Key Points
Question
Are preexisting autoimmune markers including relevant antibodies associated with outcome or with immune-related adverse events of anti–programmed cell death 1 therapy in patients with subclinical disease with advanced non–small cell lung cancer?
Findings
In this medical record analysis of 137 patients with advanced or recurrent non–small cell lung cancer treated with the programmed cell death protein 1 inhibitors nivolumab or pembrolizumab, the presence of any preexisting antibody examined was independently and significantly associated with immune-related adverse events and with clinical benefit.
Meaning
Detecting the presence of these autoimmune markers may help determine the risk-benefit ratio for individual patients with non–small cell lung cancer, maximizing therapeutic benefits while minimizing immune-related adverse events.
Abstract
Importance
Administration of anti–programmed cell death protein 1 (anti–PD-1) is now standard therapy in advanced non–small cell lung cancer (NSCLC). However, immune checkpoint inhibitors, including anti–PD-1, have not been assessed in patients with subclinical disease with advanced NSCLC, and no useful clinical biomarkers have been associated with immune-related adverse events (irAEs) among these patients treated with anti–PD-1.
Objective
To assess the safety and efficacy of anti–PD-1 treatment in patients with subclinical disease with advanced NSCLC and with or without preexisting autoimmune markers, including rheumatoid factor, antinuclear antibody, antithyroglobulin, and antithyroid peroxidase; and to assess potential clinical biomarkers that may be meaningfully and conveniently associated with clinical benefit or with irAEs following anti–PD-1 treatment.
Design, Setting, and Participants
This medical records analysis retrospectively evaluated 137 patients who received nivolumab or pembrolizumab monotherapy at Sendai Kousei Hospital in Japan between January 2016 and January 2018. Treatment efficacy and irAEs were evaluated along with candidate factors that may be associated with irAEs.
Exposures
Absence or presence of specific autoimmune markers and antibodies before treatment.
Main Outcomes and Measures
Preexisting antibodies and autoimmune markers, progression-free survival (PFS), and irAEs.
Results
Of 137 patients with advanced NSCLC, 105 were men, the median age was 68 (range, 36-88) years, 99 underwent nivolumab monotherapy, 38 underwent pembrolizumab monotherapy, and 134 had an Eastern Cooperative Oncology Group performance status of 0 or 1. The median PFS was 6.5 (95% CI, 4.4-12.9) months among patients with examined preexisting antibodies and 3.5 (95% CI, 2.4-4.1) months among patients without, suggesting significantly better prognosis in the former. The hazard ratio for disease progression or death in the presence of the examined preexisting antibodies was 0.53 (95% CI, 0.36-0.79; P = .002). The PFS was significantly longer among patients with any preexisting antibodies than among those without. The examined preexisting antibodies (48 patients [73%]) and rheumatoid factor (26 patients [39%]) were more common among patients who developed irAEs. Multivariate analysis indicated that the presence of the examined preexisting antibodies was independently associated with irAEs (odds ratio, 3.25; 95% CI, 1.59-6.65; P = .001). Skin reactions were more frequent among patients with preexisting rheumatoid factor (47% vs 24%, P = .02), whereas thyroid dysfunction was more frequent among patients with preexisting antithyroid antibodies (20% vs 1%, P < .001).
Conclusions and Relevance
The presence of the examined preexisting antibodies was associated with clinical benefit and with the development of irAEs in patients with NSCLC treated with nivolumab or pembrolizumab. Thus, the presence of these autoimmune markers may help determine the risk-benefit ratio for individual patients with NSCLC, maximizing therapeutic benefits while minimizing irAEs.
This medical records analysis from a single institution in Japan assesses anti–PD-1 treatment in patients with subclinical advanced non–small cell lung cancer and with or without preexisting autoimmune markers and evaluates potential clinical biomarkers that may be associated with clinical benefit or with immune-related adverse events.
Introduction
Therapies targeting the programmed cell death protein 1 (PD-1) pathway have improved outcomes for a variety of malignant neoplasms.1,2 An inhibitory coreceptor primarily expressed at the activated T-cell surface, PD-1 modulates T-cell effector activities, including proliferation,3 cytokine production,4 and survival5 on PD–ligand 1 (PD-L1) or PD-L2–mediated activation. Accordingly, PD-1 and PD-L1 signaling disruption by monoclonal antibodies regenerates T-cell–mediated antitumor immunity, producing durable anticancer effects in a subset of patients.
In the CheckMate-017 and CheckMate-057 phase 3 randomized clinical trials, overall survival (OS) was significantly enhanced among patients with squamous and nonsquamous non–small cell lung cancer (NSCLC) who were treated with anti–PD-1 (nivolumab) rather than with docetaxel as second-line therapy.6,7 Similarly, the Keynote-010 trial showed that second-line anti–PD-1 (pembrolizumab) treatment significantly improves OS compared with docetaxel among patients with PD-L1–positive NSCLC.8 Thus, nivolumab and pembrolizumab monotherapy have become the new treatment of choice for advanced NSCLC instead of standard chemotherapy.
However, T-cell activation may cause immune-related adverse events (irAEs) that are not triggered by conventional cytotoxic anticancer agents, including skin reactions, thyroid dysfunction, pneumonitis, and hepatitis.9 These may require systemic immunosuppression or treatment termination.10 We recently reported that irAEs are also associated with the clinical response to nivolumab.11 Hence, there is an urgent clinical need to identify patients who are more likely to develop irAEs, both to personalize patient management and to possibly provide early or prophylactic interventions that may mitigate such events. To our knowledge, no convenient and useful clinical biomarkers are currently available to predict such events in patients with advanced NSCLC. In addition, immune checkpoint inhibitors such as anti–PD-1 have not been assessed specifically in patients with subclinical disease with advanced NSCLC exhibiting preexisting rheumatoid factor, antinuclear antibodies, or antithyroid antibodies, although clinical patients with preexisting autoimmune disorders have been assessed.12,13,14,15 To address these gaps, the present study retrospectively analyzed the medical records of patients with subclinical disease with preexisting autoimmune markers and advanced NSCLC who were treated with anti–PD-1.
Methods
Patients
The medical records of patients with advanced NSCLC who had received nivolumab (3 mg/kg every 2 weeks) or pembrolizumab (200 mg every 3 weeks) monotherapy at Sendai Kousei Hospital between January 2016 and January 2018 were retrospectively reviewed. Treatments were provided until disease progression, unacceptable toxicity, or consent withdrawal. All patients were followed up until death, loss of contact, or consent withdrawal. The present study was approved by the institutional review board at Sendai Kousei Hospital, which also waived the need to obtain informed consent because data were analyzed anonymously.
Assessments
Blood samples drawn at screening were tested for preexisting rheumatoid factor, antinuclear antibody, antithyroglobulin, and antithyroid peroxidase, using a cutoff of 15 IU/mL for rheumatoid factor and 1:40 for antinuclear antibody, as previously reported.16,17 A patient was considered to have any preexisting antibodies if any of the listed antibodies were present at pretreatment and were considered to have preexisting antithyroid antibodies if either antithyroglobulin or antithyroid peroxidase was present. Patients with or without preexisting antibodies were evaluated based on objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), OS, and irAE development. In addition, patients who developed or did not develop irAEs were evaluated according to the same criteria, and factors associated with such events were assessed.
Progression-free survival and OS were measured as the time between start of treatment and documented disease progression or death owing to any cause (PFS) or to the latter (OS). Tumor response to nivolumab or pembrolizumab monotherapy was objectively assessed by pulmonary physicians (attending physicians, all authors but Y.H., and investigators Y.T. and S.S.) who evaluated using Response Evaluation Criteria in Solid Tumors, version 1.1, computed tomographic scans obtained every 8 to 9 weeks.18 The attending physicians indicated above and a nurse specialist also provided physical examinations and assessed irAEs, defined as adverse events with a potential immunologic basis and requiring potential intervention with immunosuppressive or endocrine therapy,6,7,8,19,20 every 2 to 3 weeks throughout the treatment course. To reduce bias, only objectively recognizable adverse events were considered, including skin reactions along with endocrine, gastrointestinal tract, hepatic, neurological, and pulmonary events, albeit not infusion reaction because it is universally triggered by monoclonal antibodies. The irAE clinical severity was graded according to the Common Terminology Criteria for Adverse Events, version 4.0.
Statistical Analysis
Associations between patient variables and nivolumab or pembrolizumab monotherapy response were analyzed by univariate and multivariate logistic regression using EZR, a graphical user interface for R (The R Foundation for Statistical Computing).21 Categorical variables were compared in the same platform by the χ2 test, the t test, the Mann-Whitney test, or the Welch t test, as appropriate. Progression-free survival and OS until May 10, 2018, were estimated using Kaplan-Meier curves and compared by 2-sided log-rank test. Hazard ratios (HR) were estimated using Cox proportional hazards regression analysis. All P values were 2 sided, and P < .05 was considered statistically significant.
Results
Patient Characteristics
Of 137 patients with advanced NSCLC (105 men [77%]; 32 women [23%]), 99 patients underwent nivolumab monotherapy and 38 underwent pembrolizumab monotherapy during the study period (eTable 1 in the Supplement). The median age was 68 (range, 36-88) years, and 134 patients (98%) had an Eastern Cooperative Oncology Group performance status of 0 or 1. Of 137 patients, 51 (37%) were diagnosed as having squamous cell carcinoma and 86 (63%) were diagnosed as having nonsquamous NSCLC. Mutations in epidermal growth factor receptor were present in 16 patients (12%). Eighteen patients (13%) had undergone no prior chemotherapy regimen, whereas 62 patients (45%) had received 1 course of chemotherapy, 24 patients (18%) had received 2 courses, and 33 patients (24%) had received 3 or more courses. The expression of PD-L1 was abundant (tumor proportion score [TPS] ≥50%) in 27 patients (20%), at low levels (1% ≤ TPS < 50%) in 30 patients (22%), or absent (TPS < 1%) in 15 patients (11%); expression was unknown in the remaining 65 (47%). None had active autoimmune disease although 66 patients (48%) developed irAEs. Symptoms developed within 8 weeks for 46 patients (34%), with a median onset of 4.7 weeks.
Based on Response Evaluation Criteria in Solid Tumors, version 1.1, complete response was observed in 2 patients (1%), partial response in 41 patients (30%), stable disease in 53 patients (39%), and progressive disease in 41 patients (30%). The ORR was 31% (95% CI, 24%-40%), whereas the DCR was 70% (95% CI, 61%-78%).
Analysis of irAEs
The development of irAEs is summarized in eTable 2 in the Supplement. Of 66 patients experiencing irAEs, 42 (64%) presented skin reactions, whereas 14 (21%) developed pneumonitis, 15 (23%) had hypothyroidism, 1 (2%) had hyperthyroidism, 6 (9%) had hepatitis, 5 (8%) had myositis or peripheral neuropathy, and 2 (3%) had diarrhea.
Table 1 compares patients who did or did not develop irAEs. No significant differences were observed regarding sex, age, Eastern Cooperative Oncology Group performance status, pathologic subtype, smoking history, or number of past treatment regimens. Preexisting rheumatoid factor was significantly more frequent among patients who developed irAEs. Among these, 1 patient (2%) showed complete response, 33 patients (50%) exhibited partial response, 27 patients (41%) developed stable disease, and 5 patients (8%) presented progressive disease. In the 71 patients who did not develop irAEs, 1 (1%) showed complete response, 8 (11%) exhibited partial response, 26 (37%) developed stable disease, and 36 (51%) presented progressive disease. The ORR (52% vs 13%, P < .001) and DCR (92% vs 49%, P < .001) were significantly higher among patients who developed irAEs vs those who did not.
Table 1. Characteristics of 137 Patients Who Did or Did Not Develop irAEs During Nivolumab or Pembrolizumab Monotherapy.
| Characteristic | No. (%) of Patients | P Value | |
|---|---|---|---|
| With irAE (n = 66) | Without irAE (n = 71) | ||
| Sex, male | 54 (82) | 51 (72) | .24a |
| Age, median (range), y | 68 (36-88) | 67 (31-84) | .61b |
| ECOG PS | |||
| 0 | 47 | 35 | |
| 1 | 17 | 35 | |
| ≥2 | 2 | 1 | |
| Pathologic subtype, No. | |||
| Squamous cell carcinoma | 24 | 27 | .98a |
| Nonsquamous NSCLC | 42 | 44 | |
| Smoking, never or previous/current, No. | 9/57 | 15/56 | .35a |
| Past regimens, median (range), No. | 1.5 (0-7) | 2.0 (0-9) | .05c |
| Best response | |||
| Complete response | 1 | 1 | |
| Partial response | 33 | 8 | |
| Stable disease | 27 | 26 | |
| Progress disease | 5 | 36 | |
| Objective response rated | 34 (52) | 9 (13) | <.001a |
| Disease control ratee | 61 (92) | 35 (49) | <.001a |
| Immunoglobulin, median (range), mg/dL | |||
| IgG | 1322 (481-3185) | 1219 (649-3165) | .46c |
| IgA | 275 (54-1114) | 256 (102-514) | .55f |
| IgM | 79 (14-251) | 86 (29-370) | .90c |
| IgE | 78 (54-1114) | 78 (5-8900) | .99c |
| Preexisting antibody | |||
| Anyg | 48 (73) | 32 (45) | .002a |
| RFh | 26 (39) | 12 (17) | .006a |
| ANAi | 29 (44) | 19 (27) | .05a |
| Antithyroidj | 15 (23) | 10 (14) | .28a |
Abbreviations: ANA, antinuclear antibody; ECOG PS, Eastern Cooperative Oncology Group performance status; Ig, immunoglobulin; irAE, immune-related adverse event; NSCLC, non–small cell lung cancer; RF, rheumatoid factor.
SI conversion factor: To convert immunoglobulin level to milligrams per liter, multiply by 10.
By χ2 test.
By t test
By Mann-Whitney test.
Proportion of patients achieving complete or partial response based on modified Response Evaluation Criteria in Solid Tumors version 1.1.
Proportion of patients achieving complete response, partial response, or stable disease based on modified Response Evaluation Criteria in Solid Tumors version 1.1.
By Welch t test.
A patient was considered positive if any RF, ANA, antithyroglobulin, or antithyroid peroxidase was present at pretreatment.
A patient was considered positive if RF was greater than 15 IU/mL at pretreatment.
A patient was considered positive if ANA was 1:40 or greater at pretreatment.
A patient was considered positive if either antithyroglobulin or antithyroid peroxidase was present at pretreatment.
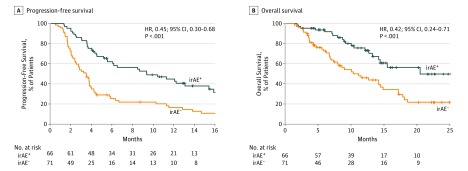
The median PFS was 10.3 (95% CI, 5.5-15.2) months for patients who developed irAEs vs 3.4 (95% CI, 2.4-3.8) months for patients who did not develop irAEs, indicating significantly better survival times in the former group (Figure 1A). Similarly, 12-month PFS was 44% (95% CI, 31%-56%) for patients who developed irAEs vs 18% (95% CI, 10%-29%) for patients who did not develop irAEs. The HR for disease progression or death due to irAEs was 0.45 (95% CI, 0.30-0.68; P < .001). The median OS was not reached (NR) (95% CI, 14.5-NR) for patients who developed irAEs but was 11.4 (95% CI, 7.5-15.2) months for those who did not, indicating significantly better survival in the former (Figure 1B). Similarly, 12-month OS was 76% (95% CI, 62%-86%) for patients who developed irAEs and 47% (95% CI, 34%-59%) for patients who did not develop irAEs. The HR for death due to irAEs was 0.42 (95% CI, 0.24-0.71; P < .001). Univariate analysis identified variables significantly associated with irAEs; further multivariate analysis revealed that the presence of any of the antibodies tested at pretreatment was independently associated with such events (odds ratio, 3.25; 95% CI, 1.59-6.65; P = .001).
Figure 1. Progression-Free Survival and Overall Survival Among Patients With or Without Immune-Related Adverse Events (irAEs).
Kaplan-Meier curves are shown for progression-free survival (A) and overall survival (B) among patients with (irAE+) or without irAEs (irAE−). Tick marks indicate patients for whom data were censored on May 10, 2018.
Contributions of Preexisting Antibodies
Table 2 stratifies patient characteristics by the presence or absence of preexisting antibodies. The ORR was significantly higher among patients with (41%) than without (18%) (P = .006) any preexisting antibodies although no significant differences were observed between patients with or without rheumatoid factor, antinuclear antibody, or antithyroid antibody. Similar trends regarding any preexisting antibodies were observed for the DCR (81% vs 54%, P = .001), but this was also comparable between the other patient groups. The irAEs were significantly more frequent among patients with any preexisting antibodies than among those without (60% vs 32%, P = .002), and among those with vs without preexisting rheumatoid factor (68% vs 40%, P = .006). By contrast, irAEs were comparable in frequency between patients with or without preexisting antinuclear or antithyroid antibodies. In any case, adverse events of grade 3 or higher were comparable among all groups. Skin reactions were more common among patients with than without any preexisting antibodies (40% vs 18%, P = .009) or preexisting rheumatoid factor (47% vs 24%, P = .02). However, the frequency was comparable between patients with or without preexisting antinuclear or antithyroid antibodies. Moreover, hypothyroidism developed more frequently among patients with than without preexisting antithyroid antibodies (20% vs 1%, P < .001) although this result was not observed between patients with or without preexisting rheumatoid factor and antinuclear antibody. Development of pneumonitis, hepatitis, and myositis or peripheral neuropathy was comparable among all groups.
Table 2. Stratification of 137 Patients by Presence or Absence of Antibodies.
| Response | No. (%) of Patients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any Preexisting Antibody | Preexisting RF | Preexisting ANA | Preexisting Antithyroid | |||||||||
| With (n = 80)a | Without (n = 57) | P Valueb | With (n = 38)c | Without (n = 99) | P Valueb | With (n = 48)d | Without (n = 89) | P Valueb | With (n = 25)e | Without (n = 112) | P Valueb | |
| Objective response ratef | 33 (41) | 10 (18) | .006 | 17 (45) | 26 (26) | .06 | 18 (38) | 25 (28) | .35 | 12 (48) | 31 (28) | .08 |
| Disease control rateg | 65 (81) | 31 (54) | .001 | 31 (82) | 65 (66) | .11 | 37 (77) | 59 (66) | .26 | 22 (88) | 74 (66) | .05 |
| Development of irAE | 48 (60) | 18 (32) | .002 | 26 (68) | 40 (40) | .006 | 29 (60) | 37 (42) | .05 | 15 (60) | 51 (46) | .28 |
| Development of irAE >grade 3 | 6 (8) | 3 (5) | .86 | 3 (8) | 6 (6) | >.99 | 4 (8) | 5 (6) | .80 | 1 (4) | 8 (7) | .90 |
| Skin reaction | 32 (40) | 10 (18) | .009 | 18 (47) | 24 (24) | .02 | 18 (38) | 24 (27) | .28 | 10 (40) | 32 (29) | .38 |
| Pneumonitis | 8 (10) | 6 (11) | >.99 | 4 (11) | 10 (10) | >.99 | 4 (8) | 10 (11) | .81 | 3 (12) | 11 (10) | >.99 |
| Hypothyroidism | 6 (8) | 0 | .07 | 2 (5) | 4 (4) | >.99 | 3 (6) | 3 (3) | .51 | 5 (20) | 1 (1) | <.001 |
| Hyperthyroidism | 1 (1) | 0 | >.99 | 0 | 1 (1) | >.99 | 1 (2) | 0 | .66 | 1 (4) | 0 | .48 |
| Hepatitis | 3 (4) | 3 (5) | >.99 | 2 (5) | 4 (4) | >.99 | 1 (2) | 5 (6) | .60 | 0 | 6 (5) | .52 |
| Myositis or peripheral neuropathy | 4 (5) | 1 (2) | .62 | 0 | 5 (5) | .38 | 3 (6) | 2 (2) | .45 | 2 (8) | 3 (3) | .58 |
| Treatment discontinued due to irAE | 9 (11) | 6 (11) | >.99 | 3 (8) | 12 (12) | .69 | 8 (17) | 7 (8) | .20 | 4 (16) | 11 (10) | .59 |
Abbreviations: ANA, antinuclear antibody; irAE, immune-related adverse event; RF, rheumatoid factor.
A patient was considered positive if any RF, ANA, antithyroglobulin, or antithyroid peroxidase was present at pretreatment.
By χ2 test.
A patient was considered positive if RF was greater than 15 IU/mL at pretreatment.
A patient was considered positive if ANA was 1:40 or greater at pretreatment.
A patient was considered positive if either antithyroglobulin or antithyroid peroxidase was present at pretreatment.
Proportion of patients achieving complete or partial response based on modified Response Evaluation Criteria in Solid Tumors, version 1.1.
Proportion of patients achieving complete response, partial response, or stable disease based on modified Response Evaluation Criteria in Solid Tumors, version 1.1.
Nivolumab or pembrolizumab monotherapy was discontinued in 15 patients owing to 14 pneumonitis cases and 1 hepatitis case, whereas systemic steroids were used to treat pneumonitis in 14 patients (100%) and hepatitis in 1 patient (17%). One patient died from pneumonitis. No significant differences were observed in discontinuation of treatment by irAE between patients with or without antibodies among all groups.
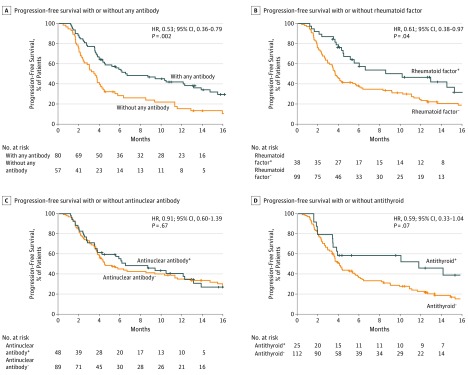
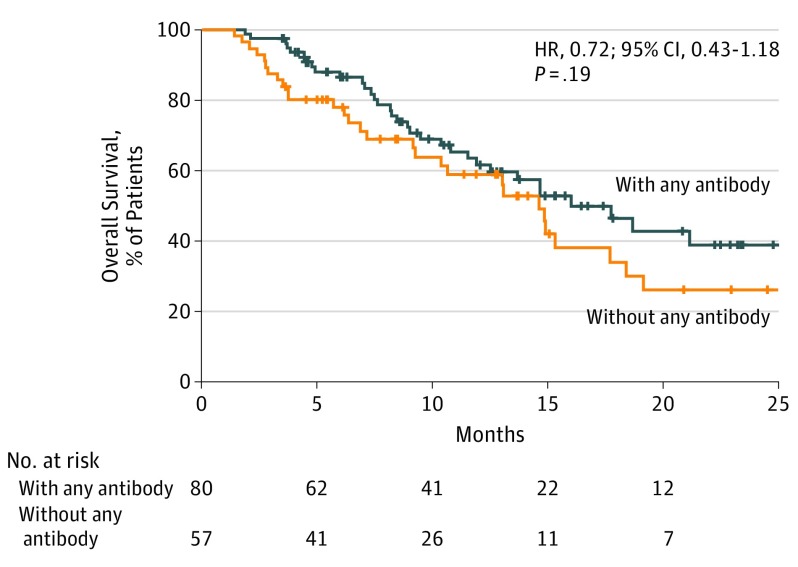
The median PFS was 6.5 (95% CI, 4.4-12.9) months among patients with any preexisting antibodies but 3.5 (95% CI, 2.4-4.1) months among those without, indicating significantly better prognosis in the former (Figure 2A). The HR for disease progression or death due to any preexisting antibodies was 0.53 (95% CI, 0.36-0.79; P = .002). The median PFS was 10.1 (95% CI, 4.4-15.2) months among patients with preexisting rheumatoid vs 3.7 (95% CI, 3.2-5.4) months among patients without preexisting rheumatoid factor, also indicating significantly better prognosis in the former (Figure 2B). The HR for disease progression or death in the presence of rheumatoid factor was 0.61 (95% CI, 0.38-0.97, P = .04). No significant differences in PFS were observed between patients with or without preexisting antinuclear or antithyroid antibody (Figure 2C and D). The median OS was 17.6 (95% CI, 11.8-NR) months among patients with any preexisting antibodies but 14.6 (95% CI, 9.2-18.3) months among those without; no significant differences in OS were observed between the 2 groups (Figure 3). Similarly, OS was comparable between patients with or without preexisting rheumatoid factor or preexisting antinuclear or antithyroid antibodies (eFigure in the Supplement).
Figure 2. Progression-Free Survival in the Cohort.
Kaplan-Meier curves are shown for progression-free survival among patients with or without any preexisting antibody (A), rheumatoid factor (B), antinuclear antibody (C), and antithyroid antibody (D). A patient was considered to have any preexisting antibodies if preexisting rheumatoid factor, antinuclear antibody, antithyroglobulin, or antithyroid peroxidase was present at pretreatment. + Indicates that the patient was positive for the indicated antibody or rheumatoid factor; −, negative for the indicated antibody or rheumatoid factor. Tick marks indicate patients for whom data were censored on May 10, 2018.
Figure 3. Overall Survival in the Cohort.
Kaplan-Meier curves are shown for overall survival among patients with or without any preexisting antibodies. A patient was considered to have any preexisting antibodies if preexisting rheumatoid factor, antinuclear antibody, antithyroglobulin, or antithyroid peroxidase was present at pretreatment. Tick marks indicate patients for whom data were censored on May 10, 2018.
Patients with TPS values 50% or greater (strong positive, n = 27) or with TPS values less than 50% (weak positive or negative, n = 45) assessed by antibody frequency are given in eTable 3 in the Supplement. No significant differences were observed for antibody frequency.
Discussion
Although anti–PD-1 is currently in common use in oncology, its safety and efficacy are unknown for patients with subclinical disease with preexisting rheumatoid factor, antinuclear antibody, antithyroglobulin, or antithyroid peroxidase, which are widely used as biomarkers of autoimmune disorders. No clinical biomarkers are available to conveniently and meaningfully predict irAEs following treatment although these would enable personalized patient management and identify those patients less prone to irAEs. To our knowledge, we are the first to evaluate the associations among preexisting autoimmune markers, irAEs, and clinical response of advanced NSCLC to anti–PD-1, using a single-center, retrospective cohort.
The development of irAEs has previously been associated with clinical benefit11,22; similar results were obtained in the present study. We believe that cautious management of irAEs (especially early detection and treatment) can facilitate achieving the maximum clinical benefit from nivolumab or pembrolizumab monotherapy. Therefore, identifying the predictors or factors associated with irAEs is important. The present study showed that the existence of preexisting antibodies represented an independent factor associated with developing irAEs. Our data showed that ORR, DCR, and PFS were significantly better among patients with rather than among those without any of the preexisting antibodies examined in the present study. For example, PFS was significantly longer among patients with preexisting rheumatoid factor although preexisting antinuclear antibody was not associated with clinical benefit. Although patients with or without preexisting antibodies did not significantly differ, OS tended to be higher in the former. Other predictive markers of response to immune checkpoint inhibitors have also been investigated23,24 although we believe that we are the first to show that the presence of any of the preexisting antibodies detected here may be associated with patient response to checkpoint inhibitors.
Biomarkers for the risk of developing irAEs to immune checkpoint inhibitors have been less thoroughly investigated. Interleukin 17, clonal expansion of CD8-positive T cells, eosinophil counts, and neutrophil activation markers have been linked to specific irAEs but were not predictive at baseline.25,26,27 Other potential baseline risk factors include family history of autoimmune disease, prior viral infections, and use of drugs with known autoimmune toxicities28,29 although these require further validation. Our data showed that irAEs were more frequent among patients with any of the preexisting antibodies examined and that such markers were independently associated with subsequent irAEs. For example, skin reactions were more common among patients with than without preexisting rheumatoid factor, and thyroid dysfunction was more common among patients with preexisting antithyroid antibody. Similarly, Osorio et al30 found that in 51 patients with advanced NSCLC who were treated with pembrolizumab, antithyroid antibodies were more common among patients who subsequently developed thyroid dysfunction. In addition, Suzuki et al31 reported 12 myasthenia gravis cases (0.12%) among 9869 patients with cancer who had been treated with nivolumab, of whom 10 had preexisting antibodies to the acetylcholine receptor. Nevertheless, we are the first to report, to our knowledge, that the presence of any of the preexisting antibodies examined here was an independent factor associated with the development of irAEs.
Why these preexisting antibodies should be linked to irAEs is unclear. Notably, PD-1 is abundantly expressed in activated B cells,32 which are also modulated by T-cell–independent and T-cell–dependent mechanisms.33,34,35 Earlier tests of PD-1 in preclinical models have also suggested antibody-dependent mitigation of immune-related toxicity.36,37 The T cells enhance PD-1 antibody therapeutic effects and may in turn induce autoantibodies in B cells, thereby triggering irAEs.31,38 This suggests that the presence of autoantibodies may correlate with irAEs and treatment response.
The patients were categorized as 2 groups on the basis of their TPS values: a TPS of 50% or higher (strong positive) or a TPS less than 50% (weak positive or negative). Both of these groups were evaluated for the frequency of preexisting autoimmunity markers. Although the frequency of any preexisting antibody and rheumatoid factor tended to be slightly higher in the strong positive group, no significant differences were observed in antibody frequency. This result may suggest that these antibodies represent independent markers of PD-L1.
To our knowledge, no reports have specified the percentage of patients positive for the antibodies examined in the present study in various types of cancer type. Some studies have reported that the positive rate for rheumatoid factor is 10% to 25% among elderly (>70 years) healthy people and 5% to 25% in malignant tumors, and 27% for antinuclear antibody and 18% for antithyroid antibody among healthy people.39,40,41 In the present study, the positive rates of rheumatoid factor and antinuclear antibodies were slightly higher than those of some previous reports39,40,41 (rheumatoid factor 27.7%, antinuclear antibodies 35.0%), whereas antithyroid antibodies were comparable (18.2%). Because the antibody positivity rate among patients with lung cancer may be high, the antibody positivity rate in each cancer type should be considered.
Limitations
The expression levels of PD-L1 were not routinely assayed in the present study because no diagnostic kits were commercially available in Japan at the time. Therefore, we were unable to fully consider PD-L1 in this study. We believe that it will be important to examine autoimmune antibodies, including PD-L1, in the future. The study was retrospective and nonrandomized and was based on a small, single-center cohort. Some further limitations to evaluating the OS also existed, for example, the low number of cases, short observation period, and presence of cases in which anti–PD-1 had been administered in a high number of regimens. However, the present results may inform future research. The duration of immunotherapy in the hospital or at follow-up may also be a potential confounder because immunologic toxicity may be more likely to develop in patients subjected to longer immune checkpoint inhibitor exposure. Nevertheless, 46 of 66 patients (70%) who developed irAEs presented symptoms within 8 weeks from the start of nivolumab or pembrolizumab monotherapy, suggesting that the observed events were unlikely to be due to higher risk of toxicity from longer therapy periods.
Conclusions
The safety and efficacy of immune checkpoint inhibitors are currently unknown among patients with subclinical disease with any of the preexisting antibodies examined. Convenient and meaningful clinical biomarkers predictive of irAEs to such inhibitors have not been developed. The presented study showed that ORR, DCR, and PFS were significantly better following nivolumab or pembrolizumab monotherapy among patients with any of the preexisting antibodies examined than among those without. Moreover, the existence of any of those preexisting antibodies was significantly and independently associated with irAEs. Although larger retrospective and prospective surveys may further illuminate the risks of immunotherapy in patients with subclinical disease with preexisting autoimmune markers, our study adds to the growing evidence supporting the use of immunotherapy in such patients although close monitoring for adverse events is recommended. In any case, additional studies are still needed to identify other risk factors for irAEs to immune checkpoint inhibitors because such studies would help determine the risk-benefit ratio for individual patients and maximize therapeutic benefits while minimizing irAEs.
eTable 1. Patient Characteristics at Baseline (n = 137)
eTable 2. Observed Immune-Related Adverse Events
eTable 3. Association Between PD-L1 Expression and Antibody Detection (n = 72)
eFigure. Overall Survival in the Cohort
References
- 1.Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315(15):1600-1609. [DOI] [PubMed] [Google Scholar]
- 2.Garon EB, Rizvi NA, Hui R, et al. ; KEYNOTE-001 Investigators . Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018-2028. [DOI] [PubMed] [Google Scholar]
- 3.Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543-9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett F, Luxenberg D, Ling V, et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation. J Immunol. 2003;170(2):711-718. [DOI] [PubMed] [Google Scholar]
- 5.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010). Lancet. 2016;387(10027):1540-1550. [DOI] [PubMed] [Google Scholar]
- 9.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697. [DOI] [PubMed] [Google Scholar]
- 10.Linardou H, Gogas H. Toxicity management of immunotherapy for patients with metastatic melanoma. Ann Transl Med. 2016;4(14):272-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toi Y, Sugawara S, Kawashima Y, et al. Association of immune-related adverse events with clinical benefit in patients with advanced non-small-cell lung cancer treated with nivolumab [published online June 22, 2018]. Oncologist. 2018;23(11):1358-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danlos FX, Voisin AL, Dyevre V, et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur J Cancer. 2018;91:21-29. [DOI] [PubMed] [Google Scholar]
- 13.Leonardi GC, Gainor JF, Altan M, et al. Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol. 2018;36(19):1905-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol. 2016;2(2):234-240. [DOI] [PubMed] [Google Scholar]
- 15.Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017;28(2):368-376. [DOI] [PubMed] [Google Scholar]
- 16.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria. Arthritis Rheum. 2010;62(9):2569-2581. [DOI] [PubMed] [Google Scholar]
- 17.Segni M, Pucarelli I, Truglia S, Turriziani I, Serafinelli C, Conti F. High prevalence of antinuclear antibodies in children with thyroid autoimmunity. J Immunol Res. 2014;2014:150239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours. Eur J Cancer. 2009;45(2):228-247. [DOI] [PubMed] [Google Scholar]
- 19.Reck M, Rodríguez-Abreu D, Robinson AG, et al. ; KEYNOTE-024 Investigators . Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. [DOI] [PubMed] [Google Scholar]
- 20.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma. Clin Cancer Res. 2016;22(4):886-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4(3):374-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17(12):e542-e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacquelot N, Roberti MP, Enot DP, et al. Predictors of responses to immune checkpoint blockade in advanced melanoma. Nat Commun. 2017;8(1):592-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shahabi V, Berman D, Chasalow SD, et al. Gene expression profiling of whole blood in ipilimumab-treated patients for identification of potential biomarkers of immune-related gastrointestinal adverse events. J Transl Med. 2013;11:75-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subudhi SK, Aparicio A, Gao J, et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc Natl Acad Sci U S A. 2016;113(42):11919-11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarhini AA, Zahoor H, Lin Y, et al. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer. 2015;3:39-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities. Ann Oncol. 2016;27(4):559-574. [DOI] [PubMed] [Google Scholar]
- 29.Manson G, Norwood J, Marabelle A, Kohrt H, Houot R. Biomarkers associated with checkpoint inhibitors. Ann Oncol. 2016;27(7):1199-1206. [DOI] [PubMed] [Google Scholar]
- 30.Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28(3):583-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki S, Ishikawa N, Konoeda F, et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology. 2017;89(11):1127-1134. [DOI] [PubMed] [Google Scholar]
- 32.Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thibult ML, Mamessier E, Gertner-Dardenne J, et al. PD-1 is a novel regulator of human B-cell activation. Int Immunol. 2013;25(2):129-137. [DOI] [PubMed] [Google Scholar]
- 34.Kawamoto S, Tran TH, Maruya M, et al. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science. 2012;336(6080):485-489. [DOI] [PubMed] [Google Scholar]
- 35.Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol. 2013;14(2):152-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141-151. [DOI] [PubMed] [Google Scholar]
- 37.Okazaki T, Tanaka Y, Nishio R, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9(12):1477-1483. [DOI] [PubMed] [Google Scholar]
- 38.Williams TJ, Benavides DR, Patrice KA, et al. Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol. 2016;73(8):928-933. doi: 10.1001/jamaneurol.2016.1399 [DOI] [PubMed] [Google Scholar]
- 39.Shmerling RH, Delbanco TL. The rheumatoid factor. Am J Med. 1991;91(5):528-534. [DOI] [PubMed] [Google Scholar]
- 40.Kumagai S, Hayashi N. Immunofluorescence–still the ‘gold standard’ in ANA testing? Scand J Clin Lab Invest Suppl. 2001;235:77-83. [DOI] [PubMed] [Google Scholar]
- 41.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994). J Clin Endocrinol Metab. 2002;87(2):489-499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Patient Characteristics at Baseline (n = 137)
eTable 2. Observed Immune-Related Adverse Events
eTable 3. Association Between PD-L1 Expression and Antibody Detection (n = 72)
eFigure. Overall Survival in the Cohort