Key Points
Question
For infants with biliary atresia, should biliary-enteric drainage remain the initial treatment of choice and liver transplant used as salvage therapy?
Findings
In this cohort study that included 626 infants with biliary atresia, patients who underwent primary liver transplant had a markedly reduced risk of long-term mortality compared with patients initially managed with biliary-enteric drainage procedure.
Meaning
Patients treated with a primary liver transplant live longer than those initially treated with biliary-enteric drainage; a multi-institutional clinical trial is necessary to identify the initial treatment optimal for patients with biliary atresia.
Abstract
Importance
Some infants with biliary atresia are treated with primary liver transplant (pLT), but most are initially treated with biliary-enteric drainage (BED) with a subsequent salvage liver transplant. Given the improvements in liver transplant outcomes, it is important to determine whether BED treatment remains the optimal surgical algorithm for patients with biliary atresia.
Objective
To compare the survival of patients with biliary atresia initially treated with BED with patients who underwent pLT.
Design, Setting, and Participants
This cohort study used deidentified records from the California Office of Statewide Health Planning and Development database to identify patients with biliary atresia (n = 1252) between January 1, 1990, through December 31, 2015. Patients were categorized into 1 of 2 cohorts: those who received BED treatment and those who underwent pLT. Excluded from the study were those born before January 1, 1995, and those without any documented operative intervention by age 5 years. Data analysis was performed from January 1, 1990, to December 31, 2015.
Main Outcomes and Measures
Overall survival was compared between the BED and pLT cohorts using the Kaplan-Meier method. The treatment’s association with treatment era was examined by comparing survival before 2002 and on or after January 1, 2002.
Results
In total, 1252 patients with biliary atresia were identified. After exclusions, 626 remained; of these patients, 351 (56.1%) were female and 275 (43.9%) were male with a median (interquartile range) age at intervention for initial BED treatment of 65 (48-81) days. Among the 626 patients studied, initial BED treatment was performed in 313 patients (50.0%), and pLT was performed in 313 patients (50.0%). Although patients who underwent pLT had a higher mortality rate within the first 3 months after the procedure, they had a reduced risk of long-term mortality compared with patients initially managed with BED treatment (hazard ratio [HR] ≥6 months after the initial procedure, 0.19; 95% CI, 0.08-0.42; P = .01). Patients requiring salvage liver transplant had a substantially higher risk of mortality than patients who received pLT (HR, 0.43; 95% CI 0.25-0.76; P = .003). Those who underwent pLT had superior survival compared with BED treatment recipients on or after 2002 (HR, 0.16; 95% CI, 0.05-0.54; P < .001), and that persisted when censoring patients who underwent salvage liver transplant (HR, 0.23; 95% CI, 0.07-0.82; P = .01).
Conclusions and Relevance
Patients who underwent pLT experienced superior long-term survival compared with patients who underwent BED treatment. Multi-institutional trials are needed to determine which initial treatment is most advantageous to patients with biliary atresia.
This study uses the California Office of Statewide Health Planning and Development database to compare the short- and long-term outcomes of biliary-enteric drainage and primary liver transplant in young children with biliary atresia.
Introduction
Biliary atresia (BA) is a progressive fibroinflammatory cholangiopathy of unclear origin affecting 1:10 000 to 1:20 000 live births in the United States.1 Prior to the availability of liver transplant (LT), surgical biliary-enteric drainage (BED), most commonly by hepatic portoenterostomy (HPE), was the only therapy that offered the potential for long-term survival in patients with BA.2 Hepatic portoenterostomy was first described in the late 1950s by the pioneering surgeon Moroi Kasai.3 The procedure involves resection of the obliterated and fibrotic extrahepatic biliary tree, the hallmark of BA, and anastomosis of a segment of small bowel to the hilar plate, allowing drainage of bile into the intestine.3 Little has changed in the surgical technique of HPE since Kasai’s initial descriptions. Alternative techniques, such as drainage using the gallbladder, have been explored but were determined to have limited application.4
The rate of survival with native liver after a BED treatment is 37.9% to 40% after 5 years and decreases to 25% to 32.1% after 15 years, even at experienced centers and despite considerable efforts to improve the long-term outcomes.5,6,7 Mortality occurs secondary to progressive hepatic injury, which leads to cirrhosis and end-stage liver disease that eventually require LT in most patients.8 Unfortunately, the poor early experience and high mortality rate associated with LT relegated it to a salvage procedure reserved for patients whose BED treatment was unsuccessful.
Advances in the perioperative care, surgical technique, and immunosuppressive management of the pediatric LT recipient over the past several decades have markedly improved short- and long-term outcomes. The current 10-year survival rate for LT recipients with end-stage liver disease associated with BA is 90%.9 Most of these LT recipients have undergone a BED procedure on the recommendation that infants with BA should receive BED as an initial treatment.1 However, a subset of infants proceed directly to primary LT (pLT).
Using data from a large, statewide database, this study compared the survival over a 25-year period of infants with BA initially treated with BED and infants treated with pLT. The 2002 change in liver allocation to a Model for End Stage Liver Disease/Pediatric End Stage Liver Disease Model (MELD/PELD)–based system10 was also considered in evaluating whether BED should remain the initial treatment for BA in the setting of improved pediatric LT outcomes.
Methods
OSHPD Database
This study was approved by the University of Southern California Institutional Review Board. Informed consent was waived by the board as the data used in the study were obtained from a state database. Deidentified records of pediatric patients with BA diagnosis (International Classification of Diseases, Ninth Revision, code 75161) between January 1, 1990, through December 31, 2015, were requested from the California Office of Statewide Health Planning and Development (OSHPD). The OSHPD collects data from more than 6000 California Department of Public Health–licensed health care facilities, including 450 hospitals. The OSHPD requires participating facilities to submit reports on a semiannual basis that detail patient information subdivided into multiple data categories on all discharged individuals. Data include admission and discharge dates; patient age, sex, and primary and secondary diagnoses; and procedure codes. This information is then reported annually in patient discharge data. The linked death file associates patient discharge data via a Social Security number and abstract record number to the Death Statistical Master File, a database maintained by the California Department of Public Health that contains death certificate information.
Patients with BA diagnosis were categorized into 1 of 2 cohorts on the basis of initial surgical procedure because this represents the primary treatment decision point: BED (International Classification of Diseases, Ninth Revision, codes 5131, 5132, 5136, 5137, and 5139) or pLT (International Classification of Diseases, Ninth Revision, code 5051 or 5059). Patients in the BED cohort were further subdivided into those who underwent only BED procedure (oBD) and those who underwent subsequent salvage LT (sLT). Patients born before January 1, 1995, and patients without any documented operative intervention by 5 years of age were excluded from further analysis. Data analysis was performed from January 1, 1990, to December 31, 2015.
Statistical Analysis
Patient demographic and clinical characteristics were summarized and compared among groups using the χ2 test for categorical factors and the Kruskal-Wallis test for continuous variables. Overall survival was calculated from the time of first procedure to the last follow-up; death from any cause was counted. Patients were censored if they were still alive at the data-extraction date. Among patients who underwent initial BED treatment, the likelihood of LT and the likelihood of death after BED treatment were estimated using the cumulative incidence curve calculations with death or LT, respectively, as the competing risk event. Overall survival between BED treatment and pLT was compared using Kaplan-Meier curves and the log-rank test; however, the hazard ratio (HR) was calculated at a time, starting 6 months (0.5 years) after the initial procedure to eliminate crossing hazards.
Patients were further categorized into 1 of 2 eras on the basis of whether the primary procedure was performed before 2002 or on or after January 1, 2002. For patients who underwent BED treatment and patients who underwent pLT, Cox proportional hazard regression, including patient characteristics, was performed to identify significant risk factors for overall survival. Among patients who underwent initial BED treatment, a test of interaction effect was performed to determine the association of era and LT with patient survival. For patients who underwent BED treatment, LT status was defined as a time-dependent variable, which was assigned as value 1 or 0 at time t measured from BED procedure data, depending on whether the patient had received LT at that time. Subsequent survival analyses were also performed on the basis of whether the primary procedure was performed before 2002 or on or after January 1, 2002. All P values were based on 2-sided statistical tests at a significance level of .05. The statistical analysis was performed in SAS, version 9.4 (SAS Institute Inc), and R, version 3.4.0 (R Foundation for Statistical Computing).
Results
In total, 1252 patients with BA were identified in the OSHPD database. Of these patients, 216 (17.3%) were excluded from further analysis because they were born before 1990. Of the 1036 remaining patients, 410 (39.6%) were excluded from further analysis because no operative intervention by age 5 years exists, because 5-year intervention-free survival is not consistent with the natural history of BA. The remaining 626 patients were included for analysis; of these patients, 351 (56.1%) were female and 275 (43.9%) were male with a median (interquartile range [IQR]) age at intervention for initial BED treatment of 65 (48-81) days. An initial BED treatment was performed in 313 patients (50.0%). An sLT was subsequently performed in 147 (46.9%) of these 313 patients, and the remaining 166 (53.0%) did not undergo an sLT. A pLT was performed in 313 patients (50.0%).
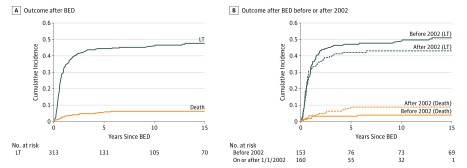
At 5 years after BED treatment, the cumulative incidence of sLT was 45% and death was 5.6% (Figure 1A). Patients who underwent sLT were significantly older than patients who had oBD at the time of diagnosis (median [IQR] age, 66 [49-88] days vs 57 [43-76] days; P = .001) and at the time of BED treatment (median [IQR] age, 71 [55-96] days vs 65 [48-81] days; P = .003). In addition, patients who received sLT had a higher incidence of cholangitis, sepsis, and bacteremia compared with oBD patients (Table 1).
Figure 1. Cumulative Incidence of Death and Liver Transplant (LT) Among Patients Who Underwent Biliary-Enteric Drainage (BED) Treatment.
Table 1. Patient Characteristics Comparison Between Groups.
| Variable | No. (%) | P Value | ||
|---|---|---|---|---|
| oBD (n = 166) | sLT (n = 147) | pLT (n = 313) | ||
| Age, median (IQR), d | ||||
| At diagnosis | 57 (43-76) | 66 (49-88) | 232 (165-358) | <.001 |
| At BED | 65 (48-81) | 71 (55-96) | NA | .003 |
| At LT | NA | 313 (221-505) | 315 (230-485) | .80 |
| BA diagnosis to 1st procedure, median (IQR), d | 4 (1-7) | 5 (1-8) | 41 (1-120) | <.001 |
| Sex | .23 | |||
| Male | 77 (46.4) | 71 (48.3) | 127 (40.6) | |
| Female | 89 (53.6) | 76 (51.7) | 186 (59.4) | |
| Procedure era | .22 | |||
| Before 2002 | 75 (45.2) | 78 (53.1) | 166 (53.0) | |
| On or after January 1, 2002 | 91 (54.8) | 69 (46.9) | 147 (46.9) | |
| Infectious complications | ||||
| Sepsis | 6 (3.6) | 14 (9.5) | 10 (3.2) | .01 |
| Bacteremia | 24 (14.5) | 44 (29.9) | 49 (15.7) | <.001 |
| Intra-abdominal abscess | 3 (1.8) | 3 (2.0) | 9 (2.9) | .73 |
| Cholangitis | 95 (57.2) | 105 (71.4) | 127 (40.6) | <.001 |
Abbreviations: BA, biliary atresia; BED, biliary-enteric drainage; IQR, interquartile range; LT, liver transplant; oBD, only biliary-enteric drainage; pLT, primary liver transplant; sLT, salvage liver transplant.
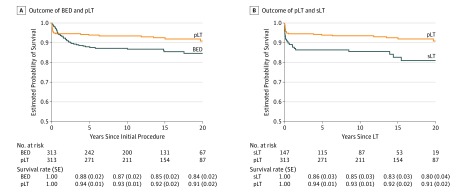
The association of initial treatment decision, BED or pLT, with overall survival was explored. The 1-, 5-, 10-, and 15-year Kaplan-Meier survival rates (SE) for all patients who received BED treatment compared with pLT patients were 94% vs 95%, 88% (0.02) vs 94% (0.01), 87% (0.02) vs 91% (0.01), and 85% (0.02) vs 92% (0.02) (Figure 2A). Although patients who underwent pLT had a higher mortality rate within the first 3 months after the procedure, they had a reduced risk of long-term mortality compared with patients initially managed with BED treatment (HR ≥6 months after the initial procedure, 0.19; 95% CI, 0.08-0.42; P = .01).
Figure 2. Association of Initial Treatment With Overall Survival.
A, Patients who received primary liver transplant (pLT) had a higher short-term mortality rate after the initial procedure but had lower risk of long-term mortality than patients who received biliary-enteric drainage (BED) treatment (hazard ratio [HR] ≥6 months after the initial procedure, 0.19; 95% CI, 0.08-0.42; P = .01). B, Patients who received salvage LT (sLT) had higher mortality risk than patients who received pLT (HR, 0.43; 95% CI 0.25-0.76; P = .003).
The association of previous BED treatment with LT was examined by comparing sLT with pLT. Patients who underwent pLT were significantly older than patients who underwent sLT at time of BA diagnosis, but the median (IQR) age at LT did not differ between pLT and sLT recipients (315 [230-485] days vs 313 [221-505] days; P = .80). Recipients of sLT had higher rates of infectious complications compared with recipients of pLT (Table 1). In addition, patients requiring sLT had a substantially higher risk of mortality than patients who received pLT (HR, 0.43; 95% CI, 0.25-0.76; P = .003) (Figure 2B).
Because of the occurrence of considerable changes to liver allocation over the course of the study period, we tested for a difference in survival after LT between the 2 eras (before 2002 and on or after 2002). For patients who underwent initial BED treatment, a substantial interaction effect was identified between era and LT, indicating that post-LT survival differed between the 2 eras (P = .008). Salvage LT was associated with a substantial increased risk of mortality before 2002 (HR, 9.34; 95% CI, 3.29-26.5; P < .001; Table 2), but this was not the case for the on-or-after 2002 cohort (HR, 0.99; 95% CI, 0.34-2.91; P = .98; Table 2). A considerable improvement in survival was also seen in pLT. Recipients of pLT on or after 2002 had a reduced risk of mortality compared with recipients of pLT before 2002 (HR, 0.19; 95% CI, 0.06-0.64; P = .007; Table 2). No statistically significant era-associated difference was found in the cumulative incidence of sLT (Gray test P = .47) or death (Gray test P = .08) after BED treatment (Figure 1B).
Table 2. Analysis of Risk Factors for Mortality.
| Variable | Death, No. (%) | Hazard Ratio (95% CI) | Wald Test P Value |
|---|---|---|---|
| Analysis of Risk Factors for Mortality in BED Before 2002 | |||
| sLT | NA | 9.34 (3.29-26.50) | <.001 |
| Sex | .18 | ||
| Male (n = 71) | 14 (20) | 1 [Reference] | |
| Female (n = 82) | 10 (12) | 0.57 (0.25-1.29) | |
| Age at BED procedure, d | .81 | ||
| ≤60 (n = 59) | 8 (14) | 1 [Reference] | |
| 61-90 (n = 61) | 11 (18) | 1.35 (0.54-3.39) | |
| >90 (n = 33) | 5 (15) | 1.20 (0.39-3.70) | |
| Days between BA diagnosis and BED treatment | .09 | ||
| ≤2 (n = 64) | 9 (14) | 1 [Reference] | |
| 3-7 (n = 53) | 5 (9) | 0.55 (0.18-1.65) | |
| >7 (n = 36) | 10 (28) | 1.79 (0.72-4.42) | |
| Analysis of Risk Factors for Mortality in BED On or After January 1, 2002 | |||
| sLT | NA | 0.99 (0.34-2.91) | .98 |
| Sex | .96 | ||
| Male (n = 77) | 9 (12) | 1 [Reference] | |
| Female (n = 83) | 10 (12) | 1.02 (0.42-2.53) | |
| Age at BED procedure, d | .86 | ||
| ≤60 (n = 58) | 6 (10) | 1 [Reference] | |
| 61-90 (n = 69) | 9 (13) | 1.32 (0.46-3.79) | |
| >90 (n = 33) | 4 (12) | 1.09 (0.29-4.09) | |
| Days between BA diagnosis and BED treatment | .59 | ||
| ≤2 (n = 58) | 6 (10) | 1 [Reference] | |
| 3-7 (n = 59) | 6 (10 | 0.89 (0.28-2.79) | |
| >7 (n = 43) | 7 (16) | 1.53 (0.51-4.61) | |
| Analysis of Risk Factors for Mortality in pLT | |||
| Procedure era | .007 | ||
| Before 2002 (n = 166) | 21 (12.6) | 1 [Reference] | |
| On or after January 1, 2002 (n = 147) | 3 (2.0) | 0.19 (0.06-0.64) | |
| Sex | .53 | ||
| Male (n = 127) | 8 (6.3) | 1 [Reference] | |
| Female (n = 186) | 16 (8.6) | 1.32 (0.56-3.10) | |
| Age at LT procedure, d | .36 | ||
| ≤240 (n = 94) | 4 (4) | 1 [Reference] | |
| 241-365 (n = 94) | 9 (10) | 2.36 (0.72-7.71) | |
| >365 (n = 125) | 11 (8.8) | 1.86 (0.57-6.02) | |
| Days between BA diagnosis and pLT procedure | .42 | ||
| ≤14 (n = 110) | 6 (5.5) | 1 [Reference] | |
| 15-90 (n = 109) | 10 (9.2) | 1.93 (0.70-5.35) | |
| >90 (n = 94) | 8 (9) | 1.74 (0.59-5.13) | |
Abbreviations: BA, biliary atresia; BED, biliary-enteric drainage; LT, liver transplant; NA, not applicable; pLT, primary liver transplant; sLT, salvage liver transplant.
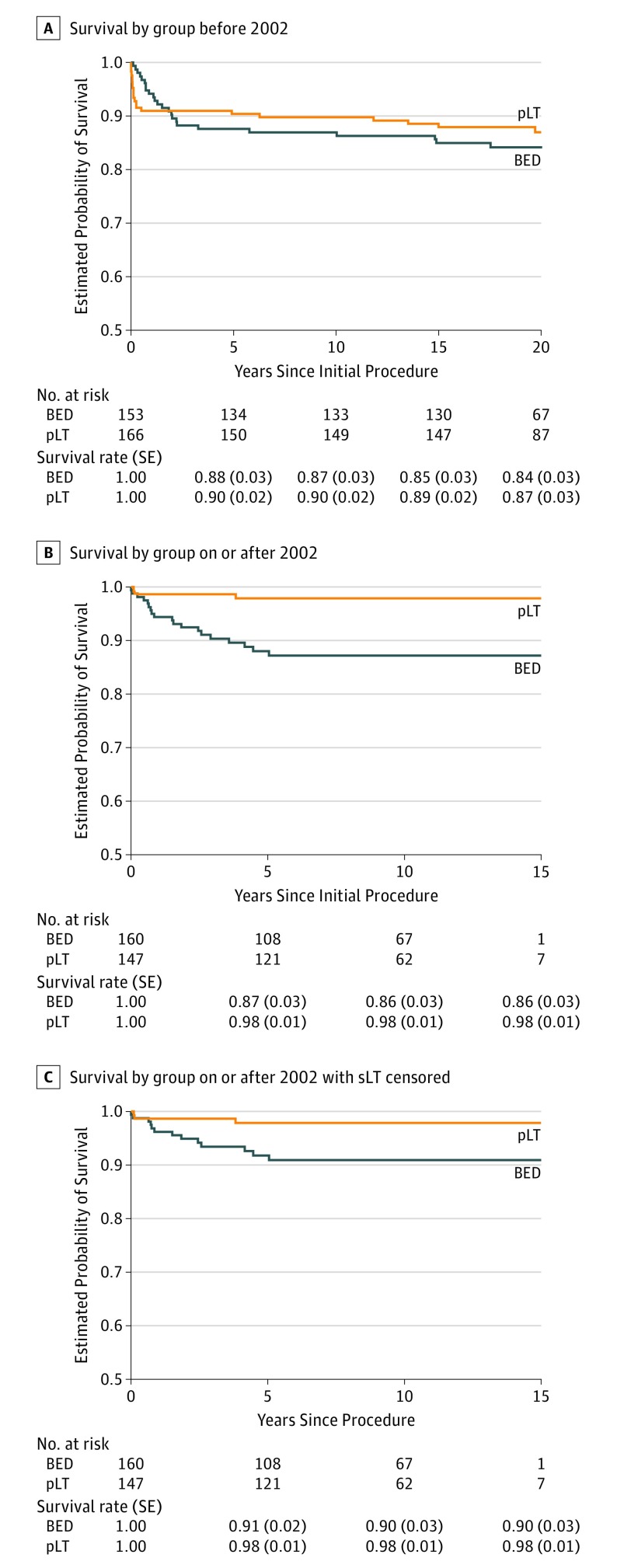
Next, we compared BED vs pLT by era. No difference was identified in the Kaplan-Meier survival of patients who received BED treatment vs pLT before 2002 (Figure 3A). However, pLT recipients had superior survival compared with BED treatment recipients on or after 2002 (HR, 0.16; 95% CI, 0.05-0.54; P < .001) (Figure 3B). The superior survival of patients who underwent pLT compared with BED treatment on or after 2002 persisted when censoring patients who underwent sLT (HR, 0.23; 95% CI, 0.07-0.82; P = .01) (Figure 3C).
Figure 3. Association of Initial Treatment With Overall Survival by Era.
A, No survival difference between patients who received biliary-enteric drainage (BED) treatment and primary liver transplant (pLT) before 2002. B, Patients who received pLT had superior survival compared with BED treatment on or after 2002 (HR, 0.16; 95% CI, 0.05-0.54; P < .001). C, Recipients of pLT compared with BED treatment on or after 2002 persisted when censoring patients who underwent sLT (HR, 0.23; 95% CI, 0.07-0.82; P = .01).
Discussion
Most children with BA in the United States will undergo an initial treatment of surgical BED, most frequently by HPE, and this operation will be unsuccessful in more than 50% of these patients 5 years after the intervention. Perioperative risk factors for the unsuccessful HPE are well characterized and include age at HPE, intrahepatic extension of cholangiopathy, treatment center experience, and failure of bilirubin levels to normalize within 3 months.5,8,11,12 Unfortunately, neither the technique nor the long-term outcomes of BED treatment have changed in the nearly 70 years since the procedure was first described.2,5,8 The use of steroids after operation to modulate the inflammatory response has also not demonstrated efficacy, and today the 20-year survival rate of 21% for patients with their native liver, as described by Altman in 1997, has only marginally improved.13 In addition, most children who do survive into adulthood experience severe liver disease. In 2 European series, 79% to 97% of patients surviving longer than 20 years with their native liver had cirrhosis, and 46% to 69% had portal hypertension.14,15 The near-universal progression of fibrosis and cholangiopathy in the native liver of long-term survivors will certainly necessitate LT as an adult in a large percentage of these patients.
Fortunately, LT has developed into a highly effective salvage therapy for those whose BED treatment was unsuccessful. The outcomes have improved year over year, such that nearly 94% of all children who received LT for the treatment of BA are alive 1 year after transplant.9 As such, BED functions as a temporizing treatment in most patients. The high failure rate of BED and the excellent success rate of LT raises the question of whether it is time for a paradigm change in the treatment of BA. Specifically, does BED treatment confer a survival advantage when compared with pLT?
We used the California OSHPD database to identify all patients with a diagnosis of BA between 1990 and 2015. Patients were categorized into 2 groups: those initially treated with BED procedure, regardless of whether they underwent sLT, and those who underwent pLT. Patients in the BED cohort had a lower early mortality rate, but long-term survival was significantly higher among patients in the pLT cohort. We then examined the association of previous BED treatment with post-LT survival. Salvage LT was found to have an increased risk of death compared with pLT. An explanation for this finding may be the well-documented increased technical difficulty of LT in the setting of previous hilar dissection and BDE operation. These patients for whom early BED treatment was unsuccessful are at increased risk of infectious complications and intestinal perforation compared with patients who did not receive BED treatment, which would be anticipated to adversely affect outcome.8,16,17
A substantial interaction effect was observed in the outcomes of LT occurring before and on or after January 1, 2002, when the MELD/PELD scoring system was implemented. Specifically, the increased mortality associated with sLT in the pre-MELD/PELD era was not observed in the post-MELD/PELD era. Substantial improvement in survival after pLT was noted. These findings are in accordance with continued advances in pediatric LT.17,18 After the implementation of the MELD/PELD score for allocation, the number of patients receiving deceased-donor livers has increased and the pediatric waitlist mortality has decreased. In addition, advances in surgical technique, such as partial liver grafts and live-donor LT, as well as improvements in the pediatric intensive care for children before and after LT have contributed to better outcomes. In addition, prophylaxis recommendations and monitoring for opportunistic infections as well as the advent of tacrolimus-based immunosuppression, leading to lower rejection rates and allowing for steroid-sparing protocols, have been instrumental in the current success of pediatric LT. As anticipated, and consistent with other published data, no substantial improvement was noted in the survival of patients who received BED treatment between the 2 eras.
Limitations
This study is limited by the lack of granularity common to large databases, particularly because the OSHPD database was designed to capture admission, procedure, and discharge diagnoses. The fidelity of the data is limited by the quality of the data entry; more than 410 patients did not have an operative intervention listed, suggesting missing data or misdiagnosis. Despite this, the OSHPD database provides a valuable resource that allows the tracking of treatment for a relatively uncommon disease in a large population over an extended period.
The OSHPD database, unlike other commonly used surgical outcomes and transplant databases, allows us to identify all patients with a BA diagnosis, regardless of treatment type. With these data, we were unable to discern the reason that patients who received pLT were older than their BED counterparts or the association of this age difference with overall outcomes. Furthermore, we were unable to evaluate the individual factors behind the decision to undergo an initial BED treatment or a pLT, and whether these procedures represent 2 differing phenotypes of BA as opposed to 1 phenotype treated differently. Additional studies will provide answers to these important questions.
Biliary-enteric drainage with HPE is performed as soon as possible after the diagnosis of BA, not because of an immediate risk of mortality but because of a greater risk of an unsuccessful BED procedure associated with increasing age. Most infants with a late diagnosis of BA who do not undergo BED treatment continue to grow considerably during the first 6 to 12 weeks of life and can undergo LT at a safe weight and with excellent outcomes. We recognize that the supply of organs suitable for pediatric LT is limited and demand continues to outpace supply. However, most patients will require an LT during their lifetime, and receiving a transplant at a young age allows for the greater use of left lateral segment grafts from living donors without affecting the deceased-donor pool. In addition, the use of deceased-donor left lateral segment grafts allows for the transplant of the right trisegments into adults, thus minimizing the strain on the deceased-donor supply.
Conclusions
The overall findings of this study highlight our responsibility to address, in a thoughtful and scientifically rigorous manner, the risks associated with unsuccessful BED procedures. There is, clearly, a subset of patients with BA for whom good and durable outcomes can be achieved with a Kasai-first approach. Conversely, a growing number of patients will have superior outcomes with a pLT approach. In the current setting emphasizing excellent outcomes in pediatric LT, our goal should no longer be to avoid LT at any cost. The characteristics of patients who would thrive with an initial BED treatment or with pLT need to be elucidated. A prospective, multi-institutional clinical trial is necessary to determine which initial treatment will be most advantageous to individual patients with BA.
References
- 1.Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology. 2007;46(2):26-32. doi: 10.1002/hep.21790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altman RP. The portoenterostomy procedure for biliary atresia: a five year experience. Ann Surg. 1978;188(3):351-362. doi: 10.1097/00000658-197809000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasai M, Suzuki H, Ohashi E, Ohi R, Chiba T, Okamoto A. Technique and results of operative management of biliary atresia. World J Surg. 1978;2(5):571-579. doi: 10.1007/BF01556048 [DOI] [PubMed] [Google Scholar]
- 4.Hery G, Gonzales E, Bernard O, Fouquet V, Gauthier F, Branchereau S. Hepatic portocholecystostomy: 97 cases from a single institution. J Pediatr Gastroenterol Nutr. 2017;65(4):375-379. doi: 10.1097/MPG.0000000000001685 [DOI] [PubMed] [Google Scholar]
- 5.Chardot C, Buet C, Serinet MO, et al. Improving outcomes of biliary atresia: French national series 1986-2009. J Hepatol. 2013;58(6):1209-1217. doi: 10.1016/j.jhep.2013.01.040 [DOI] [PubMed] [Google Scholar]
- 6.Bondoc AJ, Taylor JA, Alonso MH, et al. The beneficial impact of revision of Kasai portoenterostomy for biliary atresia: an institutional study. Ann Surg. 2012;255(3):570-576. doi: 10.1097/SLA.0b013e318243a46e [DOI] [PubMed] [Google Scholar]
- 7.Serinet MO, Wildhaber BE, Broué P, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics. 2009;123(5):1280-1286. doi: 10.1542/peds.2008-1949 [DOI] [PubMed] [Google Scholar]
- 8.Superina R, Magee JC, Brandt ML, et al. ; Childhood Liver Disease Research and Education Network . The anatomic pattern of biliary atresia identified at time of Kasai hepatoportoenterostomy and early postoperative clearance of jaundice are significant predictors of transplant-free survival. Ann Surg. 2011;254(4):577-585. doi: 10.1097/SLA.0b013e3182300950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexopoulos SP, Nekrasov V, Cao S, et al. Effects of recipient size and allograft type on pediatric liver transplantation for biliary atresia. Liver Transpl. 2017;23(2):221-233. doi: 10.1002/lt.24675 [DOI] [PubMed] [Google Scholar]
- 10.McDiarmid SV, Merion RM, Dykstra DM, Harper AM. Selection of pediatric candidates under the PELD system. Liver Transpl. 2004;10(10 suppl 2):S23-S30. doi: 10.1002/lt.20272 [DOI] [PubMed] [Google Scholar]
- 11.Davenport M, Ong E, Sharif K, et al. Biliary atresia in England and Wales: results of centralization and new benchmark. J Pediatr Surg. 2011;46(9):1689-1694. doi: 10.1016/j.jpedsurg.2011.04.013 [DOI] [PubMed] [Google Scholar]
- 12.Altman RP, Lilly JR, Greenfeld J, Weinberg A, van Leeuwen K, Flanigan L. A multivariable risk factor analysis of the portoenterostomy (Kasai) procedure for biliary atresia: twenty-five years of experience from two centers. Ann Surg. 1997;226(3):348-353. doi: 10.1097/00000658-199709000-00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bezerra JA, Spino C, Magee JC, et al. ; Childhood Liver Disease Research and Education Network (ChiLDREN) . Use of corticosteroids after hepatoportoenterostomy for bile drainage in infants with biliary atresia: the START randomized clinical trial. JAMA. 2014;311(17):1750-1759. doi: 10.1001/jama.2014.2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lykavieris P, Chardot C, Sokhn M, Gauthier F, Valayer J, Bernard O. Outcome in adulthood of biliary atresia: a study of 63 patients who survived for over 20 years with their native liver. Hepatology. 2005;41(2):366-371. doi: 10.1002/hep.20547 [DOI] [PubMed] [Google Scholar]
- 15.de Vries W, Homan-Van der Veen J, Hulscher JB, Hoekstra-Weebers JE, Houwen RH, Verkade HJ; Netherlands Study Group of Biliary Atresia Registry . Twenty-year transplant-free survival rate among patients with biliary atresia. Clin Gastroenterol Hepatol. 2011;9(12):1086-1091. doi: 10.1016/j.cgh.2011.07.024 [DOI] [PubMed] [Google Scholar]
- 16.Neto JS, Feier FH, Bierrenbach AL, et al. Impact of Kasai portoenterostomy on liver transplantation outcomes: a retrospective cohort study of 347 children with biliary atresia. Liver Transpl. 2015;21(7):922-927. doi: 10.1002/lt.24132 [DOI] [PubMed] [Google Scholar]
- 17.Alexopoulos SP, Merrill M, Kin C, et al. The impact of hepatic portoenterostomy on liver transplantation for the treatment of biliary atresia: early failure adversely affects outcome. Pediatr Transplant. 2012;16(4):373-378. doi: 10.1111/j.1399-3046.2012.01677.x [DOI] [PubMed] [Google Scholar]
- 18.Jain A, Mazariegos G, Kashyap R, et al. Pediatric liver transplantation. A single center experience spanning 20 years. Transplantation. 2002;73(6):941-947. doi: 10.1097/00007890-200203270-00020 [DOI] [PMC free article] [PubMed] [Google Scholar]