Key Points
Question
What factors are associated with opioid consumption after surgery?
Findings
In this population-based study of patients undergoing surgery in Michigan, 2392 patients used only 27% of the opioids prescribed to them. Prescription size had the strongest association with opioid consumption after surgery, with patients using an additional 5 pills for every 10 extra pills prescribed.
Meaning
Excessive opioid prescribing is associated with higher opioid consumption after surgery. Using patient-reported opioid consumption will improve postoperative opioid prescribing to better match patient opioid requirements.
Abstract
Importance
There is growing evidence that opioids are overprescribed following surgery. Improving prescribing requires understanding factors associated with opioid consumption.
Objective
To describe opioid prescribing and consumption for a variety of surgical procedures and determine factors associated with opioid consumption after surgery.
Design, Setting, and Participants
A retrospective, population-based analysis of the quantity of opioids prescribed and patient-reported opioid consumption across 33 health systems in Michigan, using a sample of adults 18 years and older undergoing surgery. Patients were included if they were prescribed an opioid after surgery. Surgical procedures took place between January 1, 2017, and September 30, 2017, and were included if they were performed in at least 25 patients.
Exposures
Opioid prescription size in the initial postoperative prescription.
Main Outcomes and Measures
Patient-reported opioid consumption in oral morphine equivalents. Linear regression analysis was used to calculate risk-adjusted opioid consumption with robust standard errors.
Results
In this study, 2392 patients (mean age, 55 years; 1353 women [57%]) underwent 1 of 12 surgical procedures. Overall, the quantity of opioid prescribed was significantly higher than patient-reported opioid consumption (median, 30 pills; IQR, 27-45 pills of hydrocodone/acetaminophen, 5/325 mg, vs 9 pills; IQR, 1-25 pills; P < .001). The quantity of opioid prescribed had the strongest association with patient-reported opioid consumption, with patients using 0.53 more pills (95% CI, 0.40-0.65; P < .001) for every additional pill prescribed. Patient-reported pain in the week after surgery was also significantly associated with consumption but not as strongly as prescription size. Compared with patients reporting no pain, patients used a mean (SD) 9 (1) more pills if they reported moderate pain and 16 (2) more pills if they reported severe pain (P < .001). Other significant risk factors included history of tobacco use, American Society of Anesthesiologists class, age, procedure type, and inpatient surgery status. After adjusting for these risk factors, patients in the lowest quintile of opioid prescribing had significantly lower mean (SD) opioid consumption compared with those in the highest quintile (5 [2] pills vs 37 [3] pills; P < .001).
Conclusions and Relevance
The quantity of opioid prescribed is associated with higher patient-reported opioid consumption. Using patient-reported opioid consumption to develop better prescribing practices is an important step in combating the opioid epidemic.
This study describes opioid prescribing and consumption for a variety of surgical procedures and investigates factors associated with opioid consumption after surgery.
Introduction
Opioids are widely overprescribed after surgery, with up to 92% of patients having leftover opioids after common operations.1,2,3,4,5,6,7 Excessive prescribing significantly contributes to the current opioid health crisis: of the 3.8 million American individuals who misuse prescription opioids, more than half get the medication from a friend or relative.8 The millions of leftover pills available for diversion, misuse, and abuse must be reduced to effectively address the opioid epidemic.9
Single-institution initiatives have successfully reduced prescribing by matching postoperative prescription size to patients’ opioid consumption in selected general surgery procedures.6,10,11 At our own institution, we surveyed patients undergoing laparoscopic cholecystectomy regarding how much opioid medication they used after surgery. Patient responses were used to develop evidence-based prescribing recommendations. These recommendations resulted in an immediate and sustained 63% reduction in opioid prescription size without an increase in refill requests or patient-reported pain scores. Despite this initial success, little is known about consumption in a wide range of procedures, or what patient factors are associated with opioid consumption. In this context, a population-based study of postoperative consumption would help surgeons tailor prescriptions to patients’ analgesic needs.12,13
To better inform efforts to improve opioid prescribing, we investigated the quantity of opioids prescribed and patient-reported opioid consumption following common surgical procedures in a statewide population. We used data from the Michigan Surgical Quality Collaborative (MSQC), a statewide consortium that shares surgical outcomes data, to develop and implement quality improvement strategies.14,15 Our 2 objectives were to compare postoperative opioid prescription size and patient consumption and to identify factors associated with postoperative opioid consumption.
Methods
Data Source and Patient Population
The University of Michigan institutional review board approved this study of deidentified data as exempt from review. Because these data were deidentified and survey participation presented no more than minimal risk, patient consent was not obtained. Data were obtained from a database maintained by the MSQC. The MSQC is a clinical registry that prospectively collects data on patient demographics, perioperative processes of care, and 30-day clinical outcomes after primarily general surgery operations, as well as a limited number of vascular and gynecologic operations. Participating hospitals receive funding from Blue Cross Blue Shield, a large private payer in the state of Michigan, to support trained nurses to abstract clinical data. Cases were reviewed using a sampling algorithm designed to minimize selection bias and represent 90% of eligible cases, approximately 50 000 cases per year.16
We retrospectively established the study cohort by identifying surgical procedures for which at least 25 patients reported opioid consumption, between January 1, 2017, and September 30, 2017. For these procedures, we included patients 18 years and older who had elective, urgent, or emergent inpatient or outpatient general surgery, vascular, and gynecologic operations.
Patient-Reported Outcome Collection
Starting in January 2017, the MSQC began a pilot effort to collect data on opioid prescriptions at the time of discharge from surgery, patient-reported opioid consumption, and patient-reported outcomes (PROs). Of the patients selected for inclusion in the MSQC database, a subset of patients was sampled for PRO collection. Because this was a pilot effort, sampling algorithms were decided by each hospital’s clinical nurse reviewer based on feasibility given fluctuating workload. This resulted in not all patients within the MSQC database being contacted for PRO collection. Patients were contacted by telephone or mail with a survey at postoperative day 30 and allowed 90 days to respond (or until postoperative day 120). Patients were asked to report how many opioid pills they had consumed. Patients were also asked to report their pain score in the first 7 days after surgery. Patients selected an answer from the following scale: 1 (no pain), 2 (minimal pain), 3 (moderate pain), and 4 (severe pain). Data regarding preoperative opioid use were not collected. In cases where patient data were incomplete (patients incompletely answered survey questions or prescription dose was missing), these patients were excluded from analysis.
Outcome and Explanatory Variables
Demographic data included patient age and sex. Opioid prescription data at the time of discharge from surgery included opioid type, dose, and quantity of pills. The primary outcome was quantity of opioid consumed after surgery. Prescription and consumption data were converted to oral morphine equivalents (OMEs) to adjust for varying potencies of different medications.17 For ease of interpretation, opioid quantity is also presented as an equivalent number of pills or tablets of hydrocodone/acetaminophen, 5/325 mg, a common opioid medication. One tablet of hydrocodone/acetaminophen, 5/325 mg (pill), is equivalent to 5 OMEs. To illustrate the burden to the local community, we also tabulated the total number of pills from the prescriptions that remained in this statewide cohort.
The primary explanatory variable of interest was the quantity of opioid prescribed in the initial postoperative prescription in OME. Additional explanatory variables included age, sex, tobacco use, alcohol use, cancer, obesity, American Society of Anesthesiologists (ASA) physical status classification, surgical procedure, elective vs emergent status, inpatient vs outpatient status, and patient-reported pain scores in the first 7 days after surgery. Outpatient status was determined for patients who had a hospital length of stay of 0 days. These explanatory variables were chosen to include a broad variety of factors that may influence postoperative opioid consumption and to account for potential cofounders. All variables were included in the analysis.
Analysis
Descriptive statistics were used to characterize the prescribing and consumption patterns for each procedure. Opioid consumption is represented as median consumption in OMEs and interquartile range (IQR). Opioid quantities prescribed and consumed were compared using the Mann-Whitney U test. A multivariable linear regression model was used to evaluate the covariate-adjusted association between the quantity of opioid prescribed and patient-reported opioid consumption. The model was also used to calculate risk-adjusted means and 95% confidence intervals (95% CIs) for patient-reported opioid consumption. Risk adjustment was performed by including the aforementioned explanatory variables in the regression model. Robust standard errors were calculated owing to violation of the heteroscedasticity assumption. The assumption of normality was visually assessed using a Q-Q plot. There was no evidence of multicollinearity, as evidenced by tolerance values greater than 0.1. Two-sided significance tests were used for all analyses, and significance was set at P less than .05. All analyses were conducted using Stata, version 15 (StataCorp).
Results
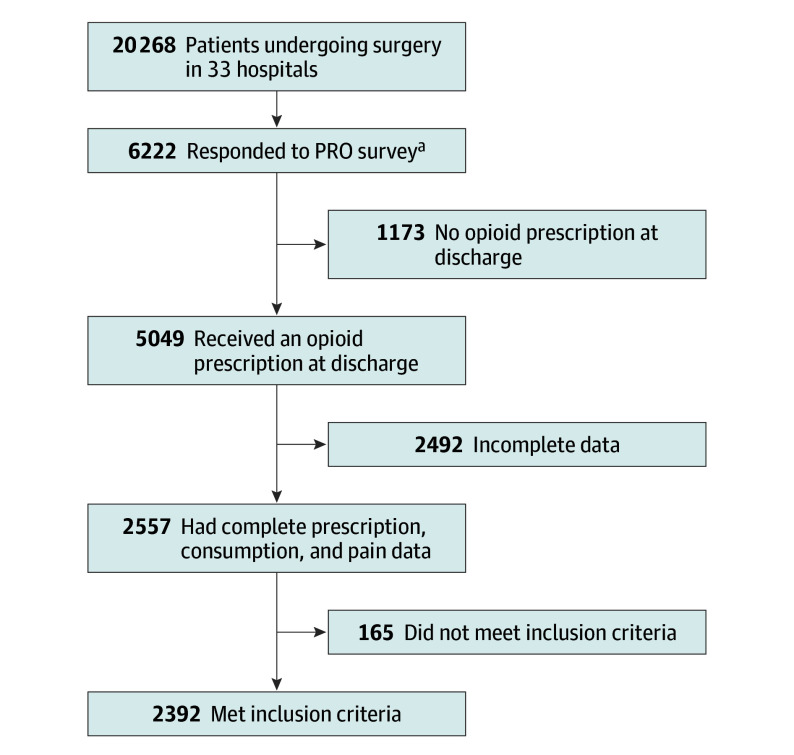
From January 1, 2017, to September 30, 2017, data from 20 268 patients across 33 hospitals were collected (Figure 1). Of these patients, an unspecified number were contacted for PRO collection based on individual hospitals’ sampling algorithms. Of this sample, 5049 received an opioid prescription at discharge. Of these patients, 2557 reported opioid consumption and had complete survey data. There were 12 surgical procedures performed in at least 25 patients, resulting in a total of 2392 patients included in this cohort for analysis (Table 1). Surgical procedures included laparoscopic cholecystectomy; laparoscopic appendectomy; inguinal and femoral hernia repair (open or laparoscopic); open incisional hernia repair; laparoscopic colectomy; open colectomy; ileostomy and colostomy takedown; small-bowel resection and/or enterolysis; thyroidectomy; vaginal hysterectomy; laparoscopic or robotic hysterectomy; and abdominal hysterectomy. There were no vascular procedures with more than 25 patients during the study period, and therefore, none were included for analysis. The most common procedures were open and laparoscopic inguinal/femoral hernia repair (n = 659 of 2392 [28%]), laparoscopic cholecystectomy (n = 25 of 2392 [25%]), and laparoscopic appendectomy (n = 224 of 2392 [9%]). Mean age in this population was 55 years, and 1353 patients were women (57%). For the entire cohort, 1833 patients (77%) underwent elective surgery while 559 (23%) underwent urgent or emergent surgery. Outpatient procedures were performed for 1100 patients (46%), and 1292 patients (54%) had a hospital length of stay of 1 day or greater.
Figure 1. Cohort of Patients Reporting Opioid Use After Surgery.
Patients at 33 health systems were surveyed for patient-reported outcomes (PROs).
aIn this pilot project, each health system was allowed to determine their own sampling algorithm to fit their unique and fluctuating workload; health systems did not report the total number of patients contacted.
Table 1. Patient and Procedure Characteristics.
| Characteristic | No. (%) |
|---|---|
| All patients | 2392 (100) |
| Age, y | |
| <45 | 670 (28) |
| 45-64 | 1001 (42) |
| ≥65 | 721 (30) |
| Sex | |
| Male | 1039 (43) |
| Female | 1353 (57) |
| Tobacco use | |
| Yes | 524 (22) |
| No | 1868 (78) |
| Alcohol use | |
| Yes | 68 (3) |
| No | 2324 (97) |
| Cancer | |
| Yes | 148 (6) |
| No | 2244 (94) |
| Obesity | |
| Yes | 1148 (48) |
| No | 1244 (52) |
| ASA class | |
| 1 | 184 (8) |
| 2-3 | 2132 (89) |
| 4-5 | 76 (3) |
| Elective/emergent status | |
| Elective | 1833 (77) |
| Emergent/urgent | 559 (23) |
| Outpatient surgery | |
| Outpatient | 1100 (46) |
| Inpatient | 1292 (54) |
| Procedure | |
| Laparoscopic cholecystectomy | 603 (25) |
| Laparoscopic appendectomy | 224 (9) |
| Inguinal/femoral hernia repair (open/laparoscopic) | 659 (28) |
| Open incisional hernia repair | 159 (7) |
| Laparoscopic colectomy | 112 (5) |
| Open colectomy | 102 (4) |
| Ileostomy/colostomy takedown | 59 (2) |
| Small bowel resection and enterolysis | 33 (1) |
| Thyroidectomy | 40 (2) |
| Vaginal hysterectomy | 113 (5) |
| Laparoscopic/robotic hysterectomy | 203 (8) |
| Abdominal hysterectomy | 85 (4) |
Abbreviation: ASA, American Society of Anesthesiologists.
Comparison of Quantity of Opioid Prescribed and Patient-Reported Opioid Consumption
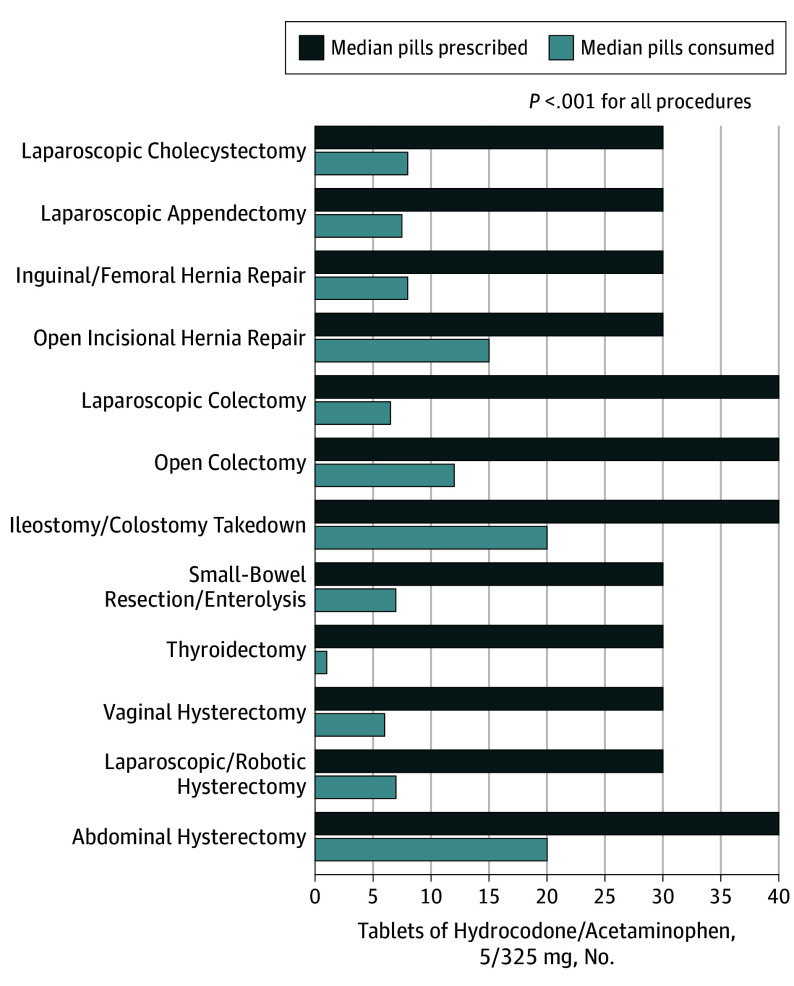
Quantity of opioid prescribed was significantly greater than quantity consumed for all 12 operations (Figure 2). Median OME prescribed overall for this cohort was 150 OME (IQR, 135-225), equivalent to 30 pills (IQR, 27-45) of hydrocodone/acetaminophen, 5/325 mg. Median OMEs consumed was 45 OMEs (IQR, 5-125) or 9 pills (IQR, 1-25) (P < .001). Overall, median consumption was 27% of the prescribed amount. For all procedures except ileostomy/colostomy takedown, median opioid consumption was less than half of quantity prescribed. Consumption ranged from 3% (95% CI, 0-25%) of quantity prescribed following thyroidectomy to 67% (95% CI, 46%-72%) of quantity prescribed following ileostomy/colostomy takedown.
Figure 2. Opioid Prescription Size and Consumption.
Median opioid prescription size and patient-reported consumption for all 12 procedures in tablets of hydrocodone/acetaminophen, 5/325 mg. Quantity of opioid prescribed was significantly greater than patient consumption for all procedures.
Overall, 582 patients (24%) reported taking no opioids after surgery. This was highest following thyroidectomy (19 patients [48%] took no opioids) and laparoscopic colectomy (38 patients [34%] took no opioids) and lowest following abdominal hysterectomy (17 patients [20%] took no opioids) and laparoscopic cholecystectomy (128 patients [21%] took no opioids). Consumption of the entire prescription occurred in only 534 patients (22%). This was highest for ileostomy/colostomy takedown (24 patients [42%] took the entire prescription) and lowest for thyroidectomy (1 patient [3%] took the entire prescription).
Median number of leftover OMEs for the entire cohort was 100 (IQR, 25-150). Median number of leftover pills was 19 (IQR, 4-29). This ranged from 10 (IQR, 0-27) following ileostomy/colostomy takedown to 24 (IQR, 13-30) following thyroidectomy (eTable in the Supplement). Overall, the 2392 patients in this cohort had a total of 42 692 pills (58%) remaining.
Variables Associated With Patient-Reported Opioid Consumption
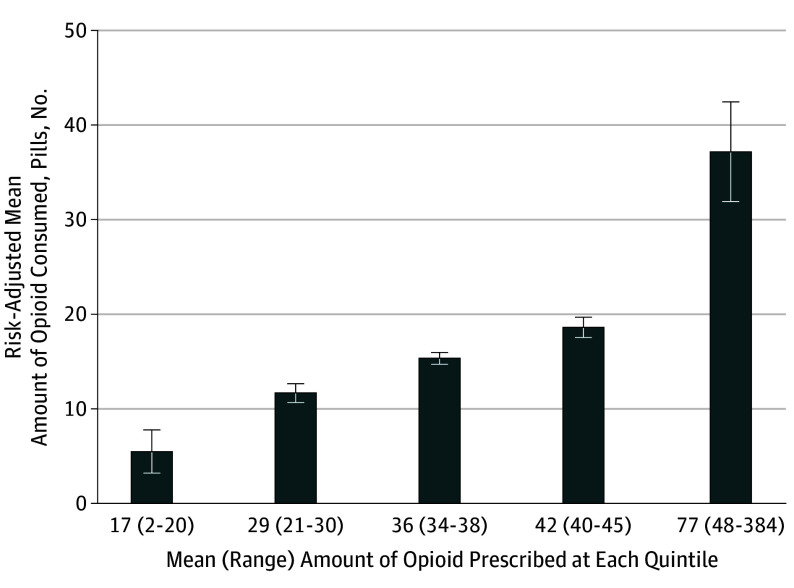
The linear regression model for opioid consumption following surgery is presented in Table 2. Although significant outliers exist in the data, the model had a good fit, with R2 = 0.47. Quantity of opioid prescribed had the strongest association with postoperative opioid consumption because it had the highest standardized β coefficient in the multiple regression model (standardized β = 0.565). For each additional OME prescribed, patients used an additional 0.53 OMEs (95% CI, 0.40-0.65; P < .001), which equates to using 5.3 more pills for every 10 additional pills prescribed. A patient prescribed 100 pills could therefore be expected to use roughly 40 more pills than a patient prescribed 20 pills. Risk-adjusted opioid consumption at the mean of each quintile of opioid prescription size in this cohort is shown in Figure 3. Specifically, patients in the lowest quintile used significantly fewer opioids compared with patients in the highest quintile (mean [SD], 5 [2] pills vs 37 [3] pills; P < .001).
Table 2. Linear Regression Model.
| Variable | β | SE | Standardized β | P Value |
|---|---|---|---|---|
| Prescribed OME | 0.529 | 0.063 | 0.565 | <.001 |
| Age | −0.773 | 0.124 | −0.104 | <.001 |
| Sex | ||||
| Male | 1 [Reference] | 1 [Reference] | 1 [Reference] | NA |
| Female | 5.922 | 4.733 | 0.024 | .22 |
| Tobacco use | 21.596 | 5.331 | 0.0727 | <.001 |
| Alcohol use | 5.833 | 11.903 | 0.008 | .62 |
| Cancer | −12.728 | 11.637 | −0.025 | .27 |
| Obesity | 7.382 | 3.977 | 0.030 | .06 |
| ASA class | .046 | |||
| 1 | 1 [Reference] | 1 [Reference] | 1 [Reference] | NA |
| 2-3 | 3.688 | 7.898 | 0.009 | .64 |
| 4-5 | 31.920 | 14.175 | 0.046 | .02 |
| Elective surgery | −2.762 | 6.923 | −0.010 | .69 |
| Outpatient surgery | −17.731 | 5.389 | −0.072 | .001 |
| Procedure | .16 | |||
| Laparoscopic cholecystectomy | 1 [Reference] | 1 [Reference] | 1 [Reference] | NA |
| Laparoscopic appendectomy | −21.106 | 7.797 | −0.050 | .007 |
| Inguinal/femoral hernia repair (open/lap) | 13.092 | 5.597 | 0.048 | .02 |
| Open incisional hernia repair | 14.707 | 8.854 | 0.030 | .08 |
| Laparoscopic colectomy | −3.714 | 11.126 | −0.007 | .74 |
| Open colectomy | 8.869 | 15.185 | 0.145 | .56 |
| Ileostomy/colostomy | 8.819 | 19.129 | 0.010 | .67 |
| Small bowel resection/enterolysis | 39.391 | 25.941 | 0.037 | .13 |
| Thyroidectomy | −32.895 | 12.061 | −0.035 | .006 |
| Vaginal hysterectomy | −12.085 | 9.980 | −0.021 | .23 |
| Laparoscopic/robotic hysterectomy | −10.622 | 6.699 | −0.024 | .11 |
| Abdominal hysterectomy | 14.887 | 11.631 | 0.022 | .20 |
| Pain score | <.001 | |||
| No pain | 1 [Reference] | 1 [Reference] | 1 [Reference] | NA |
| Minimal | 8.329 | 5.898 | 0.033 | .16 |
| Moderate | 45.541 | 6.119 | 0.184 | <.001 |
| Severe | 82.337 | 9.497 | 0.217 | <.001 |
Abbreviations: ASA, American Society of Anesthesiologists; NA, not applicable; OME, oral morphine equivalents.
Figure 3. Association of Opioid Consumption With Opioid Prescription Size.
Risk-adjusted opioid consumption at the mean of each prescription size quintile in this cohort. Amount of opioid consumed and amount of opioid prescribed are represented as tablets of acetaminophen/hydrocodone, 5/325 mg. Mean quintile values are 17 tablets (range, 2-20), 29 tablets (range, 21-30), 36 tablets (range, 34-38), 42 tablets (range, 40-45), and 77 tablets (range, 48-384), respectively. Error bars show 95% confidence intervals for each mean. Amount of opioid consumed increased significantly at each quintile of prescription size.
Higher pain scores in the week following surgery were also associated with increased opioid consumption but to a lesser degree than quantity of opioid prescribed, as evidenced by smaller standardized β coefficients (standardized β = 0.184 for moderate pain and β = 0.217 for severe pain). Compared with patients reporting no pain after surgery, patients who reported moderate pain used an additional 45 OMEs (95% CI, 33.54-57.54; P < .001) (9 pills) and patients who reported severe pain used an additional 82.34 OMEs (95% CI, 63.71-100.96; P < .001) (16 pills). There was no increase in opioid consumption in patients who reported minimal pain. Patients who reported minimal pain used an additional 8 OMEs (95% CI, −3.12 to 19.87), although this was not significant (P = .19).
Tobacco users tended to use 21.60 more OMEs (95% CI, 11.14-32.05; P < .001), or roughly 4 more pills, than nonsmokers. Obese patients used 7.38 more OMEs (95% CI, 0.42-15.18; P = .064), or roughly 2 more pills, than nonobese patients. Patients with ASA class IV through V used 31.92 more OMEs (95% CI, 4.12-59.72; P = .02), or roughly 7 more pills, than ASA class I patients. Outpatient surgery was associated with a decreased opioid use of 17.73 OMEs (95% CI −28.30 to −7.16; P = .001), or roughly 4 pills. Increasing age was associated with decreased opioid consumption after surgery, with patients using 0.77 fewer OMEs (95% CI −1.02 to −0.53; P < .001) for each year in age. Thus, a 65-year-old patient tended to use roughly 23 fewer OMEs, or 4 fewer pills, than a 35-year-old patient. Sex, alcohol use, cancer, ASA class II through III, and elective surgery status had no significant association with opioid consumption after surgery.
Three of 12 surgical procedures were significantly associated with opioid consumption after adjusting for quantity prescribed and patient factors. Compared with laparoscopic cholecystectomy, patients tended to use an additional 13.09 OMEs (95% CI, 2.12-24.07; P = .02) (3 pills) following open or laparoscopic inguinal/femoral hernia repair. Following laparoscopic appendectomy, patients used 21.11 fewer OMEs (95% CI, −36.40 to −5.82; P = .007) (4 pills) and following thyroidectomy, patients used 32.90 fewer OMEs (95% CI −56.55 to −9.24; P = .006) (7 pills), compared with laparoscopic cholecystectomy.
Discussion
The 2 major findings of this study are that opioids are significantly overprescribed after common surgical procedures, and that the quantity of opioid prescribed is associated with higher patient-reported opioid consumption even after controlling for postoperative pain and other factors. Overprescribing was universally observed in this cohort, affecting each of the 12 procedures analyzed. This phenomenon was not limited to single, outlier institutions, but was widespread across many hospitals. This resulted in increased opioid consumption among patients who received larger prescriptions, as well as tens of thousands of leftover pills in 9 months that entered communities across the state of Michigan.
Our findings build on existing work demonstrating significant disparities between the quantity of opioids prescribed and consumed after surgery, posing a risk for diversion to other patients and the community.3,4,5,7 Importantly, overprescribing occurred for all procedures included in this study, from relatively minor to major operations. Most patients who receive opioids after surgery do not dispose of leftover medication.18 It is well established that most individuals who misuse prescription opioids obtain the medication from a friend or relative as opposed to “doctor shopping” or illicit sources.8 Additionally, many heroin users first obtain opioids from a prescription that is not their own.19,20 These data highlight the importance of significantly changing the way opioids are prescribed following surgery to decrease excess medication as a source for diversion and abuse.
This study also identified several factors that were associated with opioid consumption following surgery. Patient-specific factors associated with increased opioid consumption included tobacco use and ASA classes IV through V. Obese patients also trended toward significantly higher opioid use. Smoking is known to be associated with higher pain intensity and opioid use after surgery.21 Increased opioid use in patients with the highest ASA classification may be explained by the fact that preoperative opioid use is more common in patients with a greater number of comorbodities.22 These patients also often have prolonged periods of recovery following surgery, with pain delaying their return to baseline functional status. Conversely, outpatient surgery and increasing age were associated with decreased opioid consumption. The former finding may reflect the presence of fewer comorbidities in patients who are candidates for outpatient surgery, as well as the more routine use of multimodal analgesia in this setting. These results may assist surgeons in using patient characteristics to provide opioid prescriptions that more accurately reflect a given patient’s analgesic needs following surgery. For example, for 2 patients undergoing the same operation, these data suggest that a surgeon may comfortably expect an 80-year-old male nonsmoker to require significantly less postoperative analgesia than a 30-year-old female smoker with multiple comorbidities.
Association Between Quantity of Opioids Prescribed and Consumed
This study found that the quantity of opioids prescribed had the strongest association with opioid consumption after controlling for surgical procedure and patient-specific factors including postoperative pain. Importantly, this finding represents associations found in observational data and does not confirm a causal association between larger prescriptions and increased consumption. For example, the observed association between opioid prescribing and consumption in this study could be owing to clinicians prescribing more to patients with preoperative opioid use, who are known to consume more opioids.23,24 Because preoperative opioid use data were not collected in this cohort, we were unable to adjust for this in our analysis. Evaluating the effect of preoperative opioid use will be the focus of future data collection. Despite this limitation, others have demonstrated a similar association between opioid prescription size and consumption among patients undergoing laparoscopic cholecystectomy and cesarean delivery.7,10,11 A plausible explanation for the association between prescription size and medication use is the anchoring and adjustment heuristic. This is a psychologic heuristic wherein a piece of information serves as an anchor on which adjustments are made to reach an estimation or decision. For example, obesity literature has shown that food intake increases with portion size.25,26 In this case, a larger amount of opioids may serve as a mental anchor by which patients estimate their analgesic needs.
It is unsurprising that higher pain scores were associated with increased opioid use in this cohort. However, our model suggests that the quantity of opioids prescribed is significantly associated with opioid consumption after surgery, even more so than pain. This finding may have significant clinical implications. The amount of opioids a surgeon prescribes to a patient may influence that patient’s opioid consumption after surgery. Increased opioid consumption exposes patients to increased risk of the adverse effects of opioids. In studies of patients using opioids for nonsurgical pain, higher doses were found to be associated with increased risk of nonfatal and fatal overdose.27,28 At least 1 study has shown larger index opioid prescriptions are also associated with an increased rate of late opioid use, and this may play a role in postoperative opioid consumption as well.29 Interventions designed to reduce prescribing may further elucidate its association with opioid consumption.
Next Steps and Limitations
These findings represent the critical first step of a large-scale reduction strategy and should be leveraged to recommend practice change. By using patient-reported data to define consumption norms, procedure-specific prescribing recommendations can be developed for wide use, expanding previous single-institution efforts.10 In addition to procedure-specific recommendations, patient-specific recommendations can also be made to adjust for various factors associated with postoperative opioid consumption. While these factors will likely be more nuanced in their application, they have the potential to further tailor postoperative opioid prescribing. The end result would be prescriptions that adequately treat an individual patient’s postoperative pain while resulting in the smallest possible amount of leftover medication. Using collaborative infrastructures, such as the MSQC or other professional societies, prescribing recommendations can be disseminated widely. The effect of these changes can subsequently be evaluated and refined through ongoing data collection and analysis in a continuous cycle of quality improvement.30
This study is not without its limitations. Importantly, the logistical implementation of PRO collection precludes calculation of a response rate. As part of the pilot PRO collection, the MSQC allowed each hospital’s trained nurse reviewer to determine the sampling algorithm according to their fluctuating workload. For this reason, we could not reliably estimate the true denominator of patients contacted for PRO collection, because individual hospitals contacted had different sampling strategies. This limitation prevents us from knowing precisely how representative this sample is of the overall patient population. Additionally, roughly half of patients who responded to the survey and received an opioid prescription had incomplete survey or prescription data and were excluded from analysis. This is a limitation to the study because including these patients would have doubled our sample size, likely resulting in more robust findings. Despite these limitations in determining response rate, the patient demographics and procedures in our cohort are generally similar to the MSQC cohort for the same time frame. Another limitation is that no data regarding preoperative opioid use were collected, as previously discussed. This cohort almost certainly includes a number of patients who use opioids chronically because chronic opioid use has a prevalence of 3% to 4%.31 Using these data to develop prescribing recommendations may therefore overestimate postoperative analgesic requirements for opioid-naive patients. However, intentionally keeping future recommendations liberal in quantity may ultimately aid with widespread adoption, especially for clinicians concerned that prescribing reductions may lead to increased pain and calls for refills after surgery. Our future data collection efforts will focus on evaluating preoperative opioid use. Additionally, it is clear that significant outliers exist in opioid consumption, which is likely driven by patient-specific factors. An understanding of the factors that lead to minimal or excessive opioid use after surgery will further assist in improving prescribing practices. A further limitation is that patient consumption data relied on the patient’s recollection of how many pills they consumed, which may not be accurate. Nevertheless, studies that have prospectively analyzed use of prescribing recommendations showed good association with patient-reported consumption.
Conclusions
Excessive opioid prescribing after surgery is not limited to single, outlier procedures or institutions but is widespread. In addition, prescription size is strongly associated with increased opioid consumption among surgical patients. Recognizing overprescribing and accurately identifying patient consumption after surgery is the first step in improving prescribing practices. Other, multifactorial solutions, such as predictive modeling based on patient characteristics, also have the potential to improve postoperative prescribing. The immediate success of this approach at the local level is already promising, and future work will analyze the effect of procedure-specific prescribing recommendations at a statewide level.
eTable. Leftover Opioids by Procedure
References
- 1.Bicket MC, Long JJ, Pronovost PJ, Alexander GC, Wu CL. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152(11):1066-1071. doi: 10.1001/jamasurg.2017.0831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartels K, Mayes LM, Dingmann C, Bullard KJ, Hopfer CJ, Binswanger IA. Opioid use and storage patterns by patients after hospital discharge following surgery. PLoS One. 2016;11(1):e0147972. doi: 10.1371/journal.pone.0147972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates C, Laciak R, Southwick A, Bishoff J. Overprescription of postoperative narcotics: a look at postoperative pain medication delivery, consumption and disposal in urological practice. J Urol. 2011;185(2):551-555. doi: 10.1016/j.juro.2010.09.088 [DOI] [PubMed] [Google Scholar]
- 4.Harris K, Curtis J, Larsen B, et al. Opioid pain medication use after dermatologic surgery: a prospective observational study of 212 dermatologic surgery patients. JAMA Dermatol. 2013;149(3):317-321. doi: 10.1001/jamadermatol.2013.1871 [DOI] [PubMed] [Google Scholar]
- 5.Rodgers J, Cunningham K, Fitzgerald K, Finnerty E. Opioid consumption following outpatient upper extremity surgery. J Hand Surg Am. 2012;37(4):645-650. doi: 10.1016/j.jhsa.2012.01.035 [DOI] [PubMed] [Google Scholar]
- 6.Hill MV, McMahon ML, Stucke RS, Barth RJ Jr. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265(4):709-714. doi: 10.1097/SLA.0000000000001993 [DOI] [PubMed] [Google Scholar]
- 7.Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130(1):29-35. doi: 10.1097/AOG.0000000000002093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipari RN, Hughes A. How People Obtain the Prescription Pain Relievers They Misuse: The CBHSQ Report. Rockville, MD: Center for Behavioral Health Statistics and Quality, Subtances and Metal Health Services Administration; 2013. [PubMed] [Google Scholar]
- 9.Waljee JF, Li L, Brummett CM, Englesbe MJ. Iatrogenic opioid dependence in the United States: are surgeons the gatekeepers? Ann Surg. 2017;265(4):728-730. doi: 10.1097/SLA.0000000000001904 [DOI] [PubMed] [Google Scholar]
- 10.Howard R, Waljee J, Brummett C, Englesbe M, Lee J. Reduction in opioid prescribing through evidence-based prescribing guidelines. JAMA Surg. 2018;153(3):285-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill MV, Stucke RS, McMahon ML, Beeman JL, Barth RJ Jr. An educational intervention decreases opioid prescribing after general surgical operations. Ann Surg. 2018;267(3):468-472. doi: 10.1097/SLA.0000000000002198 [DOI] [PubMed] [Google Scholar]
- 12.Dwyer CL, Soong M, Hunter A, Dashe J, Tolo E, Kasparyan NG. Prospective evaluation of an opioid reduction protocol in hand surgery. J Hand Surg Am. 2018;43(6):516-522.e1. doi: 10.1016/j.jhsa.2017.06.022 [DOI] [PubMed] [Google Scholar]
- 13.Soffin EM, Waldman SA, Stack RJ, Liguori GA. An evidence-based approach to the prescription opioid epidemic in orthopedic surgery. Anesth Analg. 2017;125(5):1704-1713. doi: 10.1213/ANE.0000000000002433 [DOI] [PubMed] [Google Scholar]
- 14.Campbell DA Jr, Kubus JJ, Henke PK, Hutton M, Englesbe MJ; The Michigan Surgical Quality Collaborative . The Michigan Surgical Quality Collaborative: a legacy of Shukri Khuri. Am J Surg. 2009;198(5)(suppl):S49-S55. doi: 10.1016/j.amjsurg.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 15.Englesbe MJ, Dimick JB, Sonnenday CJ, Share DA, Campbell DA Jr. The Michigan Surgical Quality Collaborative: will a statewide quality improvement initiative pay for itself? Ann Surg. 2007;246(6):1100-1103. doi: 10.1097/SLA.0b013e31815c3fe5 [DOI] [PubMed] [Google Scholar]
- 16.Healy MA, Regenbogen SE, Kanters AE, et al. Surgeon variation in complications with minimally invasive and open colectomy: results from the Michigan Surgical Quality Collaborative. JAMA Surg. 2017;152(9):860-867. doi: 10.1001/jamasurg.2017.1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gammaitoni AR, Fine P, Alvarez N, McPherson ML, Bergmark S. Clinical application of opioid equianalgesic data. Clin J Pain. 2003;19(5):286-297. doi: 10.1097/00002508-200309000-00002 [DOI] [PubMed] [Google Scholar]
- 18.Hasak JM, Roth Bettlach CL, Santosa KB, Larson EL, Stroud J, Mackinnon SE. Empowering post-surgical patients to improve opioid disposal: a before and after quality improvement study. J Am Coll Surg. 2018;226(3):235-240.e3. doi: 10.1016/j.jamcollsurg.2017.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154-163. doi: 10.1056/NEJMra1508490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers: United States, 2002-2004 and 2008-2010. Drug Alcohol Depend. 2013;132(1-2):95-100. doi: 10.1016/j.drugalcdep.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 21.Montbriand JJ, Weinrib AZ, Azam MA, et al. Smoking, pain intensity, and opioid consumption 1-3 months after major surgery: a retrospective study in a hospital-based transitional pain service. Nicotine Tob Res. 2018;20(9):1144-1151. [DOI] [PubMed] [Google Scholar]
- 22.Hilliard PE, Waljee J, Moser S, et al. Prevalence of preoperative opioid use and characteristics associated with opioid use among patients presenting for surgery [published online July 11, 2018]. JAMA Surg. doi: 10.1001/jamasurg.2018.2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roullet S, Nouette-Gaulain K, Biais M, et al. Preoperative opioid consumption increases morphine requirement after leg amputation. Can J Anaesth. 2009;56(12):908-913. doi: 10.1007/s12630-009-9185-8 [DOI] [PubMed] [Google Scholar]
- 24.Armaghani SJ, Lee DS, Bible JE, et al. Preoperative opioid use and its association with perioperative opioid demand and postoperative opioid independence in patients undergoing spine surgery. Spine (Phila Pa 1976). 2014;39(25):E1524-E1530. doi: 10.1097/BRS.0000000000000622 [DOI] [PubMed] [Google Scholar]
- 25.Rolls BJ, Roe LS, Meengs JS. The effect of large portion sizes on energy intake is sustained for 11 days. Obesity (Silver Spring). 2007;15(6):1535-1543. doi: 10.1038/oby.2007.182 [DOI] [PubMed] [Google Scholar]
- 26.Young LR, Nestle M. The contribution of expanding portion sizes to the US obesity epidemic. Am J Public Health. 2002;92(2):246-249. doi: 10.2105/AJPH.92.2.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. doi: 10.7326/0003-4819-152-2-201001190-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686-691. doi: 10.1001/archinternmed.2011.117 [DOI] [PubMed] [Google Scholar]
- 29.Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine (Phila Pa 1976). 2007;32(19):2127-2132. doi: 10.1097/BRS.0b013e318145a731 [DOI] [PubMed] [Google Scholar]
- 30.Campbell DA Jr, Dellinger EP. Multihospital collaborations for surgical quality improvement. JAMA. 2009;302(14):1584-1585. doi: 10.1001/jama.2009.1474 [DOI] [PubMed] [Google Scholar]
- 31.Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 2009;18(12):1166-1175. doi: 10.1002/pds.1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Leftover Opioids by Procedure