Key Points
Question
Is aspirin alone inferior to other anticoagulant medications for reducing the risk of venous thromboembolism (VTE) in patients after total knee arthroplasty?
Findings
In this cohort study, 573 of 41 537 patients after total knee arthroplasty sustained a VTE event. Of those receiving prophylaxis, 541 had a VTE event; 149 if receiving aspirin alone, 321 when prescribed anticoagulation alone, and 71 if prescribed both aspirin and anticoagulation.
Meaning
Aspirin alone may be an appropriate alternative to other pharmacologic prophylaxis in preventing VTE for patients undergoing total knee arthroplasty.
Abstract
Importance
There has been significant debate in the surgical and medical communities regarding the appropriateness of using aspirin alone for venous thromboembolism (VTE) prophylaxis following total knee arthroplasty (TKA).
Objective
To determine the acceptability of aspirin alone vs anticoagulant prophylaxis for reducing the risk of postoperative VTE in patients undergoing TKA.
Design, Setting, and Participants
Noninferiority study of a retrospective cohort of TKA cases submitted to the Michigan Arthroplasty Registry Collaborative Quality Initiative at 29 member hospitals, ranging from small community hospitals to large academic and nonacademic medical centers in Michigan. The study included 41 537 patients who underwent primary TKA between April 1, 2013, and October 31, 2015. Clinical events were monitored for 90 days after surgery. Data were analyzed between September and October 2016.
Exposures
The method of pharmacologic prophylaxis: neither aspirin nor anticoagulants for 668 patients (1.6%), aspirin only for 12 831 patients (30.9%), anticoagulant only (eg, low-molecular-weight heparin, warfarin, and Xa inhibitors) for 22 620 patients (54.5%), and both aspirin/anticoagulant for 5418 patients (13.0%). Most patients were also using intermittent pneumatic compression stockings.
Main Outcome and Measures
The primary composite outcome was the first occurrence of VTE or death. The noninferiority margin was specified as 0.3. The secondary outcome was bleeding events.
Results
Of the 41 537 patients, 14 966 were men (36%), and the mean age was 65.8 years. A VTE event occurred in 573 of 41 537 patients (1.38%); 32 of 668 (4.79%) who received no pharmacologic prophylaxis, 149 of 12 831 (1.16%) treated with aspirin alone, 321 of 22 620 (1.42%) with anticoagulation alone, and 71 of 5418 (1.31%) prescribed both aspirin and anticoagulation. Aspirin only was noninferior for the composite VTE outcome compared with those receiving other chemoprophylaxis (adjusted odds ratio, 0.85; 95% CI, 0.68-1.07, P for inferiority = .007). Bleeding occurred in 457 of 41 537 patients (1.10%), 10 of 668 (1.50%) without prophylaxis, 116 of 12 831 (0.90%) in the aspirin group, 258 of 22 620 (1.14%) with anticoagulation, and 73 of 5418 (1.35%) of those receiving both. Aspirin alone was also noninferior for bleeding complications (adjusted odds ratio, 0.80; 95% CI, 0.63-1.00, P for inferiority <.001).
Conclusions and Relevance
In this study of patients undergoing TKA, aspirin was not inferior to other anticoagulants in the postoperative rate of VTE or death. Aspirin alone may provide similar protection from postoperative VTE compared with other anticoagulation treatments.
This cohort study examines whether aspirin as a single agent is inferior to other anticoagulation agents for reducing the risk of venous thromboembolism or major bleeding events after unilateral primary total knee arthroplasty.
Introduction
In 2015, an estimated 719 000 total knee arthroplasties (TKA) were performed in the United States.1 A significant associated risk is venous thromboembolism (VTE), including fatal pulmonary embolus (PE). In an early report of cases performed from 1974 to 1979, the incidence of symptomatic PE was 1.7%.2 Anticoagulation has been recommended since. Both the American College of Chest Physicians and the American Association of Orthopaedic Surgeons (AAOS) recommended the use of pharmacologic VTE prophylaxis.3,4 The American College of Chest Physicians favored low-molecular-weight heparin (LMWH), while the AAOS felt that there was insufficient evidence for a specific recommendation. Studies, such as the Pulmonary Embolism Prevention trial,5 found a significant reduction in VTE events in patients with hip fracture receiving aspirin, but significance was not reached in patients undergoing elective hip or knee arthroplasty. Additionally, the Pulmonary Embolism Prevention trial was not designed to answer whether aspirin alone could replace anticoagulants for VTE prophylaxis. A systematic review and meta-analysis6 of aspirin as prophylaxis after TKA concluded that most studies were of low quality, and numerous studies comparing prophylaxis include cases with asymptomatic deep venous thromboembolism.6,7,8 Constructing randomized clinical trials comparing different prophylactic strategies has been difficult, given the overall low incidence of VTE events.9,10 Additionally, many studies underrepresented the risks of surgical site bleeding and reoperation after TKA.11,12 There are concerns of potential publication bias in favor of anticoagulant prophylaxis for VTE.13 Total knee arthroplasty has undergone significant changes in the last decade, resulting in a reduced VTE risk not accounted for in the current guidelines.14,15 The purpose of this study is to determine whether aspirin as a single agent is inferior to other anticoagulation agents for reducing the risk of VTE or major bleeding events after unilateral primary TKA.
Methods
Study Population
Background information on the Michigan Arthroplasty Registry Collaborative Quality Initiative (MARCQI), data sources, and variables collected have been previously described.16 This is a multihospital consortium through which all elective primary total hip and knee arthroplasties at participating sites are entered into a registry with clinical laboratory values and preoperative, perioperative, and postoperative information. Trained clinical medical record abstractors follow up each case for 90 days after surgery using available data sources, such as hospital medical records and surgeon office notes. The Michigan Inpatient Data Base of the Michigan Hospital Association is used to obtain supplemental information for diagnoses and events occurring at the index hospital, as well as at external hospitals over time. Patients who left the state of Michigan would be lost to follow-up, but because all cases were manually abstracted out to 90 days, this is likely to be a rare scenario.
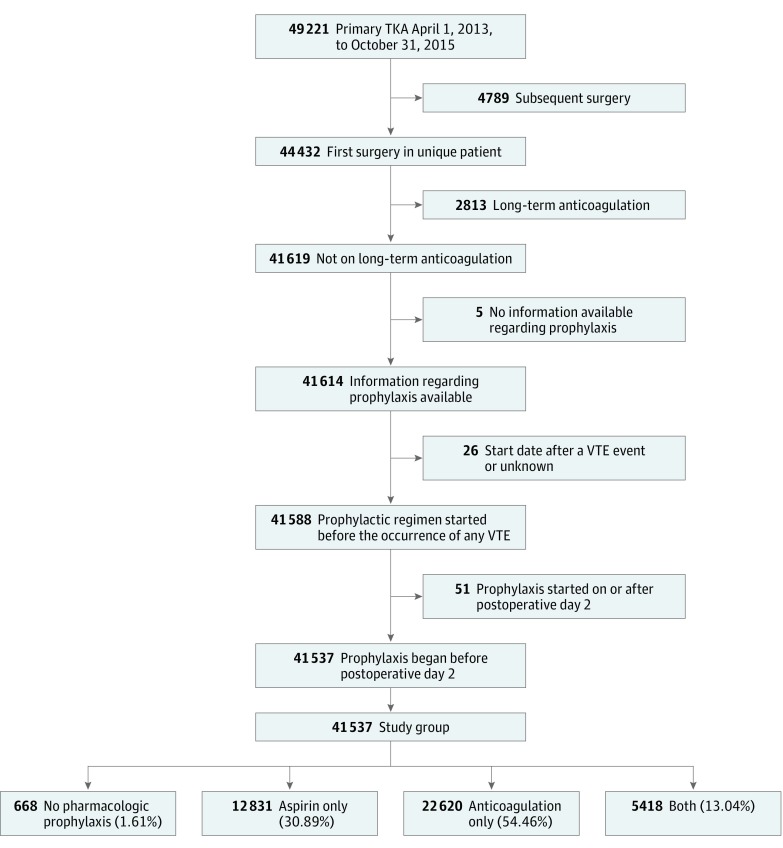
This study population was a retrospective cohort of 49 221 consecutive primary unilateral total knee arthroplasty cases performed at MARCQI=participating hospitals between April 1, 2013, and October 31, 2015. Each case was followed up for 90 days after the day of surgery, making the latest date for follow-up January 28, 2016. For patients with multiple surgeries recorded in the registry, only the first surgery was included, excluding 4789 subsequent surgeries. The final data set contained information on 41 537 cases after excluding those in which the patient was taking anticoagulants on a daily basis within 30 days prior to surgery (2813 cases), anticoagulation started after a VTE event (26 cases), the only prophylaxis used began on or after postoperative day 2 (51 cases), or no recorded information regarding the presence or absence of VTE prophylaxis (5 cases) (Figure 1).
Figure 1. Data Flowchart for Michigan Arthroplasty Registry Collaborative Quality Initiative (MARCQI) Venous Thromboembolism (VTE) Analysis.
TKA indicates total knee arthroplasty.
The MARCQI member hospitals were advised to follow the AAOS Guideline on Preventing Venous Thromboembolic Disease in Patients Undergoing Elective Hip and Knee Arthroplasty4 that included aspirin for prophylaxis and recommend screening for VTE risk. At MARCQI quarterly meetings, clinicians were encouraged to use regional anesthesia, tranexamic acid, intermittent pneumatic compression devices, and early mobilization.17
Institutional review board review was obtained from the St Joseph Mercy Health System institutional review board, and the study was deemed to be exempt on July 2, 2014. For this reason, patient consent was not obtained. The original study protocol, dated June 15, 2014, proposed a study population that included both hip and knee arthroplasties. A power analysis with the number of cases available at that time showed that there was not adequate power. A second power analysis, conducted on October 2, 2014, based on a noninferiority analysis, assuming a baseline event rate of 1.0% and a noninferiority margin of .50, showed a power of 54% and indicated there were still too few cases in the registry at that time. Because a sufficient number of additional knee cases but not hip cases could be accrued over the subsequent 12 to 18 months, the study team narrowed the study to knee cases only and deferred analysis until adequate cases were accrued. Before the final analysis was conducted, there was a change in the study personnel that led to meetings to clarify the study question and appropriate statistical approach beginning September 2016.
The final power analysis was performed October 7, 2016, and found that 41 000 cases would be needed to reach 80% power using a noninferiority margin of 0.3 (upper limit of odds ratio [OR], 1.3).18 This margin was selected by clinical experts on the research team as an acceptable difference given the significant clinical implications of VTE. For comparison, this margin is more conservative than the double increased risk chosen in a 2018 equivalence study19 of aspirin and rivaroxaban. The final analytical plan assessed the noninferiority of aspirin vs other pharmacologic agents for prevention of VTE after TKA, with a secondary outcome of bleeding events.
Main Outcomes
The primary composite outcome was the first occurrence of pulmonary embolism, deep venous thrombosis, or death during the 90-day postoperative period as determined by trained nurse abstractors. To avoid including superficial clots that were not clinically significant, a VTE event required a documented diagnosis, a confirmatory study, and treatment. Information as to location of the VTE and whether it was symptomatic was not recorded.
The secondary outcome was the occurrence of a major bleeding event within 90 days of surgery, excluding those that could be attributed to therapeutic anticoagulation for a VTE event. The date of the bleeding event was therefore part of the definition. For patients having a VTE event during the index hospitalization, a bleeding event was defined as a drop in hemoglobin of 7 g/dL or more (to convert to grams per liter, multiply by 10), a hematoma that occurred prior to the VTE, or a bleeding diagnosis recorded in the Michigan Inpatient Data Base that was not present on admission. For patients whose VTE occurred after the index hospitalization, the definition of bleeding was similar except that bleeding-related diagnoses recorded on readmissions identified in the Michigan Inpatient Data Base were required to be deemed present on admission. For patients not having a VTE during the 90 days of follow-up, the first occurrence of any of the aforementioned events was considered a major bleeding event.
Exposure
The exposure variable was the choice of agents for VTE prophylaxis initiated during the 3-day perioperative window (1 day prior to surgery, the day of surgery, and the day following surgery). There were 4 possible mutually exclusive categories: aspirin only, the use of any other nonaspirin anticoagulation agent, a combination of aspirin and anticoagulation, or no pharmacoprophylaxis. Agents administered after a VTE event were not considered. The anticoagulation category included direct-factor Xa inhibitors, direct thrombin inhibitors, LMWH, synthetic pentasaccharides, and warfarin. The association of individual agents with VTE was not studied, in part owing to numerous combinations of anticoagulants used and various durations of the individual agents. The start and stop dates of specific medications were available in the registry but not the corresponding dosages.
Statistical Analysis
SAS, version 9.4, and SAS macro language (SAS Institute) were used for the statistical analyses. The level of statistical significance was α= .05. The testing was 2-sided except for noninferiority testing, which was 1-sided. Kruskal-Wallis test was used to compare age, body mass index, preoperative creatinine levels, and hemoglobin levels and operation times between prophylaxis groups. χ2 Test was used to test the homogeneity of categorical variables with prophylaxis groups.
To minimize the loss of power and decrease bias owing to exclusion of cases that were missing 1 or more covariates, missing covariates were imputed 10 times using multivariate sequential regression approach in SAS. The final analysis controlled for numerous patient, surgical, and hospital-level variables through inverse probability of treatment weighting. With this approach, cases in which the choice of prophylaxis appears statistically to have been heavily influenced by the risk factors considered are given less weight in the final analysis than cases for which the choice of strategy appears more random. Consequently, the analysis produces results that are less confounded, although never definitive and always subject to critical appraisal. Details are provided in the eMethods in the Supplement. The weights were derived from multinomial regression that accounted for differences in the prophylactic regimens based on differences in age; sex; race/ethnicity; body mass index; Elixhauser comorbidity diagnoses20; preoperative values of hemoglobin, platelets, and creatinine; smoking history; preoperative treatment with antiplatelet agents; steroids and narcotics; preoperative history of venous thromboembolic disease; history of bleeding disorders; preoperative use of a cane or walker; American Society of Anesthesiologists status; marital status; type of insurance; surgical approach; operative duration; intermittent pneumatic compression devices; foot pumps; tranexamic acid; and anesthesia type (general or other).21,22,23 Race/ethnicity was included owing to the relative increase risk of VTE in African American individuals24 and was recorded by the clinical site abstractors as recorded in the hospital medical records. The hospital’s frequency of screening for VTE was also included in the risk adjustment, calculated as the total number of venous ultrasonography examinations performed postoperatively divided by the number of cases performed at the hospital.25 Hierarchical logistic regression models were used for the primary VTE and for the secondary bleeding analyses, weighted by the standardized inverse probability of treatment weights. The hierarchical nature of the data in the outcome model was evaluated by including a random effect for surgeon.26 To test how the trajectories of composite VTE and bleeding events changed from 2013 to 2015, logistic regression models were fitted using 11 quarterly data points for the 2 outcomes separately. The β coefficients (standard error and P value) were computed. The P value level of significance was .05, and all P values were 2-sided. Finally, a sensitivity analysis was performed excluding patients with a history of VTE from the data set and then refitting all models with an updated inverse probability of treatment weighting. Odds ratios and P values were recalculated.
Results
There were 49 221 TKA cases in the database, and 41 537 met inclusion criteria (Figure 1). The surgeries were performed in 29 hospitals by 374 surgeons. Six hundred sixty-eight patients (1.6%) were not receiving any pharmacologic VTE prophylaxis, 12 831 patients (30.9%) were receiving aspirin only, 22 620 (54.5%) were receiving at least 1 of 5 nonaspirin chemoprophylactic agents, and 5418 (13.0%) were receiving both aspirin and nonaspirin prophylaxis. Unadjusted, baseline patient demographics and characteristics are detailed in Table 1. Additional information on the study population is provided in eTables 1 and 2 the Supplement.
Table 1. Characteristics of Study Population (N = 41 537).
| Variable | Population, No. (%) (N = 41 537) | None, No./Total No. (%) (N = 668) | Aspirin Only, No./Total No. (N = 12 831) | Anticoagulant Only, No./Total No. (N = 22 620) | Both, No./Total No. (N = 5418) | P Value of Statistical Tests |
|---|---|---|---|---|---|---|
| Continuous variables | ||||||
| Age, y, No. (mean [SD]) | 41 537 (65.8 [9.8]) | 668 (65.7 [10.2]) | 12 831 (65.6 [9.8]) | 22 620 (65.5 [9.8]) | 5418 (67.4 [9.4]) | <.001a |
| BMI, No. (mean [SD]) | 41 369 (33.1 [6.9]) | 664 (32.6 [6.6]) | 12 783 (32.7 [6.7]) | 22 523 (33.4 [7.1]) | 5399 (33.1 [6.8]) | <.001a |
| Categorical variables | ||||||
| African American race | 3230 (7.8) | 54/668 (8.1) | 855/12 831 (6.7) | 1840/22 620 (8.1) | 481/5418 (8.9) | <.001b |
| Male | 14 966 (36.1) | 261/668 (39.1) | 4882/12 822 (38.1) | 7499/22 605 (33.2) | 2324/5418 (42.9) | <.001b |
| Marital status (married) | 27 462 (66.2) | 453/667 (67.9) | 8658/12 795 (67.7) | 14 652/22 595 (64.8) | 3699/5415 (68.3) | <.001b |
| Surgical approach | ||||||
| Medial parapatellar | 34 185 (82.3) | 542/668 (81.1) | 10 424/12 831 (81.2) | 19 158/22 620 (84.7) | 4061/5418 (75.0) | <.001b |
| Lateral parapatellar | 181 (0.4) | <10c | 96/12 831 (0.8) | 52/22 620 (0.2) | 27/5418 (0.5) | |
| Midvastus | 5814 (14.0) | 90/668 (13.5) | 1774/12 831 (13.8) | 2800/22 620 (12.4) | 1150/5418 (21.2) | |
| Subvastus | 1016 (2.5) | 15/668 (2.3) | 420/12 831 (3.3) | 479/22 620 (2.1) | 102/5418 (1.9) | |
| Other | 341 (0.8) | 15/668 (2.3) | 117/12 831 (0.9) | 131/22 620 (0.6) | 78/5418 (1.4) | |
| General anesthesia | 13 383 (32.2) | 265/667 (39.7) | 4211/12 819 (32.8) | 7021/22 617 (31.0) | 1886/5418 (34.8) | <.001b |
| ASA status | ||||||
| I | 702 (1.7) | <10c | 250/12 806 (2.0) | 338/22 599 (1.5) | 107/5416 (2.0) | <.001b |
| II | 21 837 (52.6) | 322/667 (48.3) | 6986/12 806 (54.6) | 11 873/22 599 (52.5) | 2656/5416 (49.0) | |
| III | 18 480 (44.5) | 323/667 (48.4) | 5438/12 806 (42.5) | 10 130/22 599 (44.8) | 2589/5416 (47.8) | |
| IV | 469 (1.1) | 15/667 (2.3) | 132/12 806 (1.0) | 258/22 599 (1.1) | 64/5416 (1.2) | |
| Bleeding disorder | 472 (1.1) | 36/667 (5.4) | 136/12 799 (1.1) | 232/22 596 (1.0) | 68/5414 (1.3) | <.001b |
| VTE | 2320 (5.6) | 39/667 (5.9) | 399/12 803 (3.1) | 1532/22 589 (6.8) | 350/5409 (6.5) | <.001b |
| Tranexamic acid given | 25 698 (61.9) | 381/668 (57.0) | 9135/12 831 (71.2) | 12 678/22 620 (56.0) | 3504/5418 (64.7) | <.001b |
| Intermittent pneumatic compression | 36 113 (86.9) | 607/668 (90.9) | 11 755/12 831 (91.6) | 19 670/22 620 (87.0) | 4081/5418 (75.3) | <.001b |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); VTE, venous thromboembolism.
Kruskal-Wallis Test: nonparametric testing of continuous variables for comparing independent group.
Pearson χ2 tests of homogeneity/independence for categorical variables with prophylaxis groups.
Actual numbers for cell size less than 10 are not shown.
A VTE event occurred in 1.38% of patients (573 of 41 537). Of the 668 patients receiving no pharmacologic prophylaxis, 4.79% (n = 32 of 668) had a composite VTE event; 2.40%, had a deep venous thromboembolism (n = 16 of 668), 1.95% had a PE (n = 13 of 668), and there were 3 deaths (0.45%). The rates of the composite VTE outcome and the components of death, pulmonary embolism, and deep venous thrombosis for the other regimens are shown in Table 2. A separate χ2 test of the history of VTE alone was significantly different in the aspirin group compared with other groups (none, n = 39 [5.9%]; aspirin, n = 399 [3.1%]; anticoagulation, n = 1532 [6.8%]; both, n = 350 [6.5%]; P < .001), likely reflecting expected differences in patient selection.
Table 2. Unadjusted Outcomes by Venous Thromboembolism Prophylaxis Treatment Group.
| Outcome | No. (%) | P Value of Pearson χ2 Testa | |||
|---|---|---|---|---|---|
| None | Aspirin Only | Anticoagulation | Both | ||
| Total No. | 668 (1.61) | 12 831 (30.89) | 22 620 (54.46) | 5418 (13.04) | NA |
| Composite VTE (death, PE, or DVT) | 32 (4.79) | 149 (1.16) | 321 (1.42) | 71 (1.31) | <.001 |
| Death | <10b | 13 (0.10) | 28 (0.12) | <10b | NA |
| Pulmonary embolism | 13 (1.95) | 41 (0.32) | 89 (0.39) | 25 (0.46) | <.001 |
| Deep venous thrombosis | 16 (2.40) | 95 (0.74) | 204 (0.90) | 41 (0.76) | <.001 |
| Bleeding outcome | 10 (1.50) | 116 (0.90) | 258 (1.14) | 73 (1.35) | .03 |
Abbrevations: DVT, deep venous thromboembolism; NA, not applicable; PE, pulmonary embolus; VTE, venous thromboembolism.
Pearson χ2 tests of homogeneity/independence for categorical variables with prophylaxis groups.
Actual numbers for cell size less than 10 are not shown.
Multivariable analyses of the primary outcome reflecting adjustment for confounding showed that the patients receiving no prophylaxis had a several-fold higher risk than those given prophylaxis (adjusted OR, 5.13; 95% CI, 3.74-7.02). Patients taking aspirin only compared with other chemoprophylactic regimens had a noninferior risk of the composite VTE outcome, with an adjusted OR of 0.85 (95% CI, 0.68-1.07; P for inferiority = .007).
Overall, 1.10% of patients had the secondary outcome of bleeding (n = 457 of 41 537). In the 668 without prophylaxis, 1.50% had a bleeding event (n = 10 of 668). In the aspirin group, 0.90% (n = 116 of 12 831) had bleeding events. Bleeding occurred in 1.14% (n = 258 of 22 620) with anticoagulation and 1.35% (n = 73 of 5418) with both. After adjustment, aspirin was not inferior in the risk for a bleeding event when compared with other chemoprophylaxis (adjusted OR, 0.80; 95% CI, 0.63-1.00; P for inferiority <.001) (Table 3). The findings were similar in the sensitivity analysis in which patients with a history of VTE were excluded (eAppendix in the Supplement).
Table 3. Odds Ratio for the Primary Outcome Venous Thromboembolism/Death and Secondary Outcome Bleeding Eventa.
| Comparison | VTE/Death | Bleeding Event | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjustedb | Unadjusted | Adjustedb | |||||||
| OR (95% CI) | P Value | OR (95% CI) | P Value | P Value for Inferiority | OR (95% CI) | P Value | OR (95% CI) | P Value | P Value for Inferiority | |
| None vs anticoagulant only | 3.52 (2.40-5.15) | <.001 | 5.13 (3.74-7.02) | <.001 | NA | 1.33 (0.70-2.53) | .38 | 1.20 (0.72-2.00) | .49 | NA |
| Aspirin only vs anticoagulant only | 0.82 (0.66-1.02) | .07 | 0.85 (0.68-1.07) | .23 | .007 | 0.81 (0.64-1.02) | .08 | 0.80 (0.63-1.00) | .05 | <.001 |
| Both vs anticoagulant only | 1.04 (0.79-1.36) | .80 | 1.01 (0.77-1.32) | >.99 | NA | 1.25 (0.95-1.65) | .11 | 0.98 (0.75-1.28) | .90 | NA |
Abbreviations: NA, not applicable; OR, odds ratio; VTE, venous thromboembolism.
Confounders considered in propensity score model include age and age squared, body mass index and body mass index squared, preoperative hemoglobin, platelets and creatinine, sex, American Society of Anesthesiologists (ASA) status, race/ethnicity, smoking status, insurance, marital status, preoperative narcotic, steroid or antiplatelet use, bleeding disorder, history of deep vein thrombosis or pulmonary embolus, use of assistive devices, Elixhauser variables, surgical approach, operative duration, pneumatic compression devices, foot pumps, tranexamic acid, and anesthesia type.
Risk-adjusted model.
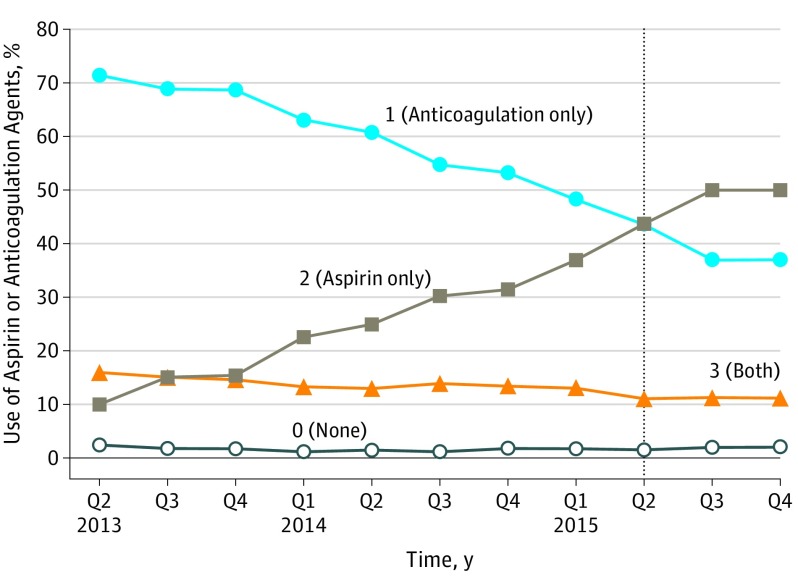
During the study period, the use of chemoprophylaxis other than aspirin, including patients receiving both, decreased from 87.4% (n = 1847) to 47.9% (n = 877) of patients, whereas aspirin use rose from 10.2% (n = 215) to 50.0% (n = 915) (Figure 2). There was no rise in the rate of VTE events (β = −0.02; SE = 0.01; P = .15), or bleeding events (β = −0.02; SE = 0.02; P = .21) during this time.
Figure 2. Trends in the Use of Aspirin and Anticoagulation Agents in Michigan Arthroplasty Registry Collaborative Quality Initiative (MARCQI) by Quarter (Q) From Second Q 2013 to Fourth Q 2015.
Discussion
In patients undergoing unilateral, primary TKA, aspirin alone was found to be noninferior to other forms of chemoprophylaxis in rates of VTE event or death, adjusting for confounders. These findings were supported by the stable incidence of composite VTE events despite a dramatic increase in the use of aspirin during the study period.
The results emphasize the need for chemoprophylaxis vs no prophylaxis to reduce the risk of VTE, given the dramatically higher odds of a VTE in the group without prophylaxis. The registry data do not capture the reason 668 patients received no prophylaxis. The AAOS guidelines do recommend this approach along with intermittent pneumatic compression devices for patients at increased risk of sustaining a bleeding event but at standard risk for a VTE event.4 This group had a 1.5% incidence of bleeding complication. However, the adjusted odds of bleeding were not significantly different for aspirin compared with anticoagulant or no prophylaxis vs anticoagulant.
There are several reasons to prefer using aspirin for VTE prophylaxis in the appropriately screened patient. Aspirin administration is simple, safe, and does not require monitoring. Although this study did not find a significant difference, a 2008 practice survey conducted by the American Association of Hip and Knee Surgeons found that while most orthopaedic surgeons felt LMWH to be most efficacious, aspirin was felt to be the easiest to use with the lowest risk of bleeding or wound complications.27 Aspirin is also much less expensive. The reported cost for a 30-day supply of rivaroxaban is approximately $379 to $450,28,29 and that of LMWH is estimated at $450 to $890.29,30 Warfarin costs a few dollars for a 30-day course, but with monitoring considered, the cost approaches that of the other anticoagulants.31 In contrast, aspirin costs approximately $2 per month, and no monitoring is needed. These cost differences could have a substantial association with a patient’s out-of-pocket expenses as well as the cost of a hospital’s overall bundle in the new episode payment models.
Limitations
The study has several limitations. First, the results may not apply to all settings outside of the MARCQI collaborative. Although this work represents the experience of a large number of patients and surgeons, it reflects an active, collaborative, quality improvement environment with motivated participants. Second, this is an observational study and can only demonstrate association and not causation. The analysis is limited by and dependent on the variables collected and the quality of the data. The surgeons chose aspirin or another method of anticoagulation based on their individual practice, determination of patient risk, and interpretation of the guidelines. Although measured potential confounders, including history of VTE, were addressed, it is not known to what extent the results were affected by unmeasured variables, such as intraoperative soft tissue handling or postoperative rehabilitation protocols. The conclusions of noninferiority were unchanged by the sensitivity analysis that excluded patients with history of VTE, but caution is needed when applying the results of this study to the treatment of patients with prior VTE. Third, we did not examine event rates for the specific agents included in the anticoagulation category. This was in part owing to the numerous combinations of agents used together as well as unknown information regarding the dosages used. Fourth, there are limitations with the definition of bleeding events. One could argue that a drop in hemoglobin level of 7 g/dL or greater is too stringent and could undercapture significant bleeding events. Also, the data collection protocol did not account for potential intra-articular bleeding that did not lead to a hospitalization, documentation of a hematoma, or a wound complication. Decreased local bleeding, swelling, and inflammation are difficult to track but may influence outcomes such as range of motion, function, and satisfaction. Future studies, including more standard definitions of bleeding events and the inclusion of patient-reported outcomes, may improve the validity of the events reported.
Despite these limitations, further evidence to support the use of aspirin is provided by the stable incidence of VTE during the study period despite a dramatic rise in aspirin use (Figure 2). There is also increasing evidence in the literature for low rates of VTE events with the use of aspirin.19,31 The increased use of aspirin is consistent with a 201632 study and is likely multifactorial. As Shah et al32 mention, a significant driver for the increased adoption is alignment of the clinical practice guidelines from AAOS and American College of Chest Physicians and the inclusion of aspirin in the Surgical Care Improvement Project recommendations in 2014.
While this observational study supports the use of aspirin, clinical judgment is still required for the ultimate selection of prophylaxis. In addition, only the pharmacologic prophylaxis was studied. This is just 1 component of a modern protocol that should also include regional anesthesia, comprehensive pain management protocols, intermittent pneumatic compression devices, tranexamic acid, and early mobilization. The expanded use of aspirin alone and the clarification of guidelines have the potential to simplify the care of these patients by surgeons, hospitalists, and primary care physicians and significantly reduce the cost of the episode of care without increasing the risk of VTE events.
Conclusions
Among patients undergoing TKA, aspirin was not inferior to other anticoagulants in the postoperative rate of VTE or death. Aspirin alone may provide similar protection from postoperative VTE compared with other anticoagulation treatments. Further prospective studies are needed to confirm these findings.
eMethods.
eTable 1. Raw Baseline Data of Study Population
eTable 2. Adjusted Odds Ratio for the Primary Outcome VTE/Death and Secondary Outcome Bleeding Event: Excluding Patients With VTE history
References
- 1.Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630. doi: 10.2106/JBJS.M.00285 [DOI] [PubMed] [Google Scholar]
- 2.Stulberg BN, Insall JN, Williams GW, Ghelman B. Deep-vein thrombosis following total knee replacement: an analysis of six hundred and thirty-eight arthroplasties. J Bone Joint Surg Am. 1984;66(2):194-201. doi: 10.2106/00004623-198466020-00005 [DOI] [PubMed] [Google Scholar]
- 3.Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2)(suppl):e278S-e325S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mont MA, Jacobs JJ, Boggio LN, et al. ; AAOS . Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg. 2011;19(12):768-776. doi: 10.5435/00124635-201112000-00007 [DOI] [PubMed] [Google Scholar]
- 5.Pulmonary Embolism Prevention (PEP) Trial Collaborative Group Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet. 2000;355(9212):1295-1302. doi: 10.1016/S0140-6736(00)02110-3 [DOI] [PubMed] [Google Scholar]
- 6.An VV, Phan K, Levy YD, Bruce WJ. Aspirin as thromboprophylaxis in hip and knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2016;31(11):2608-2616. doi: 10.1016/j.arth.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 7.Parvizi J, Jacovides CL, Bican O, et al. Is deep vein thrombosis a good proxy for pulmonary embolus? J Arthroplasty. 2010;25(6)(suppl):138-144. doi: 10.1016/j.arth.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 8.Budhiparama NC, Abdel MP, Ifran NN, Parratte S. Venous thromboembolism (VTE) prophylaxis for hip and knee arthroplasty: changing trends. Curr Rev Musculoskelet Med. 2014;7(2):108-116. doi: 10.1007/s12178-014-9207-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391. doi: 10.1302/0301-620X.88B3.17207 [DOI] [PubMed] [Google Scholar]
- 10.Freedman KB, Brookenthal KR, Fitzgerald RH Jr, Williams S, Lonner JH. A meta-analysis of thromboembolic prophylaxis following elective total hip arthroplasty. J Bone Joint Surg Am. 2000;82-A(7):929-938. doi: 10.2106/00004623-200007000-00004 [DOI] [PubMed] [Google Scholar]
- 11.Galat DD, McGovern SC, Larson DR, Harrington JR, Hanssen AD, Clarke HD. Surgical treatment of early wound complications following primary total knee arthroplasty. J Bone Joint Surg Am. 2009;91(1):48-54. doi: 10.2106/JBJS.G.01371 [DOI] [PubMed] [Google Scholar]
- 12.Treasure T, Chong L-Y, Sharpin C, Wonderling D, Head K, Hill J. Developing guidelines for venous thromboembolism for The National Institute for Clinical Excellence: involvement of the orthopaedic surgical panel. J Bone Joint Surg Br. 2010;92(5):611-616. doi: 10.1302/0301-620X.92B5.24448 [DOI] [PubMed] [Google Scholar]
- 13.Lee YK, Chung CY, Koo KH, Lee KM, Ji HM, Park MS. Conflict of interest in the assessment of thromboprophylaxis after total joint arthroplasty: a systematic review. J Bone Joint Surg Am. 2012;94(1):27-33. doi: 10.2106/JBJS.J.01033 [DOI] [PubMed] [Google Scholar]
- 14.Kjaersgaard-Andersen P, Kehlet H. Should deep venous thrombosis prophylaxis be used in fast-track hip and knee replacement? Acta Orthop. 2012;83(2):105-106. doi: 10.3109/17453674.2012.672094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearse EO, Caldwell BF, Lockwood RJ, Hollard J. Early mobilisation after conventional knee replacement may reduce the risk of postoperative venous thromboembolism. J Bone Joint Surg Br. 2007;89(3):316-322. doi: 10.1302/0301-620X.89B3.18196 [DOI] [PubMed] [Google Scholar]
- 16.Hughes RE, Hallstrom BR, Cowen ME, Igrisan RM, Singal BM, Share DA. Michigan Arthroplasty Registry Collaborative Quality Initiative (MARCQI) as a model for regional registries in the United States. Orthop Res Rev. 2015;7:47-56. doi: 10.2147/ORR.S82732 [DOI] [Google Scholar]
- 17.Hallstrom B, Singal B, Cowen ME, Roberts KC, Hughes RE. The Michigan experience with safety and effectiveness of tranexamic acid use in hip and knee arthroplasty. J Bone Joint Surg Am. 2016;98(19):1646-1655. doi: 10.2106/JBJS.15.01010 [DOI] [PubMed] [Google Scholar]
- 18.Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26(2):192-196. doi: 10.1007/s11606-010-1513-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson DR, Dunbar M, Murnaghan J, et al. Aspirin or rivaroxaban for VTE prophylaxis after hip or knee arthroplasty. N Engl J Med. 2018;378(8):699-707. doi: 10.1056/NEJMoa1712746 [DOI] [PubMed] [Google Scholar]
- 20.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 21.Li F, Zaslavsky AM, Landrum MB. Propensity score weighting with multilevel data. Stat Med. 2013;32(19):3373-3387. doi: 10.1002/sim.5786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13(2):273-277. doi: 10.1111/j.1524-4733.2009.00671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee BK, Lessler J, Stuart EA. Weight trimming and propensity score weighting. PLoS One. 2011;6(3):e18174. doi: 10.1371/journal.pone.0018174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dua A, Desai SS, Lee CJ, Heller JA. National trends in deep vein thrombosis following total knee and total hip replacement in the United States. Ann Vasc Surg. 2017;38(38):310-314. doi: 10.1016/j.avsg.2016.05.110 [DOI] [PubMed] [Google Scholar]
- 25.Bilimoria KY, Chung J, Ju MH, et al. Evaluation of surveillance bias and the validity of the venous thromboembolism quality measure. JAMA. 2013;310(14):1482-1489. doi: 10.1001/jama.2013.280048 [DOI] [PubMed] [Google Scholar]
- 26.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387-398. doi: 10.1016/S0895-4356(00)00321-8 [DOI] [PubMed] [Google Scholar]
- 27.Markel DC, York S, Liston MJ Jr, Flynn JC, Barnes CL, Davis CM III; AAHKS Research Committee . Venous thromboembolism: management by American Association of Hip and Knee Surgeons. J Arthroplasty. 2010;25(1):3-9.e1, 2. [DOI] [PubMed] [Google Scholar]
- 28.Kwong LM. Cost-effectiveness of rivaroxaban after total hip or total knee arthroplasty. Am J Manag Care. 2011;17(1)(suppl):S22-S26. [PubMed] [Google Scholar]
- 29.Duran A, Sengupta N, Diamantopoulos A, Forster F, Kwong L, Lees M. Cost effectiveness of rivaroxaban versus enoxaparin for prevention of post-surgical venous thromboembolism from a US payer’s perspective. Pharmacoeconomics. 2012;30(2):87-101. doi: 10.2165/11599370-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 30.Schousboe JT, Brown GA. Cost-effectiveness of low-molecular-weight heparin compared with aspirin for prophylaxis against venous thromboembolism after total joint arthroplasty. J Bone Joint Surg Am. 2013;95(14):1256-1264. doi: 10.2106/JBJS.L.00400 [DOI] [PubMed] [Google Scholar]
- 31.Mostafavi Tabatabaee R, Rasouli MR, Maltenfort MG, Parvizi J. Cost-effective prophylaxis against venous thromboembolism after total joint arthroplasty: warfarin versus aspirin. J Arthroplasty. 2015;30(2):159-164. doi: 10.1016/j.arth.2014.08.018 [DOI] [PubMed] [Google Scholar]
- 32.Shah SS, Satin AM, Mullen JR, Merwin S, Goldin M, Sgaglione NA. Impact of recent guideline changes on aspirin prescribing after knee arthroplasty. J Orthop Surg Res. 2016;11(1):123. doi: 10.1186/s13018-016-0456-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Raw Baseline Data of Study Population
eTable 2. Adjusted Odds Ratio for the Primary Outcome VTE/Death and Secondary Outcome Bleeding Event: Excluding Patients With VTE history