Key Points
Question
Can implementing a group of evidence-informed transitional care services in a publicly funded health care system improve outcomes among patients discharged after hospitalization for heart failure?
Findings
In this pragmatic stepped-wedge cluster randomized trial that included 2494 patients in 10 hospitals in Ontario, Canada, there were no significant differences between patients who were randomized to receive a care transition program vs usual care in the primary composite outcome of time to all-cause readmission, emergency department visit, or death at 3 months (hazard ratio, 0.99) or the co–primary composite outcome of all-cause readmission or emergency department visit at 30 days (hazard ratio, 0.92).
Meaning
This patient-centered transitional care service model did not improve a composite of clinical outcomes in patients hospitalized for heart failure.
Abstract
Importance
Health care services that support the hospital-to-home transition can improve outcomes in patients with heart failure (HF).
Objective
To test the effectiveness of the Patient-Centered Care Transitions in HF transitional care model in patients hospitalized for HF.
Design, Setting, and Participants
Stepped-wedge cluster randomized trial of 2494 adults hospitalized for HF across 10 hospitals in Ontario, Canada, from February 2015 to March 2016, with follow-up until November 2016.
Interventions
Hospitals were randomized to receive the intervention (n = 1104 patients), in which nurse-led self-care education, a structured hospital discharge summary, a family physician follow-up appointment less than 1 week after discharge, and, for high-risk patients, structured nurse homevisits and heart function clinic care were provided to patients, or usual care (n = 1390 patients), in which transitional care was left to the discretion of clinicians.
Main Outcomes and Measures
Primary outcomes were hierarchically ordered as composite all-cause readmission, emergency department (ED) visit, or death at 3 months; and composite all-cause readmission or ED visit at 30 days. Secondary outcomes were B-PREPARED score for discharge preparedness (range: 0 [most prepared] to 22 [least prepared]); the 3-Item Care Transitions Measure (CTM-3) for quality of transition (range: 0 [worst transition] to 100 [best transition]); the 5-level EQ-5D version (EQ-5D-5L) for quality of life (range: 0 [dead] to 1 [full health]); and quality-adjusted life-years (QALY; range: 0 [dead] to 0.5 [full health at 6 months]).
Results
Among eligible patients, all 2494 (mean age, 77.7 years; 1258 [50.4%] women) completed the trial. There was no significant difference between the intervention and usual care groups in the first primary composite outcome (545 [49.4%] vs 698 [50.2%] events, respectively; hazard ratio [HR], 0.99 [95% CI, 0.83-1.19]) or in the second primary composite outcome (304 [27.5%] vs 408 [29.3%] events, respectively; HR, 0.93 [95% CI, 0.73-1.18]). There were significant differences between the intervention and usual care groups in the secondary outcomes of mean B-PREPARED score at 6 weeks (16.6 vs 13.9; difference, 2.65 [95% CI, 1.37-3.92]; P < .001); mean CTM-3 score at 6 weeks (76.5 vs 70.3; difference, 6.16 [95% CI, 0.90-11.43]; P = .02); and mean EQ-5D-5L score at 6 weeks (0.7 vs 0.7; difference, 0.06 [95% CI, 0.01 to 0.11]; P = .02) and 6 months (0.7 vs 0.6; difference, 0.06 [95% CI, 0.01-0.12]; P = .02). There was no significant difference in mean QALY between groups at 6 months (0.3 vs 0.3; difference, 0.00 [95% CI, −0.02 to 0.02]; P = .98).
Conclusions and Relevance
Among patients with HF in Ontario, Canada, implementation of a patient-centered transitional care model compared with usual care did not improve a composite of clinical outcomes. Whether this type of intervention could be effective in other health care systems or locations would require further research.
Trial Registration
ClinicalTrials.gov Identifier: NCT02112227
This cluster randomized trial compares the effects of a patient-centered transitional care service intervention vs usual care on a composite outcome of all-cause readmission, emergency department (ED) visit, or death at 3 months, and a composite of all-cause readmission or ED visit at 30 days among patients hospitalized for heart failure.
Introduction
Heart failure (HF) is a leading cause of hospitalization in older adults.1 Nearly 80% of HF costs are due to hospitalizations and readmissions, which reduce quality of life (QOL) and are independently associated with death.2 In a retrospective chart review, approximately 40% of early readmissions following HF hospitalization were related to suboptimal transitional care (ie, actions that promote care coordination and continuity as patients transfer between health care settings).3,4 In an observational study, patients discharged from hospitals with the lowest 1-week follow-up rates experienced the highest 30-day readmission rates.5
Transitional care services can improve outcomes, but have not been systematically implemented. A network meta-analysis of randomized clinical trials (RCTs) was undertaken to inform the design of a transitional care model.6 Nurse-led home visits and multidisciplinary heart function clinics (HFCs) were associated with a reduction in all-cause readmissions and death relative to other services following hospitalization for HF, with benefits evident within 30 days of discharge.7 Shared features included self-care education and multidisciplinary care.6
In an effort to translate knowledge to action,8 evidence-informed services6 were combined with guideline recommendations9,10 and a patient-centered approach11,12 to form the Patient-Centered Care Transitions in HF (PACT-HF) service model. With an integrated knowledge translation research approach13 and a pragmatic stepped-wedge cluster randomized trial design for sequential implementation across hospitals,14,15 this study tested the effect of the intervention on a composite outcome of all-cause readmission, emergency department (ED) visit, or death at 3 months and all-cause readmission or ED visit at 30 days.
Methods
The protocol and statistical analysis plan are included in Supplement 1 and Supplement 2.16 Because services were evidence-informed and considered quality improvement,6,7 the study was approved by all institutional research ethics boards with waiver of written consent. Patients provided verbal informed consent for study participation.
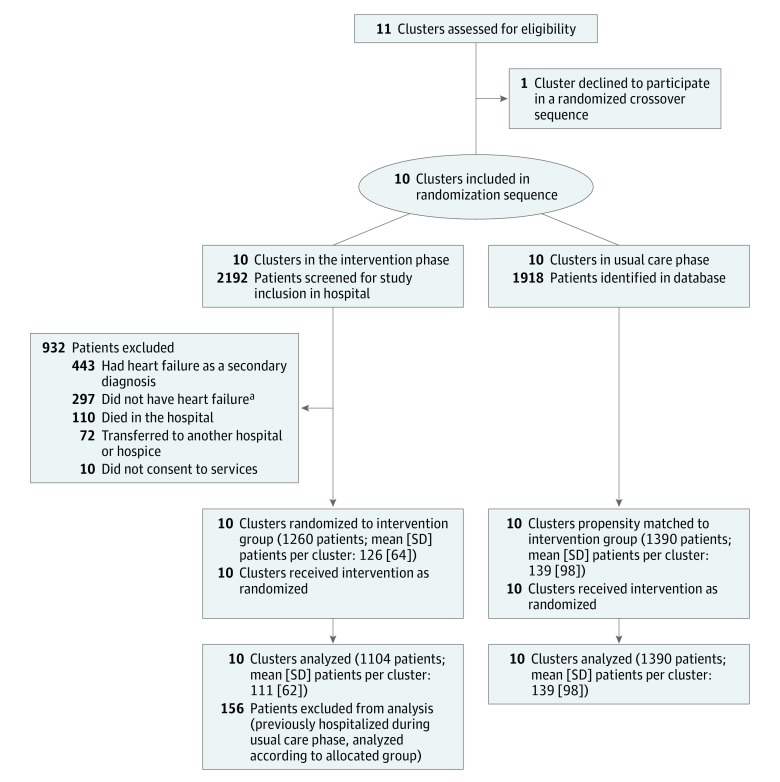
We considered 11 tertiary or quaternary care urban hospitals across southern Ontario for inclusion. We excluded 1 hospital corporation that could not agree to a randomized implementation sequence. We incorporated feedback from patients, clinicians, and policy makers during the planning phase.16,17
Patients
We included patients whose primary reason for hospitalization was HF. We excluded patients who did not have HF, did not consent to inclusion, died during hospitalization, were transferred to another hospital, or were discharged with a primary diagnosis other than HF. During the intervention phase, Boston criteria18 and/or serum thresholds of brain natriuretic peptide (BNP) or N-terminal prohormone brain natriuretic peptide were used to exclude a diagnosis of HF (eFigure 1 in Supplement 3).19
Randomization
Using a stepped-wedge design, we introduced the intervention to 10 hospitals in a randomized sequence, determined by a number generator, at monthly intervals until all hospitals received the intervention (eFigure 2 in Supplement 3).16 We measured clinical outcomes in each hospital during the baseline month. We remeasured outcomes whenever a hospital crossed over from usual care to the intervention, making within- and between-hospital comparisons. We measured secondary outcomes in a nested sample of 8 hospitals. The study domains were pragmatic (eFigure 3 in Supplement 3), designed to assess effectiveness rather than efficacy.16
Intervention
For hospitals undergoing the intervention, a hospital nurse navigator provided the following at the time of discharge: (1) a needs assessment based on the patient’s self-reported QOL,20 in addition to multidisciplinary referrals (eg, physiotherapy) as needed; (2) HF self-care education21 to the patient and informal caregiver; (3) a structured patient-centered discharge summary with a symptom-driven action plan to the patient and the family physician; (4) family physician follow-up arrangements within 1 week of discharge; and (5) referrals to postdischarge nurse-led home visits and HFC care for patients with length of stay, acuity of presentation, comorbidities, and ED visits in the preceding 6 months (LACE index)22,23 of at least 13 (eFigure 1 in Supplement 3). The nurse-led visits included weekly, structured, face-to-face and telephone assessments lasting 4 to 6 weeks until patients were seen in the HFC. In the event of deterioration, the home-care nurse helped the patient follow the discharge action plan and contacted the HFC for expedited care. HF guidelines9 were distributed, but management was left to clinicians’ discretion.
In the usual care group, transitional care occurred at the discretion of clinicians. In 1 hospital, a nurse provided education and a home visit to select patients. Eight hospitals had access to regional HFCs, while 2 did not.
Blinding
Clinicians were unblinded to treatment allocation and patients were considered unblinded. Personnel who collected patient-reported outcomes were blinded. Clinical outcomes were adjudicated independent of this trial. To avoid contamination, we concealed the randomization sequence and crossover date from each institution until 3 months before crossover, designed month-long steps to shorten the trial length and minimize changes in usual care, and withheld intervention details, training, and toolkits until the month before crossover.
Outcomes
Primary clinical outcomes were hierarchically ordered as time to first composite all-cause readmission, ED visit, or death at 3 months and all-cause readmission or ED visit at 30 days among patients.
Secondary patient-reported outcomes were the B-PREPARED score for discharge preparedness24 at 6 weeks (range: 0-22; a higher score indicates higher level of preparedness); the 3-Item Care Transitions Measure (CTM-3)25 score for quality of care transition at 6 weeks (range: 0-100; a higher score indicates a higher quality of care); the 5-level EQ-5D version (EQ-5D-5L)26 scores for QOL at discharge, 6 weeks, and 6 months (range: 0-1; 0 indicates dead and 1 indicates full health); and quality-adjusted life-years (QALY),27 a measure of life span weighted by EQ-5D-5L health utilities,26 at 6 months (range: 0-0.5; 0 indicates dead and, for this study, 0.5 indicates full health at 6 months). While minimally important differences have not been established for the B-PREPARED and CTM-3 scores, a mean (SD) difference of 0.037 (0.001) in EQ-5D-5L is considered clinically relevant.28 Standard scripts were used for the surveys. Secondary health care cost outcomes will be reported separately.
Post hoc exploratory clinical outcomes included individual components of the composite clinical outcomes at 3 months and 30 days, as well as number of clinical events at 3 months and 30 days. In a post hoc sensitivity analysis, we reassessed the effect of the intervention on the primary composite clinical outcomes among all patients hospitalized with HF and discharged alive at participating hospitals during the study, as identified in administrative databases.16
All outcomes were measured relative to the discharge date of the index HF hospitalization, defined as the first unplanned hospitalization for HF in a participating hospital during the study period.16 To identify the cohort for analysis,16 we used the Canadian Institute for Health Information database accessed at the Institute for Clinical Evaluative Sciences (ICES). For the main analysis, eligible intervention patients identified prospectively in the hospital were matched to a comparator group identified in the database with propensity scores of at least 0.4, derived using logistic regression with the stepwise selection of the following variables: age, sex, admission through the ED, length of stay greater than 2 days, and presence of diabetes, chronic kidney disease, myocardial infarction, or atrial fibrillation. We obtained linkages to databases (eTable 1 in Supplement 3) using unique encoded identifiers accessed and analyzed at ICES.
Sample Size
Assuming 320 patients per cluster, a 0.01 intraclass correlation coefficient, and a 28% composite 1-year event rate, we expected 80% statistical power (2-sided P < .05) to detect a 25% change in primary outcomes (10 clusters) and 90% power to detect a 5% change in patient-reported outcomes (8 clusters).16,29,30 The 25% target in primary outcomes was based on the proportion of readmissions estimated to be preventable,3,31 as well as the anticipated risk reduction of services included in the transitional care model based on systematic reviews.6,7
Statistical Analysis
The unit of analysis was patients, analyzed according to their allocated group regardless of treatment received. Data were summarized using mean (SD) and median (interquartile range [IQR]) for continuous variables, and counts with percentages for categorical variables. Baseline variables were compared using the standardized difference with a significant threshold of 0.10. A 2-sided P < .05 significance threshold was used for all analyses. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory.
Regression models for all primary and secondary outcomes were adjusted for the stepped-wedge design, with the intervention and steps (time) as fixed effects and hospitals as random effects. We analyzed primary clinical outcomes using shared frailty survival models nested within hospitals, with a fixed-sequence procedure for sequentially testing hierarchical clinical outcomes; if the first null hypothesis in the hierarchy was not rejected at a nominal level of 5%, the second hypothesis was tested as an exploratory analysis.29 We plotted Kaplan-Meier curves for the primary outcomes. We described effects on survival and count outcomes using hazard ratio (HR) and relative risk (RR), respectively, with 95% CIs. We tested the proportionality assumptions for the primary outcomes using the Kolmogorov-type supremum test; the assumptions were not violated. We used Poisson regression to analyze the number of events among surviving patients. We tested for between-hospital heterogeneity in the intervention’s effect on primary outcomes in a post hoc analysis by computing the HR with 95% CI at each hospital and using the type III test (shared frailty survival models).
We measured the effect of the intervention on B-PREPARED, CTM-3, and EQ-5D-5L using generalized linear mixed models, reporting mean differences and 95% CIs. We imputed missing data for living patients using age, sex, and LACE score; we used the Markov chain Monte Carlo method and assumed that all the variables followed a joint multivariate normal distribution. We imputed missing EQ-5D-5L scores at either 6 weeks or 6 months if baseline and 1 of 2 follow-up measures were collected. We calculated QALY using the area-under-the-curve approach with EQ-5D-5L–derived utilities and corresponding survival duration.27 We analyzed the intervention’s effect on QALY using mixed linear regression, adjusting for discharge EQ-5D-5L.
We conducted all analyses using SAS version 9.4 for UNIX (SAS Institute).
Results
Hospitals
The 10 clusters included in the analysis were urban tertiary or quaternary care hospitals with onsite cardiologists, cardiac critical care units, and cardiac imaging facilities; 5 had cardiac catheterization laboratories and 3 offered cardiac surgery onsite. All had onsite or regional HFCs during the intervention phase.
Patients
Patients were enrolled from February 1, 2015, to March 30, 2016 (Figure 1). Among 2494 eligible patients included in the primary analysis (1104 receiving the intervention and 1390 receiving usual care), the mean (SD) age was 77.7 (12.1) years, 1258 (50.4%) were women, 2488 (99.8%) were admitted to the hospital via the ED, and 706 (28.3%) had at least 3 ED visits in the preceding 6 months. Mean (SD) length of hospital stay was 7.7 (5.6) days. The intervention and usual care groups were similar in baseline demographics, QOL, comorbidities, estimated prognosis based on the Charlson32 index, and health care utilization based on length of stay and mean resource intensity weights33 during the index hospitalization (standardized difference ≤0.10, Table 1). While the median resource intensity weights were the same in both groups, the standardized difference was 0.20. Characteristics of the groups prior to propensity matching are presented in eTable 2 in Supplement 3.
Figure 1. Flow Diagram for the Patient-Centered Care Transitions in HF Pragmatic Stepped-Wedge Cluster Randomized Trial.
Ten hospitals crossed over unidirectionally from usual care to the intervention in a randomized sequence. During the intervention phase, patients were excluded if they had Boston18 score < 5, N-terminal prohormone brain-type natriuretic peptide19 <300 pg/mL, or brain natriuretic peptide19 <50 pg/mL. Appropriate comparator patients were identified in administrative databases using propensity scores to balance the risk profile of patients between groups.
aBased on Boston or biomarker criteria.
Table 1. Characteristics of Patients During the Index Hospitalization for Heart Failure (N = 2494)a.
| No. (%) | Standardized Difference | ||
|---|---|---|---|
| Intervention (n = 1104) | Usual Care (n = 1390) | ||
| Age, mean (SD), y | 77.77 (12.42) | 77.59 (11.89) | 0.02 |
| Sex | |||
| Men | 560 (50.7) | 676 (48.6) | 0.04 |
| Women | 544 (49.3) | 714 (51.4) | |
| Resides in long-term care | 164 (14.9) | 222 (16.0) | 0.06 |
| EQ-Visual Acuity score, mean (SD)b | 52.6 (22.7) | 53.7 (22.2) | 0.05 |
| Comorbiditiesc | |||
| Hypertension uncomplicated | 787 (71.3) | 1002 (72.1) | 0.02 |
| Atrial fibrillation | 583 (52.8) | 684 (49.2) | 0.07 |
| Diabetes with chronic complication | 524 (47.5) | 704 (50.6) | 0.06 |
| Diabetes without chronic complication | 301 (27.3) | 438 (31.5) | 0.09 |
| Chronic kidney disease | 242 (21.9) | 316 (22.7) | 0.02 |
| Myocardial infarction | 240 (21.7) | 295 (21.2) | 0.01 |
| Chronic pulmonary disease | 235 (21.3) | 334 (24.0) | 0.07 |
| Peripheral vascular disease | 107 (9.7) | 135 (9.7) | 0.00 |
| Cerebrovascular disease | 101 (9.1) | 129 (9.3) | 0.00 |
| Dementia | 98 (8.9) | 123 (8.8) | 0.00 |
| Gastrointestinal bleeding | 79 (7.2) | 97 (7.0) | 0.01 |
| Hypertension complicated | 57 (5.2) | 82 (5.9) | 0.03 |
| Mild liver disease | 32 (2.9) | 42 (3.0) | 0.01 |
| Moderate or severe liver disease | 15 (1.4) | 10 (0.7) | 0.06 |
| Cancer (any) | 19 (1.7) | 22 (1.6) | 0.01 |
| Resource utilization, median (IQR) | |||
| ED visits in prior 6 mo | 2 (1-3) | 2 (1-3) | 0.07 |
| Acute length of stay, d | 6 (4-10) | 6 (4-10) | 0.06 |
| RIWd | |||
| Mean (SD) | 1.45 (1.25) | 1.44 (0.81) | 0.02 |
| Median (IQR) | 1 (1-2) | 1 (1-2) | 0.20 |
| Estimated risk | |||
| LACE index, median (IQR)e | 12 (10-14) | 12 (10-14) | 0.10 |
| Charlson comorbidity index, mean (SD)f | 2.43 (1.27) | 2.45 (1.34) | 0.02 |
Abbreviations: ED, emergency department; IQR, interquartile range.
Other than self-reported QOL, all data were obtained from administrative databases.
EQ-Visual Acuity score, measured by the EuroQoL visual scale, is a self-reported quality of life or health status measure ranging from 0-100, with higher scores reflecting better health status. This was measured on hospital admission.
Baseline comorbidities were obtained using a 5-y retrospective review of databases.
Resource intensity weights (RIW)33 provide an estimate of the cost of resources used in the care of a patient relative to the average hospitalized patient. The higher the RIW, the higher the resource utilization relative to the average inpatient.
LACE21,22 index is derived from length of stay, acuity of presentation, comorbidities, and ED visits in the preceding 6 months. Range: 1 to 19, with higher scores associated with a higher risk of readmission or death following hospitalization.
Charlson comorbidity index32 is a method of predicting mortality and assessing disease burden based on comorbidities. The severity of comorbidity is categorized into 3 grades: mild (scores of 1-2); moderate (scores of 3-4); and severe (scores ≥5).
Based on 2140 patients whose drug administrative data were available, there was no significant difference between the intervention and usual care groups in the proportion that filled postdischarge prescriptions for angiotensin-converting enzyme inhibitors, β-blockers, mineralocorticoid receptor antagonists, or diuretics at 7 days (82.5% vs 79.8%; P = .11) or 30 days (92.8% vs 92.7%: P = .95).
Intervention Fidelity
Retrospective administrative data revealed that 2525 patients were hospitalized with HF during the intervention phase; however, only 2192 (86.8%) were screened for eligibility while in the hospital, indicating that at least 13.2% of patients were missed. Of 1043 patients in the intervention group for whom information was available, 916 (87.8%) had a discharge summary faxed to their family physician within a day of discharge. Among the 1104 patients in the intervention group, 485 (43.9%) had a LACE index of at least 13 on the day of discharge; of these patients, 35 (7.2%) died and could not be reached within 2 weeks of discharge. Among the remainder 450 patients with a LACE index of at least 13, including patients who were readmitted, 322 (71.6%) were scheduled to be seen in an HFC and 370 (82.2%) received nurse-led home visits within a month of the index discharge (median [IQR] of 3 [2-3] structured home visits and 4 [2-6] telephone calls during the postdischarge month). The possible uptake of the intervention in the usual care group was not audited.
Outcomes
Prespecified Primary Outcomes
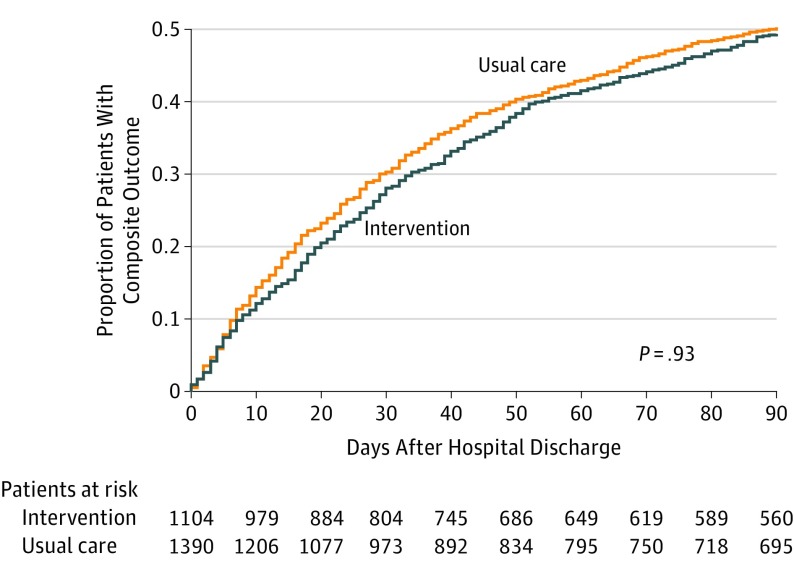
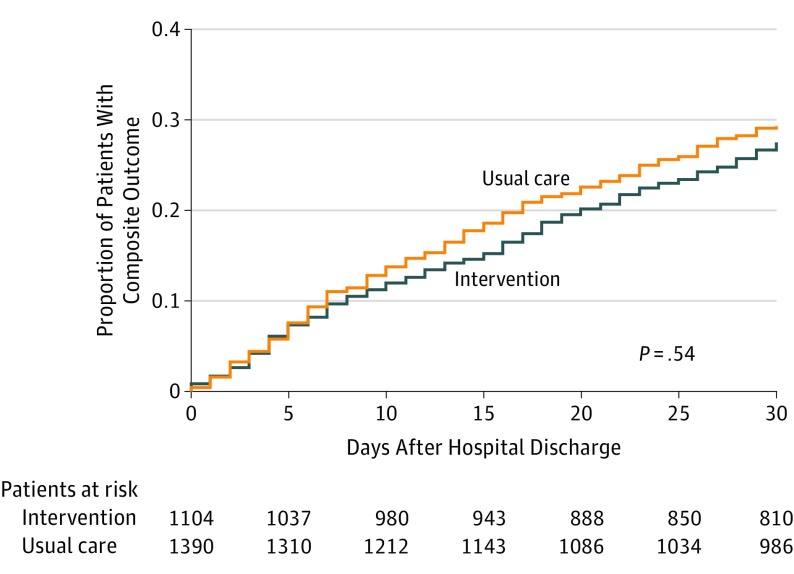
The incidence of 3-month composite all-cause readmission, ED visit, or death in the intervention and usual care group was 545 of 1104 (49.4%) and 698 of 1390 (50.2%), respectively, with a difference of 0.8%. There was no significant difference between the intervention and usual care group in the first primary outcome of time to first composite all-cause readmission, ED visit, or death at 3 months (HR, 0.99 [95% CI, 0.83-1.19]; P = .93) (Table 2 and Figure 2). Because there was no significant between-group difference in the first primary outcome in the hierarchy, analysis on the second co–primary outcome was considered exploratory. The incidence of 30-day composite all-cause readmission or ED visits in the intervention and usual care group was 304 of 1104 (27.5%) and 408 of 1390 (29.3%), respectively, with a difference of 1.8%. There was no significant difference between the intervention and usual care group in time to first composite all-cause readmission or ED visit at 30 days (HR, 0.93 [95% CI, 0.73-1.18]; P = .54) (Figure 3).
Table 2. Time-to-Event Analysis of Clinical Outcomes at 3 Months and 30 Days Following Hospitalization for Heart Failure.
| No. (%) | Hazard Ratio (95% CI)a | P Value | ||
|---|---|---|---|---|
| Intervention Group (n = 1104) | Usual Care Group (n = 1390) | |||
| Primary outcomes | ||||
| Composite all-cause readmission, ED visit, or death at 3 months | 545 (49.4) | 698 (50.2) | 0.99 (0.83-1.19) | .93 |
| Composite all-cause readmission or ED visit at 30 daysb | 304 (27.5) | 408 (29.3) | 0.93 (0.73-1.18) | .54 |
| Post hoc exploratory outcomes | ||||
| All-cause readmission at 3 months | 400 (36.2) | 500 (36.0) | 1.10 (0.91-1.34) | .32 |
| All-cause ED visit at 3 monthsc | 248 (22.5) | 334 (24.0) | 0.88 (0.68-1.15) | .36 |
| All cause death at 3 months | 111 (10.1) | 136 (9.8) | 1.18 (0.83-1.68) | .36 |
| All-cause readmission at 30 days | 225 (20.4) | 265 (19.1) | 1.23 (0.95-1.59) | .12 |
| All-cause ED visit at 30 days | 113 (10.2) | 190 (13.7) | 0.65 (0.45-0.95) | .03 |
Abbreviation: ED, emergency department.
Hazard ratio is derived using shared frailty survival models nested within hospitals, with intervention and steps as fixed effects (stepped-wedge design).
Given the lack of significant difference between intervention and usual care groups in the first composite clinical outcome of the prespecified hierarchy, analysis of the second composite clinical outcome is considered exploratory.
To prevent duplicate counting, ED visits were defined as those encounters in the ED that did not result in hospitalization.
Figure 2. Time to First Composite Readmission, Emergency Department Visit, or Death at 3 Months in the Intervention and Usual Care Groups.
Outcomes are measured relative to the date of hospital discharge following index hospitalization for heart failure, with patients analyzed in their allocated treatment group. Median (interquartile range) days of follow-up was 90 (81-90) for the intervention group and 90 (76-90) for the usual care group.
Figure 3. Time to First Composite Readmission or Emergency Department Visit at 30 Days in the Intervention and Usual Care Groups.
Outcomes are measured relative to the date of hospital discharge following index hospitalization for heart failure, with patients analyzed in their allocated treatment group. Median (interquartile range) days of follow-up was 30 (30-30) for the intervention and usual care groups.
Secondary Patient-Reported Outcomes
We collected patient-reported data from 986 patients across 8 hospitals (Table 3). There were significant differences between the intervention and usual care groups in mean B-PREPARED scores (difference, 2.65 [95% CI, 1.37-3.92]; P < .001); mean CTM-3 scores (difference, 6.16 [95% CI, 0.90-11.43]; P = .02); and mean EQ-5D-5L scores at hospital discharge (difference, 0.18 [95% CI, 0.14-0.23]; P < .001), 6 weeks (difference, 0.06 [95% CI, 0.01-0.11]; P = .02), and 6 months (difference, 0.06 [95% CI, 0.01-0.12]; P = .02). There was no significant difference between the groups in mean QALY (difference, 0.00 [95% CI, −0.02 to 0.02]; P = .98) during the 6-month follow-up period.
Table 3. Secondary Patient-Reported Outcomes Following Hospitalization for Heart Failure.
| Mean (SD) | Least-Squares Mean (95% CI)a | P Value | ||||
|---|---|---|---|---|---|---|
| Intervention (n = 606) | Usual Care (n = 380) | Intervention (n = 606) | Usual Care (n = 380) | Difference | ||
| B-PREPARED 6-week scoreb | 15.31 (4.83) | 13.67 (5.30) | 16.55 (15.50-17.59) | 13.91 (12.87-14.93) | 2.65 (1.37-3.92) | <.001 |
| CTM-3 6-week scorec | 74.34 (20.85) | 68.73 (17.83) | 76.47 (72.12-80.81) | 70.30 (65.97-74.63) | 6.16 (0.90-11.43) | .02 |
| EQ-5D-5L scored | ||||||
| Discharge | 0.70 (0.24) | 0.56 (0.28) | 0.73 (0.70-0.76) | 0.55 (0.52-0.58) | 0.18 (0.14-0.23) | <.001 |
| 6-week | 0.71 (0.24) | 0.69 (0.24) | 0.73 (0.70-0.76) | 0.67 (0.64-0.70) | 0.06 (0.01-0.11) | .02 |
| 6-month | 0.69 (0.26) | 0.66 (0.27) | 0.71 (0.67-0.74) | 0.64 (0.61-0.68) | 0.06 (0.01-0.12) | .02 |
| QALYs for the first 6 monthse | 0.34 (0.11) | 0.32 (0.11) | 0.34 (0.33-0.36) | 0.34 (0.33-0.35) | 0.00 (−0.02 to 0.02) | .98 |
Abbreviations: CTM-3, 3-Item Care Transitions Measure; EQ-5D-5L, 5-level EQ-5D version; QALY, quality-adjusted life-year.
Least-square mean models are adjusted for the stepped-wedge design. The 6-week and 6-month EQ5D5L scores and QALYs are adjusted for discharge EQ-5D-5L scores.
B-PREPARED score24 is a measure of discharge preparedness, ranging from 0 (worst) to 22 (best).
CTM-325 is a measure for quality of care transition, ranging from 0 (worst) to 100 (best).
EQ-5D-5L26 is a measure of quality of life based on domains of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The scale ranges from 0 (dead) to 1 (best quality of life).
QALY,27 a measure of both quantity and quality of life, is obtained by multiplying the value associated with a given state of health by the years lived in that state. All postdischarge measures were obtained via the telephone. A QALY of 1 implies perfect health for 1 year; QALY was measured over 6 months, so it is anchored at 0 (dead) and 0.5 (best health at 6 months).
Post Hoc Exploratory Clinical Outcomes
Among the exploratory outcomes at 3 months, there was no significant difference between the intervention and usual care group in time to first all-cause readmission (incidence, 36.2% vs 36.0%; HR, 1.10 [95% CI, 0.91-1.34]; P = .32), ED visit (incidence, 22.5% vs 24.0%; HR, 0.88 [95% CI, 0.68-1.15]; P = .36), or death (incidence, 10.1% vs 9.8%; HR, 1.18 [95% CI, 0.83-1.68]; P = .36) (Table 2 and eFigure 4 in Supplement 3). At 30 days, there was no significant difference between the intervention and usual care groups in time to first all-cause readmission (incidence, 20.4% vs 19.1%; HR, 1.23 [95% CI, 0.95-1.59]; P = .12), but a significant improvement was noted in the intervention group in time to first ED visit (incidence, 10.2% vs 13.7%; HR, 0.65 [95% CI, 0.45-0.95]; P = .03) (eFigure 5 in Supplement 3).
In an unadjusted before-and-after exploratory analysis, there were significant associations between the intervention group and first primary outcome of time to first composite all-cause readmission, ED visit, or death at 3 months at 2 of 10 hospitals (eFigure 6 in Supplement 3). However, no statistically significant between-hospital heterogeneity was found in the effect of the intervention on the primary composite outcomes at 3 months (P = .06) and 30 days (P = .07) when accounting for the stepped-wedge design.
Among 2247 patients (993 in the intervention and 1254 in the usual care group) alive at 3 months, there was no significant difference between the intervention and usual care group, respectively, in the number of composite all-cause readmissions or ED visits (797 vs 992 events; RR, 1.03 [95% CI, 0.86-1.25]; P = .73), all-cause readmissions (434 vs 566 events; RR, 1.09 [95% CI, 0.86-1.37]; P = .49), all-cause ED visits (363 vs 426 events; RR, 0.97 [95% CI, 0.79-1.19]; P = .77), or readmission for HF (163 vs 223 events; RR, 1.10 [95% CI, 0.83-1.46]; P = .50) (eTable 3 in Supplement 3). Among 2415 patients (1065 in the intervention and 1350 in the usual care group) alive at 30 days, there was no significant difference between the groups, respectively, in the number of all-cause readmissions or ED visits (352 vs 492 events; RR, 1.00 [95% CI, 0.82-1.21]; P = .98), all-cause readmissions (215 vs 279 events; RR, 1.14 [95% CI, 0.89-1.46]; P = .31), all-cause ED visits (137 vs 213 events; RR, 0.83 [95% CI, 0.58-1.18]; P = .30), or readmissions for HF (83 vs 110 events; RR, 1.07 [95% CI, 0.71-1.60]; P = .76).
In the post hoc sensitivity analysis of all patients hospitalized for HF during the study period (2525 in the intervention and 1918 in the usual care group), there was no significant difference between the intervention and usual care groups in the primary composite outcomes at 3 months (incidence, 51.6% vs 51.8%; HR, 1.00 [95% CI, 0.88-1.15]; P = .93) or 30 days (incidence, 31.1% vs 32.1%; HR, 0.91 [95% CI, 0.76-1.09]; P = .32).
Discussion
In this pragmatic stepped-wedge cluster randomized trial, a patient-centered transitional care model did not improve time to first all-cause readmission, ED visit, or death at 3 months or time to first all-cause readmission or ED visit at 30 days following HF hospitalization.
An implication of these results is that health services with demonstrated efficacy in explanatory RCTs may not be effective in improving clinical outcomes when implemented at the level of the health care system. Unlike explanatory RCTs, this pragmatic trial used broad eligibility criteria and included patients regardless of age, left ventricular function, comorbidities, or prognosis. The trial included patients without a fixed address, patients who are homebound or reside in nursing homes, and patients with language differences and suboptimal health literacy or self-care. The trial population, which was older and had a higher prevalence of comorbid conditions than populations in a majority of published transitional care RCTs,6,7 represents patients in health care settings who are often excluded from RCTs28 because of anticipated nonadherence, barriers to care, or illnesses that decrease an intervention’s efficacy.
This trial involved multicenter recruitment that included both tertiary and quaternary care centers and use of personnel managed by their institutions rather than by the research team; it is possible that this pragmatic approach introduced variation between hospitals and modified the intervention’s effect. For example, while there was no statistically significant heterogeneity in the effect of the intervention on the primary composite outcomes across hospitals, exploratory before-and-after analysis revealed significant associations between the intervention and the first primary composite outcome in 2 of the 10 hospitals.
Another reason that results may have diverged from past clinical trials is that services were titrated to risk. Nurse-led home visits and HFC care were offered to less than 40% of the intervention group, which may have diluted the effect of these interventions. The neutral findings may also represent a floor effect among high-risk patients who are at a more advanced disease stage and face a higher proportion of unavoidable admissions than low-risk patients.1 An additional pitfall of using risk prediction to guide resource allocation is that risk models are not always reliable. While the LACE index is a simple tool, validated for risk prediction at the bedside, its ability to discriminate risk in HF is modest.21
Health services often depend on contextual factors for delivery and uptake.30,33 Just before this trial, Ontario introduced a funding incentive for hospitals to improve quality of care and reduce readmissions in HF10; while this facilitated engagement of policy makers in the research, it may have also motivated hospitals to improve baseline health care quality, thereby producing a ceiling effect and minimizing the benefit of the intervention.
This study was designed to detect a 25% improvement in the first primary composite clinical outcome, arguably an ambitious target, and the possibility that the intervention improved outcomes below this threshold of detection cannot be excluded. Findings of post hoc exploratory analyses were largely consistent with a nonsignificant difference in primary outcomes between the intervention and usual care groups. Among the individual components of the primary composite clinical outcomes at 3 months and 30 days, a benefit in the intervention group was noted only in time to first ED visit at 30 days, with no reduction in number of readmissions or ED visits during the follow-up period.
Health care interventions that do not improve clinical outcomes can improve outcomes that are meaningful to patients; this tension can pose a challenge to policy makers regarding program funding. The intervention improved the secondary outcomes of discharge preparedness, quality of care transition, and QOL, but these outcomes were exploratory, and the lack of improvement in QALY, which measures both quality and quantity of life, may indicate the need for stronger evidence to support funding this intervention.
This study has several strengths. Patients, clinicians, and policy makers were engaged in this work, and the selected outcomes were meaningful to patients and the health care system. The intervention was tested using a stepped-wedge design that is novel in cardiovascular research and less subject to bias than typical quality improvement research methods.14,15 In applying a pragmatic6 approach and using existing resources within the publicly funded system, the study’s results reflect effectiveness in clinical settings.33,34 The use of administrative databases to measure clinical outcomes minimized measurement burden on patients, and the use of time-to-event analysis and nested substudies in the context of a stepped-wedge trial advanced the science of trial design.
Limitations
This study has several limitations. First, the clinical trial was confined to urban hospitals in a single-payer health care system, and results may not be generalizable to other health care systems. Second, while the baseline characteristics in the intervention and usual care groups were similar, there was a wider distribution of resource intensity weights, a measure of resource utilization, in the intervention group. Third, while certain process-of-care indicators were audited, the quality and duration of each episode of care and patients’ adherence to discharge recommendations were not assessed. Fourth, improvements in usual care just prior to the onset of the trial cannot be excluded, and these improvements may have caused a ceiling effect. Fifth, while steps were taken to avoid contamination, uptake of the intervention during the usual care phase cannot be excluded. Sixth, the focus of services on high-risk patients with little modifiable risk may have produced a floor effect.
Conclusions
Among patients hospitalized for HF in Ontario, Canada, implementation of a patient-centered transitional care model compared with usual care did not improve a composite of clinical outcomes. Whether this type of intervention could be effective in other health care systems or locations requires further research.
Trial protocol and statistical analysis plan
Summary of protocol changes
eTable 1. Provincial databases used for baseline characteristics and outcomes
eTable 2. Baseline characteristics prior to propensity scorea matching of intervention and 4 usual care groups
eTable 3. Post-hoc clinical outcomes: number of readmissions and ED visits among patients alive at 30 days and 3 months
eFigure 1. Study protocol and outcome measures
eFigure 2. Study design of the Patient-Centered Care Transitions in HF trial
eFigure 3. Pragmatic study design choices of the Patient-Centered Care Transitions in HF trial
eFigure 4a. Kaplan-Meier curves for the post-hoc outcomes of time-to-first all-cause death at 3 months in the intervention and usual care groups
eFigure 4b. Kaplan-Meier curves for the post-hoc outcomes of time-to-first all-cause readmission at 3 months in the intervention and usual care groups
eFigure 4c. Kaplan-Meier curves for the post-hoc outcomes of time-to-first all-cause ED visit at 3 months in the intervention and usual care groups
eFigure 5a. Kaplan-Meier curves for the post-hoc outcomes of time-to-first all-cause readmission at 30 days in the intervention and usual care groups
eFigure 5b. Kaplan-Meier curves for the post-hoc outcomes of time-to-first all-cause ED visit at 30 days in the intervention and usual care groups
eFigure 6. Before-after hospital level subgroup analysis of the primary composite outcome of (a) time-to-first composite readmission, ED visit, or death at 3 months and (b) time-to-first composite readmission or ED visit at 30 days
Data sharing statement
References
- 1.Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126(4):501-506. doi: 10.1161/CIRCULATIONAHA.112.125435 [DOI] [PubMed] [Google Scholar]
- 2.Shafie AA, Tan YP, Ng CH. Systematic review of economic burden of heart failure. Heart Fail Rev. 2018;23(1):131-145. doi: 10.1007/s10741-017-9661-0 [DOI] [PubMed] [Google Scholar]
- 3.Phelan D, Smyth L, Ryder M, et al. Can we reduce preventable heart failure readmissions in patients enrolled in a Disease Management Programme? Ir J Med Sci. 2009;178(2):167-171. doi: 10.1007/s11845-009-0332-6 [DOI] [PubMed] [Google Scholar]
- 4.Naylor MD, Aiken LH, Kurtzman ET, Olds DM, Hirschman KB. The care span: the importance of transitional care in achieving health reform. Health Aff (Millwood). 2011;30(4):746-754. doi: 10.1377/hlthaff.2011.0041 [DOI] [PubMed] [Google Scholar]
- 5.Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716-1722. doi: 10.1001/jama.2010.533 [DOI] [PubMed] [Google Scholar]
- 6.Van Spall HGC, Rahman T, Mytton O, et al. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta-analysis. Eur J Heart Fail. 2017;19(11):1427-1443. doi: 10.1002/ejhf.765 [DOI] [PubMed] [Google Scholar]
- 7.Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160(11):774-784. doi: 10.7326/M14-0083 [DOI] [PubMed] [Google Scholar]
- 8.Graham IDG, Logan J, Harrison MB, et al. Lost in knowledge translation: time for a map? J Contin Educ Health Prof. 2006;26(1):13-24. doi: 10.1002/chp.47 [DOI] [PubMed] [Google Scholar]
- 9.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the management of heart failure. J Card Fail. 2017;23(8):628-651. doi: 10.1016/j.cardfail.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 10.Health Quality Ontario, Ministry of Health and Long-Term Care Quality-Based Procedures: Clinical Handbook for Heart Failure (Acute and Postacute). Toronto, ON: Health Quality Ontario; 2015. http://www.hqontario.ca/Portals/0/Documents/evidence/clinical-handbooks/heart-failure-02042015-en.pdf.
- 11.World Health Organization Regional Office for Europe Integrated Care Models: An Overview. Copenhagen, Denmark: World Health Organization; 2016. http://www.euro.who.int/__data/assets/pdf_file/0005/322475/Integrated-care-models-overview.pdf.
- 12.Wolfe A. Institute of Medicine report: crossing the quality chasm: a new health care system for the 21st century. Policy Polit Nurs Pract. 2001;2:233-235. doi: 10.1177/152715440100200312 [DOI] [Google Scholar]
- 13.Canadian Institutes of Health Research Guide to Knowledge Translation Planning at CIHR and End-Of-Grant Approaches. Ottawa, ON: Canadian Institutes of Health Research; 2012. http://www.cihr-irsc.gc.ca/e/documents/kt_lm_ktplan-en.pdf.
- 14.Brown CA, Lilford RJ. The stepped wedge trial design: a systematic review. BMC Med Res Methodol. 2006;6:54. doi: 10.1186/1471-2288-6-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials. 2007;28(2):182-191. doi: 10.1016/j.cct.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 16.Van Spall HGC, Lee SF, Xie F, et al. Knowledge to action: rationale and design of the Patient-Centered Care Transitions in Heart Failure (PACT-HF) stepped wedge cluster randomized trial. Am Heart J. 2018;199:75-82. doi: 10.1016/j.ahj.2017.12.013 [DOI] [PubMed] [Google Scholar]
- 17.Smith LR, Ashok M, Dy SM, et al. Contextual Frameworks for Research on the Implementation of Complex System Interventions. Rockville, MD: US Agency for Healthcare and Quality; 2014: 8-26. [PubMed] [Google Scholar]
- 18.Roberts E, Ludman AJ, Dworzynski K, et al. The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and meta-analysis in the acute care setting. BMJ. 2015;350:h910. doi: 10.1136/bmj.h910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.EuroQol Research Foundation EQ-5D-5L User Guide: Basic information on how to use the EQ-5D-5L instrument. Rotterdam, The Netherlands; EuroQol Research Foundation; 2015. https://euroqol.org/wp-content/uploads/2016/09/EQ-5D-5L_UserGuide_2015.pdf.
- 20.Harrison MB, Graham ID, Logan J, Toman C, Friederg E. Evidence to practice: pre-post-implementation study of a patient/provider resource for self-management with heart failure. Int J Evid Based Healthc. 2007;5(1):92-101. [DOI] [PubMed] [Google Scholar]
- 21.Yazdan-Ashoori P, Lee SF, Ibrahim Q, Van Spall HG. Utility of the LACE index at the bedside in predicting 30-day readmission or death in patients hospitalized with heart failure. Am Heart J. 2016;179:51-58. doi: 10.1016/j.ahj.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 22.van Walraven C, Dhalla IA, Bell C, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551-557. doi: 10.1503/cmaj.091117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yale New Haven Health Services Corporation/Center for Outcomes Research and Evaluation 2013 Measures Updates and Specifications Report: Acute Myocardial Infarction, Heart Failure, and Pneumonia 30-Day Risk-Standardized Mortality Measure. Baltimore, MD: Centers for Medicare & Medicaid Services; 2013. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Mortality_AMI-HF-PN_Measures_Updates_Report_FINAL_06-13-2013.pdf.
- 24.Care Transitions Program Tools and resources. The Care Transitions Program website. http://caretransitions.org/tools-and-resources/. Accessed February 7, 2019.
- 25.Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96:5-21. doi: 10.1093/bmb/ldq033 [DOI] [PubMed] [Google Scholar]
- 26.Hemming K, Taljaard M. Sample size calculations for stepped wedge and cluster randomised trials: a unified approach. J Clin Epidemiol. 2016;69:137-146. doi: 10.1016/j.jclinepi.2015.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woertman W, de Hoop E, Moerbeek M, Zuidema SU, Gerritsen DL, Teerenstra S. Stepped wedge designs could reduce the required sample size in cluster randomized trials. J Clin Epidemiol. 2013;66(7):752-758. doi: 10.1016/j.jclinepi.2013.01.009 [DOI] [PubMed] [Google Scholar]
- 28.McClure NS, Sayah FA, Xie F, Luo N, Johnson JA. Instrument-defined estimates of the minimally important difference for EQ-5D-5L Index Scores. Value Health. 2017;20(4):644-650. doi: 10.1016/j.jval.2016.11.015 [DOI] [PubMed] [Google Scholar]
- 29.Stukel TA, Fisher ES, Alter DA, et al. Association of hospital spending intensity with mortality and readmission rates in Ontario hospitals. JAMA. 2012;307(10):1037-1045. doi: 10.1001/jama.2012.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shanbhag D, Graham ID, Harlos K, et al. Effectiveness of implementation interventions in improving physician adherence to guideline recommendations in heart failure: a systematic review. BMJ Open. 2018;8(3):e017765. doi: 10.1136/bmjopen-2017-017765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Walraven C, Jennings A, Forster AJ. A meta-analysis of hospital 30-day avoidable readmission rates. J Eval Clin Pract. 2012;18(6):1211-1218. doi: 10.1111/j.1365-2753.2011.01773.x [DOI] [PubMed] [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 33.Eccles M, Grimshaw J, Campbell M, Ramsay C. Research designs for studies evaluating the effectiveness of change and improvement strategies. Qual Saf Health Care. 2003;12(1):47-52. doi: 10.1136/qhc.12.1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Spall HGC, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297(11):1233-1240. doi: 10.1001/jama.297.11.1233 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
Summary of protocol changes
eTable 1. Provincial databases used for baseline characteristics and outcomes
eTable 2. Baseline characteristics prior to propensity scorea matching of intervention and 4 usual care groups
eTable 3. Post-hoc clinical outcomes: number of readmissions and ED visits among patients alive at 30 days and 3 months
eFigure 1. Study protocol and outcome measures
eFigure 2. Study design of the Patient-Centered Care Transitions in HF trial
eFigure 3. Pragmatic study design choices of the Patient-Centered Care Transitions in HF trial
eFigure 4a. Kaplan-Meier curves for the post-hoc outcomes of time-to-first all-cause death at 3 months in the intervention and usual care groups
eFigure 4b. Kaplan-Meier curves for the post-hoc outcomes of time-to-first all-cause readmission at 3 months in the intervention and usual care groups
eFigure 4c. Kaplan-Meier curves for the post-hoc outcomes of time-to-first all-cause ED visit at 3 months in the intervention and usual care groups
eFigure 5a. Kaplan-Meier curves for the post-hoc outcomes of time-to-first all-cause readmission at 30 days in the intervention and usual care groups
eFigure 5b. Kaplan-Meier curves for the post-hoc outcomes of time-to-first all-cause ED visit at 30 days in the intervention and usual care groups
eFigure 6. Before-after hospital level subgroup analysis of the primary composite outcome of (a) time-to-first composite readmission, ED visit, or death at 3 months and (b) time-to-first composite readmission or ED visit at 30 days
Data sharing statement