Abstract
This study assesses the differences in composition of human milk oligosaccharides of mothers who received either probiotic supplements or placebos in the late stages of pregnancy.
Human milk oligosaccharides (HMOs) are complex glycans and the third-largest solid component in human milk. They are typically nondigestible by humans but are a major substrate for infants’ gut microbiota and affect the maturation of the intestinal mucosal immune system. All HMOs are extensions of lactose generated by the action of a series of glycosyltransferases. Depending on the mother’s Secretor and Lewis blood groups, the fucosyltransferases FUT2 (the Secretor gene) and/or FUT3 (the Lewis gene) are available for the synthesis of HMOs. The resulting heterogeneity implies that some breastfed infants are not being exposed to certain structures, which may affect their microbiome composition and thereby disease risk for illnesses in which gut microbiome plays a role. Infants fed by mothers who lack a Secretor gene and therefore lack a functional FUT2 enzyme and all α-1-2-fucosylated oligosaccharides have delayed development of bifidobacteria-laden microbiota.1 Also, if these infants are born via cesarean delivery, they have a higher risk to manifest IgE-associated eczema.2 We recently showed that certain HMOs are associated with protection against cow-milk allergy in infants.3 Besides genetic differences that are responsible for differences in HMO profiles, HMO abundance changes throughout lactation.4 However, no other factors have been associated with variation in HMO abundances. This study sought to assess the association of maternal probiotic supplementation with HMO concentrations.
Methods
We used stored frozen colostrum samples from a previously published large, double-blind, placebo-controlled randomized clinical trial5,6 of probiotic supplementation study of 1223 pregnant mothers from Helsinki, Finland, carrying fetuses at hereditary risk for allergy (ie, the offspring had 1 or both parents with physician-diagnosed allergic rhinitis, eczema, and/or asthma). The children born to these mothers were followed up for 10 years for the development of allergic disease. In the probiotic group, mothers took twice-daily doses of Lactobacillus rhamnosus GG, Lactobacillus rhamnosus LC705, Bifidobacterium breve Bb99, and Propionibacterium freudenreichii subspecies shermanii JS in capsules from 36 weeks’ gestation until the birth of the infant. Mature milk samples were available for more than 500 mothers; we randomly selected 81 colostrum samples (stored frozen at −62.2°C), with 30 samples from the placebo group and 51 from the probiotic group. There was an equal distribution of tobacco-smoking mothers and mothers with a history of atopic disease between the 2 groups. The concentrations of 19 HMOs were determined using high-performance liquid chromatography after 2-aminobenzamide labeling.3 Raffinose was added to milk samples as an internal standard to allow for absolute quantification. Freezing does not affect HMO levels, which are very stable in full-term milk from day to day and diurnally.4
The study was approved by the institutional review board of the Hospital for Children and Adolescents, Helsinki University Central Hospital, Finland. All mothers signed a written informed consent form.
Statistical analysis was carried out using R software version 3.5.0 (R Foundation for Statistical Computing), and 2-sided P values less than .05 were considered significant in all comparisons. Data collection occurred from November 2000 to March 2003, and data analysis from March 2018 to June 2018.
Results
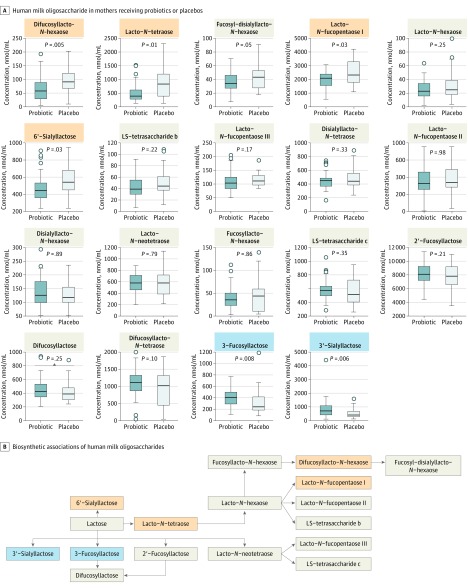
The concentrations of 3-fucosyllactose (Figure, A) were significantly higher in colostrum from the mothers in the probiotic supplementation group (mean [SD], 413 [164] μmol/mL) compared with those in the nonsupplemented group (mean [SD], 312 [232] μmol/mL; P = .008). Likewise, 3′-sialyllactose was higher in colostrum from the probiotic supplementation group (mean [SD], 833 [679] μmol/mL) than in control participants (mean [SD], 516 [378] μmol/mL; P = .006).
Figure. Probiotics and Human Milk Oligosaccharide Levels.
A, Human milk oligosaccharide levels in mothers who received a mixture of probiotics or placebos. Boxplots show median and quartiles of concentrations of each of the human milk oligosaccharides and the P value of the t test comparing log-transformed concentrations of the 2 groups. Human milk oligosaccharides that were found in a significantly lower concentration in the milk of mothers receiving the probiotic are difucosyllacto-N-hexaose, lacto-N-tetraose, lacto-N-fucopentaose I, and 6′-sialyllactose (marked with orange); those found in higher concentrations are 3-fucosyllactose and 3′-sialyllactose (marked with blue). B, Biosynthetic associations of human milk oligosaccharides are shown with probiotic-induced decreases and increases as indicated in part A.
However, mean [SD] levels of HMOs were lower in colostrum from mothers who received probiotic supplementation than in the control group; this was found in difucosyllacto-N-hexaose (probiotic group, 62.0 [43.4] μmol/mL; control group, 93.7 [47.7] μmol/mL; P = .005), lacto-N-tetraose (probiotic group, 509 [339] μmol/mL; control group, 861 [563] μmol/mL; P = .01), lacto-N-fucopentaose I (probiotic group, 2000 [585] μmol/mL; control group, 2450 [836] μmol/mL; P = .03), and 6′-sialyllactose (probiotic group, 472 [159] μmol/mL; control group, 567 [179] μmol/mL; P = .03). These changes are consistent with a change in select pathways in overall HMO biosynthetic machinery (Figure, B).
Conclusions
Although HMO composition is largely genetically determined, maternal probiotic supplementation during late stages of pregnancy may change the HMO composition in human milk. It is unknown whether the change is specific to the probiotics used in this study, and findings need to be confirmed by other studies. In this case, we hypothesize a shift from 6′-sialylation to 3′-sialylation and toward type-2 core structures in HMOs. Maternal diet, medications, and microbiome may be important factors modulating a mother’s colostrum HMO levels that should be considered when assessing biological effects suspected to be mediated by HMO levels. Ultimately, modifying mothers’ milk HMO composition may open new avenues for disease modification in infants who are breastfed.
References
- 1.Lewis ZT, Totten SM, Smilowitz JT, et al. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome. 2015;3:13. doi: 10.1186/s40168-015-0071-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sprenger N, Odenwald H, Kukkonen AK, Kuitunen M, Savilahti E, Kunz C. FUT2-dependent breast milk oligosaccharides and allergy at 2 and 5 years of age in infants with high hereditary allergy risk. Eur J Nutr. 2017;56(3):1293-1301. doi: 10.1007/s00394-016-1180-6 [DOI] [PubMed] [Google Scholar]
- 3.Seppo AE, Autran CA, Bode L, Järvinen KM. Human milk oligosaccharides and development of cow’s milk allergy in infants. J Allergy Clin Immunol. 2017;139(2):708-711.e5. doi: 10.1016/j.jaci.2016.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thurl S, Munzert M, Henker J, et al. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br J Nutr. 2010;104(9):1261-1271. doi: 10.1017/S0007114510002072 [DOI] [PubMed] [Google Scholar]
- 5.Kukkonen K, Savilahti E, Haahtela T, et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2007;119(1):192-198. doi: 10.1016/j.jaci.2006.09.009 [DOI] [PubMed] [Google Scholar]
- 6.Kuitunen M, Kukkonen K, Juntunen-Backman K, et al. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J Allergy Clin Immunol. 2009;123(2):335-341. doi: 10.1016/j.jaci.2008.11.019 [DOI] [PubMed] [Google Scholar]