(2):267-275. doi: 10.1016/j.ygyno.2017.08.022 [DOI] [PubMed] [Google Scholar]
- 11.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244-250. doi: 10.1200/JCO.2014.56.2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pujade-Lauraine E, Ledermann JA, Selle F, et al. ; SOLO2/ENGOT-Ov21 investigators . Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274-1284. doi: 10.1016/S1470-2045(17)30469-2 [DOI] [PubMed] [Google Scholar]
- 13.Mirza MR, Monk BJ, Herrstedt J, et al. ; ENGOT-OV16/NOVA Investigators . Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154-2164. doi: 10.1056/NEJMoa1611310 [DOI] [PubMed] [Google Scholar]
- 14.Coleman RL, Oza AM, Lorusso D, et al. ; ARIEL3 investigators . Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10106):1949-1961. doi: 10.1016/S0140-6736(17)32440-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goode EL, Block MS, Kalli KR, et al. ; Ovarian Tumor Tissue Analysis (OTTA) Consortium . Dose-response association of CD8+ tumor-infiltrating lymphocytes and survival time in high-grade serous ovarian cancer. JAMA Oncol. 2017;3(12):e173290. doi: 10.1001/jamaoncol.2017.3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203-213. doi: 10.1056/NEJMoa020177 [DOI] [PubMed] [Google Scholar]
- 17.Li J, Wang J, Chen R, Bai Y, Lu X. The prognostic value of tumor-infiltrating T lymphocytes in ovarian cancer. Oncotarget. 2017;8(9):15621-15631. doi: 10.18632/oncotarget.14919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124(2):192-198. doi: 10.1016/j.ygyno.2011.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gatalica Z, Snyder C, Maney T, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2965-2970. doi: 10.1158/1055-9965.EPI-14-0654 [DOI] [PubMed] [Google Scholar]
- 20.Darb-Esfahani S, Kunze CA, Kulbe H, et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget. 2016;7(2):1486-1499. doi: 10.18632/oncotarget.6429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974-1982. doi: 10.1200/JCO.2014.59.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longoria TC, Eskander RN. Immune checkpoint inhibition: therapeutic implications in epithelial ovarian cancer. Recent Pat Anticancer Drug Discov. 2015;10(2):133-144. doi: 10.2174/1574892810666150504121000 [DOI] [PubMed] [Google Scholar]
- 23.Menderes G, Schwab CL, Black J, Santin AD. The role of the immune system in ovarian cancer and implications on therapy. Expert Rev Clin Immunol. 2016;12(6):681-695. doi: 10.1586/1744666X.2016.1147957 [DOI] [PubMed] [Google Scholar]
- 24.Heery CR, O’Sullivan Coyne GH, Madan RA, et al. Phase I open-label, multiple ascending dose trial of MSB0010718C, an anti–PD-L1 monoclonal antibody, in advanced solid malignancies. J Clin Oncol. 2014;32(15 suppl):3064. doi: 10.1200/jco.2014.32.15_suppl.3064 [DOI] [Google Scholar]
- 25.Boyerinas B, Jochems C, Fantini M, et al. Antibody-dependent cellular cytotoxicity activity of a novel anti–PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res. 2015;3(10):1148-1157. doi: 10.1158/2326-6066.CIR-15-0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an anti–programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase 1b study. J Clin Oncol. 2017;35(19):2117-2124. doi: 10.1200/JCO.2016.71.6795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gulley JL, Rajan A, Spigel DR, et al. Avelumab for patients with previously treated metastatic or recurrent non–small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol. 2017;18(5):599-610. doi: 10.1016/S1470-2045(17)30240-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly K, Infante JR, Taylor MH, et al. Safety profile of avelumab in patients with advanced solid tumors: a pooled analysis of data from the phase 1 JAVELIN solid tumor and phase 2 JAVELIN Merkel 200 clinical trials. Cancer. 2018;124(9):2010-2017. doi: 10.1002/cncr.31293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bavencio (avelumab) injection [summary of product characteristics]. Darmstadt, Germany: Merck KGaA; 2017.
- 30.Bavencio (avelumab) injection [package insert]. Darmstadt, Germany: Merck KGaA; 2017.
- 31.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471-1474. doi: 10.1245/s10434-010-0985-4 [DOI] [PubMed] [Google Scholar]
- 32.Prat J; FIGO Committee on Gynecologic Oncology . Staging classification for cancer of the ovary, fallopian tube, and peritoneum: abridged republication of guidelines from the International Federation of Gynecology and Obstetrics (FIGO). Obstet Gynecol. 2015;126(1):171-174. doi: 10.1097/AOG.0000000000000917 [DOI] [PubMed] [Google Scholar]
- 33.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 34.National Cancer Institute, National Institutes of Health, US Dept of Health and Human Services Common terminology criteria for adverse events (CTCAE) version 4.03. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Published June 14, 2010. Accessed June 18, 2018.
- 35.Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20(2):109-117. doi: 10.2165/00002018-199920020-00002 [DOI] [PubMed] [Google Scholar]
- 36.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412-7420. doi: 10.1158/1078-0432.CCR-09-1624 [DOI] [PubMed] [Google Scholar]
- 37.Feng Z, Schlichting M, Helwig C, et al. Comparative study of two PD-L1 expression assays in patients with non-small cell lung cancer (NSCLC). J Clin Oncol. 2017;35(15 suppl):e20581. doi: 10.1200/JCO.2017.35.15_suppl.e20581 [DOI] [Google Scholar]
- 38.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1–positive, advanced non–small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. doi: 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 39.Rittmeyer A, Barlesi F, Waterkamp D, et al. ; OAK Study Group . Atezolizumab versus docetaxel in patients with previously treated non–small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265. doi: 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non–small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35(35):3924-3933. doi: 10.1200/JCO.2017.74.3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly K, Manitz J, Patel MR, et al. Association of efficacy and adverse events of special interest of avelumab in the JAVELIN solid tumor and JAVELIN Merkel 200 trials. J Clin Oncol. 2018;36(15_suppl):3057. doi: 10.1200/JCO.2018.36.15_suppl.3057 [DOI] [Google Scholar]
- 42.Varga A, Piha-Paul SA, Ott PA, et al. Pembrolizumab in patients (pts) with PD-L1–positive (PD-L1+) advanced ovarian cancer: updated analysis of KEYNOTE-028. J Clin Oncol. 2017;35(suppl):5513. doi: 10.1200/JCO.2017.35.15_suppl.5513 [DOI] [Google Scholar]
- 43.Matulonis UA, Shapira-Frommer R, Santin A, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: interim results from the phase 2 KEYNOTE-100 study. J Clin Oncol. 2018;36(15_suppl):5511. doi: 10.1200/JCO.2018.36.15_suppl.5511 [DOI] [PubMed] [Google Scholar]
- 44.Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of anti–PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015-4022. doi: 10.1200/JCO.2015.62.3397 [DOI] [PubMed] [Google Scholar]
- 45.Infante JR, Braiteh F, Emens LA, et al. Safety, clinical activity and biomarkers of atezolizumab (atezo) in advanced ovarian cancer (OC). Ann Oncol. 2016;27(suppl_6):871P. doi: 10.1093/annonc/mdw374.18 [DOI] [Google Scholar]
- 46.Pujade-Lauraine E, Fujiwara K, Dychter SS, Devgan G, Monk BJ. Avelumab (anti–PD-L1) in platinum-resistant/refractory ovarian cancer: JAVELIN Ovarian 200 phase III study design. Future Oncol. 2018;14(21):2103-2113. doi: 10.2217/fon-2018-0070 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Sites of Enrollment
eTable 2. Clinical Activity Based on PD-L1 Status in Evaluable Patients (N = 114)
eFigure. Kaplan-Meier Estimates of (A) Progression-Free Survival and (B) Overall Survival (N = 125)
Data Sharing Statement