Key Points
Question
What is the clinical and therapeutic significance of magnetic resonance imaging markers of covert vascular brain injury (white matter hyperintensities of presumed vascular origin, magnetic resonance imaging–defined covert brain infarcts, cerebral microbleeds, and perivascular spaces) in community-dwelling older adults?
Findings
In this systematic review and meta-analysis of more than 16 000 participants, there was evidence that white matter hyperintensities, brain infarcts, and cerebral microbleeds have a major clinical significance in community-dwelling older adults; they were associated with an increased risk of stroke (both hemorrhagic and ischemic for all markers), dementia, and death.
Meaning
This research highlights the urgent need for randomized clinical trials to assess the benefit–risk ratio of prevention strategies for individuals carrying these markers, such as aspirin and intensive blood pressure–lowering treatment.
This systematic review and meta-analysis investigates the clinical and therapeutic significance of magnetic resonance imaging markers of covert vascular brain injury (white matter hyperintensities, brain infarcts, and cerebral microbleeds) associated with stroke, dementia, and death in community-dwelling older adults.
Abstract
Importance
Covert vascular brain injury (VBI) is highly prevalent in community-dwelling older persons, but its clinical and therapeutic implications are debated.
Objective
To better understand the clinical significance of VBI to optimize prevention strategies for the most common age-related neurological diseases, stroke and dementia.
Data Source
We searched for articles in PubMed between 1966 and December 22, 2017, studying the association of 4 magnetic resonance imaging (MRI) markers of covert VBI (white matter hyperintensities [WMHs] of presumed vascular origin, MRI-defined covert brain infarcts [BIs], cerebral microbleeds [CMBs], and perivascular spaces [PVSs]) with incident stroke, dementia, or death.
Study Selection
Data were taken from prospective, longitudinal cohort studies including 50 or more adults.
Data Extraction and Synthesis
We performed inverse variance–weighted meta-analyses with random effects and z score–based meta-analyses for WMH burden. The significance threshold was P < .003 (17 independent tests). We complied with the Meta-analyses of Observational Studies in Epidemiology guidelines.
Main Outcomes and Measures
Stroke (hemorrhagic and ischemic), dementia (all and Alzheimer disease), and death.
Results
Of 2846 articles identified, 94 studies were eligible, with up to 14 529 participants for WMH, 16 012 participants for BI, 15 693 participants for CMB, and 4587 participants for PVS. Extensive WMH burden was associated with higher risk of incident stroke (hazard ratio [HR], 2.45; 95% CI, 1.93-3.12; P < .001), ischemic stroke (HR, 2.39; 95% CI, 1.65-3.47; P < .001), intracerebral hemorrhage (HR, 3.17; 95% CI, 1.54-6.52; P = .002), dementia (HR, 1.84; 95% CI, 1.40-2.43; P < .001), Alzheimer disease (HR, 1.50; 95% CI, 1.22-1.84; P < .001), and death (HR, 2.00; 95% CI, 1.69-2.36; P < .001). Presence of MRI-defined BIs was associated with higher risk of incident stroke (HR, 2.38; 95% CI, 1.87-3.04; P < .001), ischemic stroke (HR, 2.18; 95% CI, 1.67-2.85; P < .001), intracerebral hemorrhage (HR, 3.81; 95% CI, 1.75-8.27; P < .001), and death (HR, 1.64; 95% CI, 1.40-1.91; P < .001). Presence of CMBs was associated with increased risk of stroke (HR, 1.98; 95% CI, 1.55-2.53; P < .001), ischemic stroke (HR, 1.92; 95% CI, 1.40-2.63; P < .001), intracerebral hemorrhage (HR, 3.82; 95% CI, 2.15-6.80; P < .001), and death (HR, 1.53; 95% CI, 1.31-1.80; P < .001). Data on PVS were limited and insufficient to conduct meta-analyses but suggested an association of high PVS burden with increased risk of stroke, dementia, and death; this requires confirmation.
Conclusions and Relevance
We report evidence that MRI markers of VBI have major clinical significance. This research prompts careful evaluation of the benefit–risk ratio for available prevention strategies in individuals with covert VBI.
Introduction
Large-scale brain imaging studies in the general population have shown that radiological evidence of covert vascular brain injury (VBI) is much more frequent than clinical stroke and is highly prevalent in community-dwelling older persons.1,2,3 Such incidental findings are also often detected on magnetic resonance imaging (MRI) performed in routine clinical practice, and how they should be interpreted and acted on presents a common clinical challenge. Individual studies have suggested that MRI markers of covert VBI predict an increased risk of stroke, dementia, and death, but other studies did not show any association. Better understanding of the association of specific MRI markers of VBI with outcomes is crucial to optimize prevention strategies. Indeed, detection of covert VBI on MRI may provide a unique opportunity to prevent the occurrence of stroke and dementia, the 2 most common age-related neurological diseases, representing a major source of disability and mortality.4
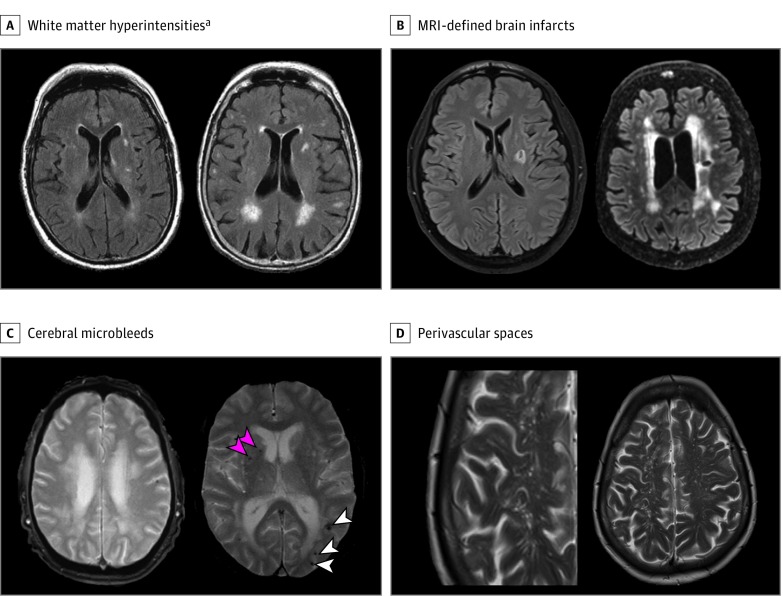
Four radiological features of VBI are seen on routine MRI scans (Figure 1).5 Magnetic resonance imaging–defined covert brain infarcts (BIs) represent areas of infarction, most commonly small and in subcortical regions, and are usually asymptomatic.6 White matter hyperintensities (WMHs) of presumed vascular origin are seen as areas of high signal on T2-weighted MRI in the periventricular and deep white matter and represent areas of gliosis, axonal loss, and ischemic demyelination.7 Cerebral microbleeds (CMBs) are seen as areas of low signal on gradient echo MRI sequences and represent susceptibility effects due to hemosiderin from previous microbleeds.8 Perivascular spaces (PVSs) correspond to the dilation of spaces surrounding small perforating vessels filled with cerebrospinal fluid–like signal and lined by leptomeningeal cells.9 These MRI markers of covert VBI are thought to primarily reflect consequences of underlying cerebral small vessel disease (SVD). The most common pathological substrates of SVD are arteriolosclerosis or lipohyalinosis and cerebral amyloid angiopathy.10 We conducted a systematic review and meta-analyses of published studies to explore associations of the 4 main MRI markers of covert VBI with risk of incident stroke, dementia, and death.
Figure 1. Magnetic Resonance Imaging (MRI) Markers of Covert Vascular Brain Injury.
A, Minor (left) and extensive (right) white matter hyperintensities of presumed vascular origin on axial fluid-attenuated inversion recovery MRI sequences.a B, Magnetic resonance imaging–defined covert brain infarct without (left) and with (right) white matter hyperintensities on axial fluid-attenuated inversion recovery MRI sequences. C, Single cerebral microbleed (left) or multiple cerebral microbleeds (right), including lobar (white arrowheads) and deep (pink arrowheads) microbleeds, on gradient echo T2-weighted axial MRI sequences. D, Perivascular spaces following the shape of deep penetrating arteries on T2-weighted MRI.
aThe definitions of extensive white matter hyperintensity burden differed across studies, ie, top half, tertile, quartile, or quintile; or moderate to severe white matter hyperintensity burden (or corresponding grades) on the following visual semiquantitative rating scales: Fazekas scale, Scheltens scale, or Age-Related White Matter Changes Scale (eTables 4-6 in the Supplement).
Methods
Search Strategy and Selection Criteria
We did a systematic search of PubMed from 1966 to December 22, 2017, for English-only publications using predefined search terms (eMethods in the Supplement) and reviewed the reference list of relevant articles. Studies were searched and selected by 3 independent researchers (S.S., M.-G.D., and S.C.L.); differences were solved by discussion. We included published prospective studies with longitudinal data exploring the association of WMH, BI, CMB, and PVS with risk of incident stroke, dementia, or death, limited to studies in adults and in English language. We included studies carried out in the general population and in populations at high risk for vascular disease or dementia and present results separately for each. We excluded studies without effect estimates and confidence intervals or raw numbers enabling the calculation of these estimates. We also excluded studies with computed tomography evaluation only; with fewer than 50 individuals (considering these could not provide reliable effect estimates); and with WMH occurring in inflammatory conditions (eg, multiple sclerosis, lupus, or Sneddon syndrome), monogenic neurodegenerative or cerebrovascular diseases (eg, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy), leukodystrophies, or studies on postthrombolysis outcome after ischemic stroke (IS). If several studies provided results on the same outcome and used overlapping groups of individuals, we included the study with the longest follow-up. If follow-up periods were equivalent, we included the study with the largest number of individuals. This review was not registered but complied with Meta-analyses of Observational Studies in Epidemiology guidelines.
Data Analysis
Data were extracted independently by S.S. and S.C.L. Extracted data consisted of population type (general or high-risk population), length of follow-up, MRI characteristics and sequence, definition of MRI marker, outcome definition (ie, stroke, IS, intracerebral hemorrhage [ICH], all-cause dementia, Alzheimer disease [AD], and death), number of incident events, and relative risk estimate for the association of the MRI marker with the outcome. Variable definitions are provided in the eMethods in the Supplement. The relative risk estimate was a hazard ratio (HR), a relative risk, or an odds ratio; odds ratios were used in the meta-analysis as an approximation of the HR.11 We extracted quality criteria of included studies (eTable 1 in the Supplement) and used the Newcastle-Ottawa Scale to quantify study quality (eTable 2 in the Supplement).12
Meta-analyses were carried out when 3 or more studies were available for the same main outcome or 2 or more studies for the same outcome subtype (and only when possible for 3 or more MRI markers of covert VBI). We calculated pooled HRs using inverse variance–weighted meta-analysis with random effects to account for potential heterogeneity for associations with BI, CMB, or dichotomized WMH burden. Statistically significant heterogeneity was defined by a heterogeneity P value less than .05 or an I2 greater than 50%. We also performed sample size–weighted z score–based meta-analyses (providing significance values but no effect estimates) to combine studies using continuous WMH burden only with those using dichotomized WMH burden. Whenever available, we used the model adjusted for vascular risk factors. Primary meta-analyses combined all available studies, and secondary meta-analyses included studies in general or high-risk populations only (eMethods in the Supplement). P values less than .003 after applying a Bonferroni correction for 17 meta-analyses were considered statistically significant (this threshold is conservative, as MRI markers are correlated with each other), and all P values were 2-tailed.
Meta-regression analysis was conducted in Stata version 14.2 (StataCorp) to assess the effect of length of follow-up and potential confounding factors (ie, age, smoking, hypertension, diabetes, and education) on associations. Small-study bias, such as publication bias, was evaluated using Egger test.13 P values less than .006 (accounting for 9 MRI marker × outcome associations) were considered statistically significant.
Results
The initial search in PubMed identified 2846 articles. Of these, 94 articles met our inclusion criteria (eFigure 1 in the Supplement). Some articles explored several MRI markers and associations with more than 1 outcome. Five articles, all on PVS burden, could not be included in the meta-analyses, as too few studies tested associations of the same MRI marker with the same outcome.
For WMH, 52 studies met our inclusion criteria: 25 for stroke,6,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 22 for dementia,25,35,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57 and 16 for death.14,15,17,20,22,33,36,37,39,58,59,60,61,62,63,64 For BI, we included 24 studies: 14 for stroke,16,17,21,25,33,36,39,65,66,67,68,69,70,71 10 for dementia,25,39,40,48,51,54,57,72,73,74 and 8 for death.17,33,36,39,58,59,71,73 For CMB, 38 studies were retained: 28 for stroke,16,18,19,24,28,30,31,32,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92 6 for dementia,54,57,93,94,95,96 and 10 for death.58,76,77,83,88,90,91,97,98,99 Of note, for 7 studies, association results of CMB with recurrent stroke or ICH risk were obtained from 3 previously published meta-analyses.78,100,101 Regarding PVS, 5 studies were included: 3 for stroke,18,102,103 2 for dementia,104,105 and 2 for death.102,103
White Matter Hyperintensity Burden
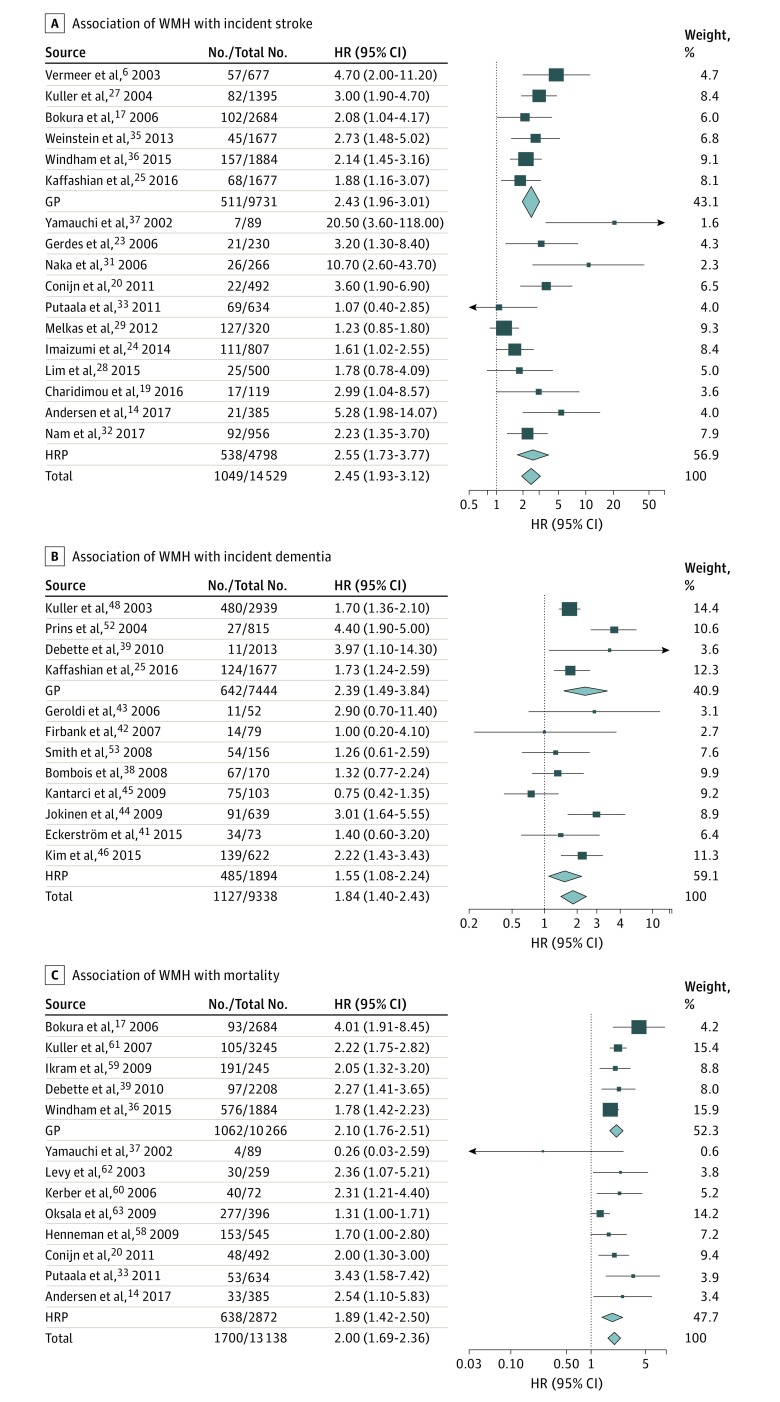
In 14 529 participants from 17 studies, we found a significant association of extensive WMH burden (n = 2859 participants) with risk of incident stroke (n = 1049 events) overall (heterogeneity test results, I2 = 56%; P = .003), in the general population,6,17,25,27,35,36 and in high risk populations14,19,20,23,24,28,29,31,32,33,37 (Table; Figure 2) (eTables 3 and 4 in the Supplement). Adding 3 studies reporting associations with continuous WMH burden in a sample size–weighted meta-analysis confirmed the association with incident stroke (n = 14 913 participants; n = 1114 events; P < .001).15,22,30 Regarding stroke subtypes, extensive WMH burden was significantly associated with increased risk of incident IS overall (I2 = 67%; P = .002), in the general population,26,27,36 and in high-risk populations14,20,23,29,31,33 and with increased risk of incident ICH overall (I2 = 65%; P = .008)16,18,21,24,26,31,34 and in the general population16,21,26 (Table) (eTables 3 and 4 and eFigure 2 in the Supplement).
Table. Summary of Meta-analysis Results for the Association of Magnetic Resonance Imaging Markers of Vascular Brain Injury With Incident Stroke, Dementia, and Death.
| Vascular Brain Injury Type | Stroke | Ischemic Stroke | Intracerebral Hemorrhage | Dementia | Alzheimer Disease | Death |
|---|---|---|---|---|---|---|
| Extensive WMH burden | ||||||
| Studies included, No. | 17 | 9 | 7 | 12 | 6 | 13 |
| No./total No. | 2859/14 529 | 1159/7320 | 1572/7976 | 2402/9338 | 572/5206 | 1496/13 138 |
| Events, No. | 1049 | 696 | 148 | 1127 | 572 | 1700 |
| HR (95% CI) | 2.45 (1.93-3.12) | 2.39 (1.65-3.47) | 3.17 (1.54-6.52) | 1.84 (1.40-2.43) | 1.50 (1.22-1.84) | 2.00 (1.69-2.36) |
| P value | <.001 | <.001 | .002 | <.001 | <.001 | <.001 |
| BI presence | ||||||
| Studies, No. | 12 | 6 | 5 | 9 | 3 | 8 |
| No./total No. | 3018/16 012 | 936/6873 | 878/8847 | 2759/10 772 | 1125/3429 | 1311/10 007 |
| Events, No. | 881 | 333 | 88 | 1029 | 414 | 1212 |
| HR (95% CI) | 2.38 (1.87-3.04) | 2.18 (1.67-2.85) | 3.81 (1.75-8.27) | 1.29 (1.02-1.65) | 1.06 (0.83-1.36) | 1.64 (1.40-1.91) |
| P value | <.001 | <.001 | <.001 | .04a | .64 | <.001 |
| CMB presence | ||||||
| Studies, No. | 22 | 20 | 23 | 5 | 6 | 10 |
| No./total No. | 3131/15 693 | 2557/13 125 | 3174/14 280 | 1498/8736 | 1514/8875 | 1433/9942 |
| Events, No. | 831 | 459 | 218 | 338 | 290 | 1134 |
| HR (95% CI) | 1.98 (1.55-2.53) | 1.92 (1.40-2.63) | 3.82 (2.15-6.80) | 1.41 (0.90-2.21) | 1.18 (0.73-1.89) | 1.53 (1.31-1.80) |
| P value | <.001 | <.001 | <.001 | .13 | .49 | <.001 |
Abbreviations: BI, brain infarct; CMB, cerebral microbleed; HR, hazard ratio; WMH, white matter hyperintensity.
Of note, 1 population-based study72 provided only effect estimates of dementia risk either comparing participants with at least 1 prevalent but no incident magnetic resonance imaging–defined BI with participants with neither prevalent nor incident magnetic resonance imaging–defined BI or comparing participants with at least 1 prevalent and 1 incident magnetic resonance imaging–defined BI with participants with neither prevalent nor incident magnetic resonance imaging–defined BI. By default, the effect estimates of the first comparison were included, but meta-analysis results were substantially unchanged overall when using effect estimates from the second comparison (HR, 1.52; 95% CI, 1.15-2.00; P = .003).
Figure 2. Association of Extensive White Matter Hyperintensity (WMH) Burden With Incident Stroke, Dementia, and Death.
The association of extensive WMH of presumed vascular origin with incident stroke (A) (overall: I2 = 56%; P = .003; in the general population [GP]: I2 = 0%; P = .43; in high-risk populations [HRP]: I2 = 67%; P < .001), incident dementia (B) (overall: I2 = 64%; P = .001; GP: I2 = 79%; P = .003; HRP: I2 = 53%; P = .04), and mortality (C) (overall: I2 = 41%; P = .06). Results correspond to hazard ratios (HRs) with 95% CIs for each study; the meta-analysis results (inverse variance–weighted meta-analysis with random effects) are shown in diamonds. The No./total No. corresponds to the number of individuals with the outcome of interest and the total sample size.
In 9338 participants from 12 studies, extensive WMH burden (n = 2402 participants) was significantly associated with incident dementia risk (n = 1127 events) overall (I2 = 64%; P = .001) and in the general population,25,39,48,52 while the association was only nominally significant in high-risk populations38,41,42,43,44,45,46,53 (Table; Figure 2) (eTables 3 and 4 in the Supplement). Adding 6 studies reporting associations with continuous WMH burden in a sample size–weighted meta-analysis confirmed the association with incident dementia (n = 11 093 participants; n = 1163 events; P < .001).40,47,49,51,55,57 Concerning dementia subtypes, we found a significant association of extensive WMH burden with risk of incident AD overall (I2 = 0%; P = .79) and in the general population,35,48 the association being only nominally significant in high-risk populations38,41,46,54 (Table) (eTables 3 and 5 and eFigure 2 in the Supplement). Adding 4 studies reporting associations with continuous WMH burden in a sample size–weighted meta-analysis confirmed the association with incident AD (n = 7123 participants; n = 708 events; P < .001).49,50,52,56
We found a significant association in 13 138 participants from 13 studies of extensive WMH (n = 1496 participants) with mortality (n = 1700 deaths) overall (I2 = 41%; P = .06), in the general population,17,36,39,59,61 and in high-risk populations14,20,33,37,58,60,62,63 (Table; Figure 2) (eTables 3 and 6 in the Supplement). Adding 3 studies reporting associations with continuous WMH burden in a sample size–weighted meta-analysis confirmed the association with increased mortality (n = 13 939 participants; n = 1818 events; P < .001).15,22,64
Magnetic Resonance Imaging–Defined Covert Brain Infarcts
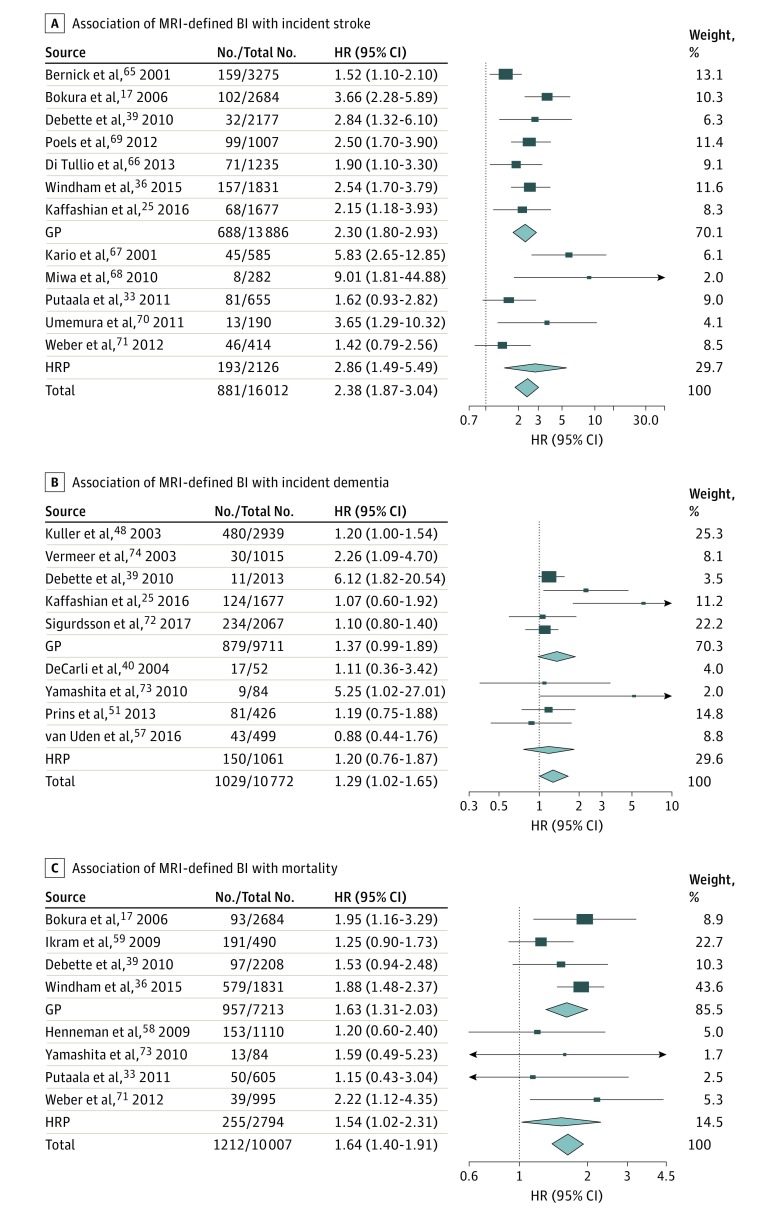
In 16 012 participants from 12 studies, presence of BI (n = 3018 participants) was associated significantly with incident stroke risk (n = 881 events) overall (I2 = 54%; P = .01), in the general population,17,25,36,39,65,66,69 and in high-risk populations33,67,68,70,71 (Table; Figure 3) (eTables 3 and 7 in the Supplement). When considering stroke subtypes, presence of BI was significantly associated with increased risk of IS overall (I2 = 0%; P = .70), in the general population,16,26,36 and in high-risk populations33,70,71 and with increased risk of incident ICH overall (I2 = 40%; P = .15)16,21,26,33,71 and in the general population16,21,26 (Table) (eTables 3 and 7 and eFigure 3 in the Supplement).
Figure 3. Association of Magnetic Resonance Imaging (MRI)–Defined Brain Infarct (BI) With Incident Stroke, Dementia, and Death.
The association of MRI-defined covert BI with incident stroke (A) (overall: I2 = 54%; P = .01; in the general population [GP]: I2 = 45%; P = .09; in high-risk populations [HRP]: I2 = 69%; P = .01), incident dementia (B) (overall: I2 = 44%; P = .08), and mortality (C) (overall: I2 = 0%; P = .48). Results correspond to hazard ratios (HRs) with 95% CIs for each study; the meta-analysis results (inverse variance–weighted meta-analysis with random effects) are shown in diamonds. The No./total No. corresponds to the number of individuals with the outcome of interest and the total sample size.
In 10 772 participants from 9 studies,25,39,40,48,51,57,72,73,74 presence of BI (n = 2759 participants) showed nominally significant association with incident dementia risk (n = 1029 events), which did not withstand correction for multiple testing (I2 = 44%; P = .08) (Table; Figure 3) (eTables 3 and 8 in the Supplement). Presence of BI was not associated with incident AD, both in 1 general population study48 and in 2 high-risk population studies54,57 (eTable 8 and eFigure 3 in the Supplement).
In 10 007 participants from 8 studies, presence of BI (n = 1311 participants) was significantly associated with mortality (n = 1212 deaths) overall (I2 = 0%; P = .48) and in the general population,17,36,39,59 with the association in high-risk populations being only nominally significant33,58,71,73 (Table; Figure 3) (eTables 3 and 9 in the Supplement).
Cerebral Microbleeds
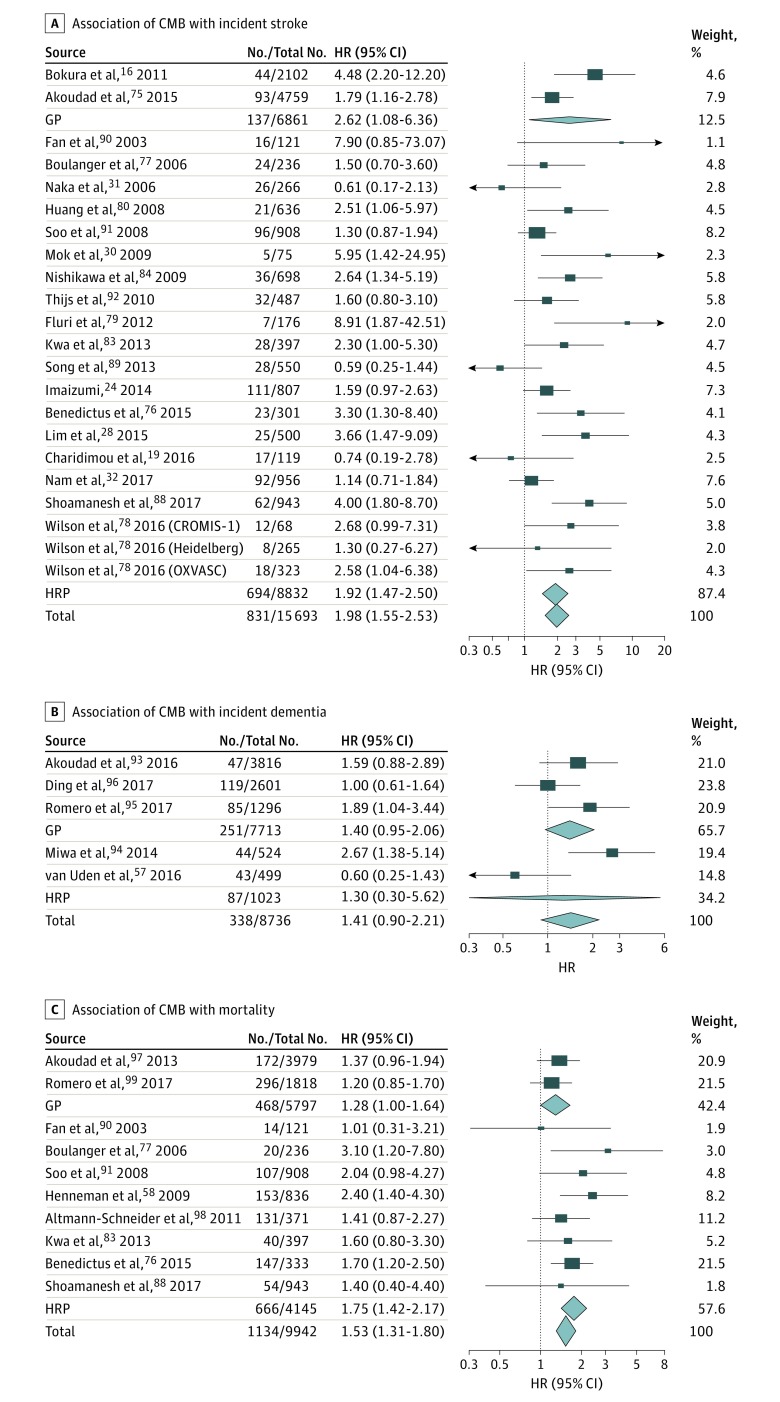
In 15 693 participants from 22 studies, presence of CMB (n = 3131 participants) was significantly associated with incident stroke risk (n = 831 events) overall (I2 = 49%; P = .005) and in high-risk populations,19,24,28,30,31,32,76,77,78,79,80,83,84,88,89,90,91,92 the association in the general population being only nominally significant16,75 (Table; Figure 4) (eFigure 2 in the Supplement). With respect to stroke subtypes, presence of CMB was significantly associated with increased risk of incident IS overall (I2 = 50%; P = .006)16,19,24,28,30,31,75,77,78,79,80,83,84,89,90,91,92 and in high-risk populations16,19,24,28,30,31,75,76,77,78,79,80,83,84,89,90,91,92 and with increased risk of incident ICH overall (I2 = 60%; P < .001) and in high-risk populations,18,19,24,30,31,76,77,78,80,81,82,83,84,85,86,87,88,89,90,91,92 with nominally significant associations in the general population16,75 (eTables 3 and 10 and eFigure 4 in the Supplement).
Figure 4. Association of Cerebral Microbleeds (CMB) With Incident Stroke, Dementia, and Death.
Association of CMB with incident stroke (A) (overall: I2 = 49%; P = .005; in the general population [GP]: I2 = 71%; P = .06; in high-risk populations [HRP]: I2 = 49%; P = .008), incident dementia (B) (overall: I2 = 61%; P = .04; GP: I2 = 32%; P = .23; HRP: I2 = 86%; P = .007), and mortality (C) (overall: I2 = 0%; P = .48). Results correspond to hazard ratios (HRs) with 95% CIs for each study; the meta-analysis results (inverse variance–weighted meta-analysis with random effects) are shown in diamonds. The No./total No. corresponds to the number of individuals with the outcome of interest and the total sample size.
In 8736 participants from 5 studies,57,93,94,95,96 presence of CMB (n = 1498 participants) was not significantly associated with increased risk of incident dementia (Table; Figure 4) (eTables 3 and 11 in the Supplement). Presence of CMB was also not associated with risk of incident AD54,57,93,94,95,96 (Table) (eTables 3 and 11 and eFigure 4 in the Supplement). In 9942 participants from 10 studies, presence of CMB (n = 1433 participants) was significantly associated with increased mortality (1134 deaths) overall (I2 = 0%; P = .48) and in high-risk populations58,76,77,83,88,90,91,97,98,99 (Table; Figure 4) (eTables 3 and 12 in the Supplement).
Information on lobar vs deep location of CMB was available only in a small minority of studies (eTables 10-12 in the Supplement). The results of these secondary meta-analyses are included in the eResults in the Supplement.
Perivascular Spaces
There were too few eligible studies on the clinical significance of PVSs to conduct meta-analyses. In one study in high-risk populations with IS or transient ischemic attack (n = 2002 participants),103 high PVS burden (>20 vs <11 PVSs) in basal ganglia was associated with recurrent stroke and IS but not with ICH. In another study in 229 patients with cerebral amyloid angiopathy–related ICH,18 high PVS burden in the centrum semiovale (≥20 PVSs) was associated with increased risk of ICH recurrence. In a single population-based study (n = 1228 participants),102 participants in the highest tertile of PVS burden did not have a significantly higher risk of incident stroke after adjusting for vascular risk factors (eTable 13 in the Supplement).
In one population-based study (n = 1778 participants),105 the highest grade of PVS burden in the basal ganglia and white matter was associated with increased risk of incident dementia. In another population-based study (n = 2612 participants),104 the presence of large PVS (greater than 3 mm) overall and in the basal ganglia was significantly associated with increased risk of incident vascular dementia but not all dementia or AD (eTable 14 in the Supplement).
One study in high-risk patients with IS or transient ischemic attack (n = 2002 participants)103 did not observe any significant association of high PVS burden (>20 vs <11 PVSs) with mortality. In a single population-based study (n = 1228 participants),102 participants in the highest tertile of PVS burden had a higher rate of vascular death but not death overall after adjusting for vascular risk factors (eTable 15 in the Supplement).
Sensitivity Analyses
Study quality was mostly high (eTable 2 in the Supplement). After removing studies with medium to low quality (less than 7 stars on the Newcastle-Ottawa scale) or studies using ORs only, the main findings of meta-analyses were unchanged (eTable 16 in the Supplement). Meta-regression analyses indicated no association of length of follow-up or adjustment for potential confounders with associations (eTable 17 in the Supplement). Regarding assessment of possible publication bias, Egger test was only nominally significant for WMH and stroke (coefficient, 2.5; SE, 0.8; P = .006), with all HRs greater than 1 (eFigures 5-10 in the Supplement).
Discussion
In this systematic review and meta-analysis, we summarized data from 94 prospective studies with up to 14 529 participants for WMH, 16 012 participants for BI, 15 693 participants for CMB, and 4587 participants for PVS. We observed significant associations of extensive WMH burden, BI, and CMB with increased risk of incident stroke and both IS (risk more than doubled) and ICH (risk more than tripled). White matter hyperintensity burden was also associated with increased risk of incident dementia and AD, with hazard ratios between 1.5 and 1.8. White matter hyperintensity burden, BI, and CMB were all associated with increased risk of death, with hazard ratios between 1.5 and 2.0. These associations were mostly seen both in the general population and in high-risk individuals. Data on high PVS burden were limited and insufficient to conduct meta-analyses, with some but not all studies reporting a significant association with increased risk of stroke, dementia, and vascular death.
To our knowledge, this is the first systematic review and meta-analysis simultaneously gathering published data on the association of the 4 main MRI markers of VBI (ie, WMH, BI, CMB, and PVS) with risk of incident stroke, dementia, and death. Previous meta-analyses focused on 1 MRI marker at once (ie, WMH or CMB)3,78,100,101,106,107 and mostly 1 outcome, sometimes in subpopulations. As well as integrating information on multiple MRI markers of VBI, our review adds numerous new studies.14,16,18,19,20,21,24,25,26,28,29,32,33,35,36,41,46,49,50,51,57,58,64,72,75,84,88,95,96,99,102,103,104,105 We also present, to our knowledge, the first meta-analyses on the association of BI with stroke subtypes, dementia, and mortality and the first systematic review on the association of PVS with stroke, dementia, and mortality.
White matter hyperintensities, BI, CMB, and PVS are common incidental findings on MRI brain scans carried out for other reasons. They occur in about 10% to 28% of individuals 70 years or older for BI and CMB and in more than 80% for WMH and PVS, leading to study of them as extensive vs nonextensive burden (with frequencies of extensive WMH and PVS burden varying depending on definitions [Figure 1] but ranging approximately between 10% and 50%).1,2,3,87,108,109,110,111 These MRI markers of VBI predominantly reflect underlying cerebral SVD, and in the vast majority of individuals, they are covert, ie, not associated with clinical stroke. The clinical and therapeutic significance of these findings is debated. Our analysis demonstrates that extensive WMH, BI, and CMB are predictors of a markedly increased risk of stroke and death and an increased risk of dementia for extensive WMH. Perivascular spaces have recently been suggested as a further manifestation of cerebral SVD,112,113,114 and some studies indicated that PVS burden correlates with cognitive outcomes.105,115,116 We found only 5 longitudinal studies,18,102,103,104,105 some of which reported a significant association of PVS burden with risk of stroke, dementia, and vascular death. Additional studies are warranted to explore the clinical significance of high PVS burden.
There are no guidelines for the management of covert, MRI-defined VBI, leading to wide variation in clinical practice. Often, these lesions are ignored, likely representing an important missed opportunity for prevention of stroke and dementia. When such findings are detected, it seems reasonable to give healthy cardiovascular lifestyle advice. However, it remains uncertain whether specific drug therapies should be given or more stringent lowering of risk factors be targeted to limit progression of VBI and prevent or slow down cognitive decline.117 The major treatable risk factor for VBI is hypertension,118 and some evidence from epidemiological studies and secondary outcomes of clinical trials suggests that treating hypertension in patients with extensive WMH reduces WMH progression119,120 and stroke risk120 and may reduce dementia risk.121 However, it remains uncertain how intensively blood pressure should be lowered. Recently, trials have been launched to look at the effect of intensive blood pressure lowering on lesion progression in extensive WMH, either in stroke-free patients (NCT01650402; NCT02472028) or patients with lacunar stroke.122 In patients with lacunar stroke included in the Secondary Prevention of Small Subcortical Strokes (SPS3) trial123 (not selected to have extensive WMH burden), no significant association of intensive blood pressure lowering with reduction in stroke risk was observed.
Our finding that WMH and BI increase the risk of not only IS but also ICH is consistent with recent data suggesting that a similar small vessel arteriopathy underlies both ischemic and hemorrhagic SVD.10,124 This has important potential implications for therapy. Antiplatelet agents such as aspirin are often empirically given to patients with WMH and BI detected on MRI. That they also predict ICH suggests that this strategy could lead to an increased hemorrhage rate. Antiplatelet agents also tend to be avoided in persons with CMB detected on MRI; however, our results highlight that persons with CMB are also at increased risk of IS. The SPS3 trial117 reported no reduction in stroke recurrence when adding clopidogrel to aspirin in patients with symptomatic lacunar stroke but a significant increase in bleeding and death. Randomized clinical trials are required to determine whether any antiplatelet therapy is indicated in stroke-free persons with extensive WMH or BI and whether the benefit–risk ratio is modified by the presence of CMB.
Magnetic resonance imaging markers of VBI are correlated with each other, and using the data in our analysis, it is not possible to determine whether observed associations are independent of other MRI markers of VBI. Studies using multimodal MRI in patients with symptomatic SVD have demonstrated that BI and WMH as well as more subtle and diffuse changes in white matter microstructure detected using diffusion tensor imaging are the strongest predictors of cognitive impairment compared with other MRI features.125,126 Results for CMB have been less conclusive, with some studies finding that they are independent predictors93,127,128,129 while others found the associations with cognition to disappear when WMH and BI are controlled for.130 Further studies, ideally combining individual-level data from large cohort studies, are needed to systematically explore the respective independent associations of MRI markers of VBI with vascular and cognitive outcomes. If valid genetic instruments become available in the near future, the causal relation of each MRI marker with clinical outcomes may also be explored by mendelian randomization. Exploring interactions of VBI markers with biomarkers of neurodegeneration in relation to dementia risk is another important future step, although specific biomarkers are still lacking for large cohort studies.
Although BI and CMB were associated with risk of stroke and death, they did not significantly predict dementia risk after correction for multiple testing. The lack of association in this data set may reflect that most individuals had a small number of BIs and CMBs, which therefore made a modest contribution to overall disease burden. This does not preclude an effect of BI or CMB on dementia risk in a subset of individuals with more extensive or rapidly progressing lesions.125,131 In line with this hypothesis, recent population-based studies reported that dementia risk was significantly increased in individuals with both prevalent and incident BI72 or with 3 or more CMBs.96 Small vessel disease is thought to cause cognitive decline and dementia at least partly by disruption of white matter pathways underlying complex cortical–subcortical networks.132,133 Recent studies have shown that the degree of network disruption, measured using advanced MRI techniques, is strongly correlated with cognitive decline125,133 and subsequent dementia risk.134 One or few BIs or CMBs are unlikely to have a significant effect on network disruption in contrast to extensive WMH, which may affect multiple white matter tracks. White matter hyperintensity volume may also be more linked with dementia-related neurodegenerative processes, including potential reverse causation with Wallerian degeneration secondary to cortical atrophy or with the frequent coexistence of AD and cerebral amyloid angiopathy.135,136
Limitations
Our study has limitations. Meta-analyses were conducted post hoc, based on available data with various sources of heterogeneity (eg, study population, measurement method of MRI markers and cutoffs used, MRI field strength, methods of incident event ascertainment, analytical model, and length of follow-up). We used random-effects meta-analyses and secondary sample size–weighted meta-analyses to account for this heterogeneity. Indeed, heterogeneity measures were statistically significant for several of the meta-analyses, although less so when looking at more homogeneous outcome subtypes, and mostly driven by differences in effect size rather than directionality of effect. Moreover, we conducted secondary analyses stratified by population type (general population vs high-risk populations, where heterogeneity was highest), excluding studies with lower methodological quality, and observed similar findings. Similarly, meta-regression analyses did not suggest any significant effect of differences between studies in length of follow-up or adjustment for confounders. Nevertheless, we acknowledge residual intrinsic sources of heterogeneity, such as differences in definition of extensive WMH burden across studies. While most studies used HRs (or few relative risks), a few studies used ORs. The latter were pooled with HRs in meta-analyses, assuming that they numerically approximate one another, but we acknowledge this may not be perfectly verified for studies in high-risk populations with a higher incidence of events and higher risks.11 Excluding studies that used ORs in sensitivity analyses led to similar results. All these sensitivity analyses suggest robustness of our findings. Because of a small number of studies, we did not include analyses of associations of longitudinal change in MRI markers of VBI with risk of clinical events. These may be important to better establish the potential role of these MRI markers as surrogate end points for prevention trials. We limited our review to articles published in English in peer-reviewed journals found in PubMed and through the reference list of relevant articles and did not systematically search other databases nor include articles published in languages other than English. This may have led to the omission of a small number of articles, most likely of lower methodological quality. We did not contact the authors of the 4 studies that did not provide an effect estimate or raw data to calculate one (4% of eligible studies); results of these studies are described in eTables 5 and 10 in the Supplement. Finally, although we did not identify any significant evidence for small-study bias, confirmation and in-depth exploration of our findings could be obtained by future prospective meta-analyses within large consortia using harmonized phenotypic criteria and analytical models, which would also enable estimation of age-specific population attributable risk.
Conclusions
In conclusion, this systematic review and meta-analysis provides evidence that WMH, BI, and CMB, all highly prevalent in the general population, have major clinical significance in that they indicate a significant increased risk of stroke, dementia, and death. From a practical perspective, the discovery of these MRI markers should prompt detailed assessment of a person’s risk for stroke and dementia and careful evaluation of the benefit–risk ratio for available preventive strategies. Randomized clinical trials are required to determine whether specific therapies, particularly aspirin therapy and intensive blood pressure lowering, are beneficial when these MRI markers are noted as incidental findings.
eMethods. Search terms and variables definition.
eResults. Additional results regarding cerebral microbleeds locations.
eTable 1. Quality criteria of included studies.
eTable 2. Assessment of quality of included studies using the Newcastle-Ottawa scale.
eTable 3. Summary of meta-analysis results for the association of magnetic resonance imaging (MRI) markers of vascular brain injury (VBI) with incident stroke, dementia, and death in the general population and in high-risk populations.
eTable 4. Studies testing the association of burden of white matter hyperintensity (WMH) of presumed vascular origin with incident stroke.
eTable 5. Studies testing the association of burden of white matter hyperintensity (WMH) of presumed vascular origin with incident dementia.
eTable 6. Studies testing the association of burden of white matter hyperintensity (WMH) of presumed vascular origin with mortality.
eTable 7. Studies testing the association of magnetic resonance imaging (MRI)–defined covert brain infarcts (BI) with incident stroke.
eTable 8. Studies testing the association of magnetic resonance imaging (MRI)–defined covert brain infarcts (BI) with incident dementia.
eTable 9. Studies testing the association of magnetic resonance imaging (MRI)–defined covert brain infarcts (BI) with mortality.
eTable 10. Studies testing the association of cerebral microbleed (CMB) with incident stroke.
eTable 11. Studies testing the association of cerebral microbleed (CMB) with incident dementia.
eTable 12. Studies testing the association of cerebral microbleed (CMB) with mortality.
eTable 13. Studies testing the association of perivascular spaces (PVS) with incident stroke.
eTable 14. Studies testing the association of perivascular spaces (PVS) with incident dementia.
eTable 15. Studies testing the association of perivascular spaces (PVS) with mortality.
eTable 16. Sensitivity analyses after exclusion of studies with medium-quality to low-quality scores on the Newcastle-Ottawa Scale (NOS) or studies reporting odds ratios only.
eTable 17. Meta-regression showing the P value for the regression coefficient.
eFigure 1. Flow chart of article selection for the systematic review and meta-analyses.
eFigure 2. Association of extensive white matter hyperintensity (WMH) of presumed vascular origin with incident stroke and dementia subtypes.
eFigure 3. Association of magnetic resonance imaging (MRI)–defined covert brain infarcts (BI) with incident stroke subtypes.
eFigure 4. Association of cerebral microbleeds with incident stroke and dementia subtypes.
eFigure 5. Funnels plots of the association of extensive white matter hyperintensity (WMH) burden with incident stroke, dementia, and mortality.
eFigure 6. Funnels plots of the association of extensive white matter hyperintensity (WMH) burden with incident stroke and dementia subtypes.
eFigure 7. Funnels plots of the association of magnetic resonance imaging (MRI)–defined brain infarct (BI) with incident stroke, dementia, and mortality.
eFigure 8. Funnels plots of the association of magnetic resonance imaging (MRI)–defined brain infarct (BI) with incident stroke subtypes.
eFigure 9. Funnels plots of the association of cerebral microbleeds (CMB) with incident stroke, dementia, and mortality.
eFigure 10. Funnels plots of the association of cerebral microbleeds (CMB) with incident stroke and dementia subtypes.
eReferences.
References
- 1.Vermeer SE, Longstreth WT Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6(7):611-619. doi: 10.1016/S1474-4422(07)70170-9 [DOI] [PubMed] [Google Scholar]
- 2.Poels MM, Ikram MA, van der Lugt A, et al. Incidence of cerebral microbleeds in the general population: the Rotterdam Scan Study. Stroke. 2011;42(3):656-661. doi: 10.1161/STROKEAHA.110.607184 [DOI] [PubMed] [Google Scholar]
- 3.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seshadri S, Wolf PA. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol. 2007;6(12):1106-1114. doi: 10.1016/S1474-4422(07)70291-0 [DOI] [PubMed] [Google Scholar]
- 5.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12(5):483-497. doi: 10.1016/S1474-4422(13)70060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM; Rotterdam Scan Study . Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34(5):1126-1129. doi: 10.1161/01.STR.0000068408.82115.D2 [DOI] [PubMed] [Google Scholar]
- 7.Schmidt R, Schmidt H, Haybaeck J, et al. Heterogeneity in age-related white matter changes. Acta Neuropathol. 2011;122(2):171-185. doi: 10.1007/s00401-011-0851-x [DOI] [PubMed] [Google Scholar]
- 8.Shoamanesh A, Kwok CS, Benavente O. Cerebral microbleeds: histopathological correlation of neuroimaging. Cerebrovasc Dis. 2011;32(6):528-534. doi: 10.1159/000331466 [DOI] [PubMed] [Google Scholar]
- 9.Marín-Padilla M, Knopman DS. Developmental aspects of the intracerebral microvasculature and perivascular spaces: insights into brain response to late-life diseases. J Neuropathol Exp Neurol. 2011;70(12):1060-1069. doi: 10.1097/NEN.0b013e31823ac627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg SM. Small vessels, big problems. N Engl J Med. 2006;354(14):1451-1453. doi: 10.1056/NEJMp068043 [DOI] [PubMed] [Google Scholar]
- 11.Symons MJ, Moore DT. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol. 2002;55(9):893-899. doi: 10.1016/S0895-4356(02)00443-2 [DOI] [PubMed] [Google Scholar]
- 12.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed July 30, 2018.
- 13.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen SD, Larsen TB, Gorst-Rasmussen A, Yavarian Y, Lip GY, Bach FW. White matter hyperintensities improve ischemic stroke recurrence prediction. Cerebrovasc Dis. 2017;43(1-2):17-24. doi: 10.1159/000450962 [DOI] [PubMed] [Google Scholar]
- 15.Appelros P, Samuelsson M, Lindell D. Lacunar infarcts: functional and cognitive outcomes at five years in relation to MRI findings. Cerebrovasc Dis. 2005;20(1):34-40. doi: 10.1159/000086202 [DOI] [PubMed] [Google Scholar]
- 16.Bokura H, Saika R, Yamaguchi T, et al. Microbleeds are associated with subsequent hemorrhagic and ischemic stroke in healthy elderly individuals. Stroke. 2011;42(7):1867-1871. doi: 10.1161/STROKEAHA.110.601922 [DOI] [PubMed] [Google Scholar]
- 17.Bokura H, Kobayashi S, Yamaguchi S, et al. Silent brain infarction and subcortical white matter lesions increase the risk of stroke and mortality: a prospective cohort study. J Stroke Cerebrovasc Dis. 2006;15(2):57-63. doi: 10.1016/j.jstrokecerebrovasdis.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 18.Boulouis G, Charidimou A, Pasi M, et al. Hemorrhage recurrence risk factors in cerebral amyloid angiopathy: comparative analysis of the overall small vessel disease severity score versus individual neuroimaging markers. J Neurol Sci. 2017;380:64-67. doi: 10.1016/j.jns.2017.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charidimou A, Inamura S, Nomura T, Kanno A, Kim SN, Imaizumi T. Cerebral microbleeds and white matter hyperintensities in cardioembolic stroke patients due to atrial fibrillation: single-centre longitudinal study. J Neurol Sci. 2016;369:263-267. doi: 10.1016/j.jns.2016.08.050 [DOI] [PubMed] [Google Scholar]
- 20.Conijn MM, Kloppenborg RP, Algra A, et al. ; SMART Study Group . Cerebral small vessel disease and risk of death, ischemic stroke, and cardiac complications in patients with atherosclerotic disease: the Second Manifestations of Arterial Disease-Magnetic Resonance (SMART-MR) study. Stroke. 2011;42(11):3105-3109. doi: 10.1161/STROKEAHA.110.594853 [DOI] [PubMed] [Google Scholar]
- 21.Folsom AR, Yatsuya H, Mosley TH Jr, Psaty BM, Longstreth WT Jr. Risk of intraparenchymal hemorrhage with magnetic resonance imaging-defined leukoaraiosis and brain infarcts. Ann Neurol. 2012;71(4):552-559. doi: 10.1002/ana.22690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu JH, Lu CZ, Hong Z, Dong Q, Luo Y, Wong KS. Extent of white matter lesions is related to acute subcortical infarcts and predicts further stroke risk in patients with first ever ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005;76(6):793-796. doi: 10.1136/jnnp.2003.032771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerdes VE, Kwa VI, ten Cate H, Brandjes DP, Büller HR, Stam J; Amsterdam Vascular Medicine Group . Cerebral white matter lesions predict both ischemic strokes and myocardial infarctions in patients with established atherosclerotic disease. Atherosclerosis. 2006;186(1):166-172. doi: 10.1016/j.atherosclerosis.2005.07.008 [DOI] [PubMed] [Google Scholar]
- 24.Imaizumi T, Inamura S, Nomura T. The severities of white matter lesions possibly influence the recurrences of several stroke types. J Stroke Cerebrovasc Dis. 2014;23(7):1897-1902. doi: 10.1016/j.jstrokecerebrovasdis.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 25.Kaffashian S, Soumaré A, Zhu YC, Mazoyer B, Debette S, Tzourio C. Long-term clinical impact of vascular brain lesions on magnetic resonance imaging in older adults in the population. Stroke. 2016;47(11):2865-2869. doi: 10.1161/STROKEAHA.116.014695 [DOI] [PubMed] [Google Scholar]
- 26.Kaffashian S, Tzourio C, Zhu YC, Mazoyer B, Debette S. Differential effect of white-matter lesions and covert brain infarcts on the risk of ischemic stroke and intracerebral hemorrhage. Stroke. 2016;47(7):1923-1925. doi: 10.1161/STROKEAHA.116.012734 [DOI] [PubMed] [Google Scholar]
- 27.Kuller LH, Longstreth WT Jr, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ Jr; Cardiovascular Health Study Collaborative Research Group . White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke. 2004;35(8):1821-1825. doi: 10.1161/01.STR.0000132193.35955.69 [DOI] [PubMed] [Google Scholar]
- 28.Lim JS, Hong KS, Kim GM, et al. Cerebral microbleeds and early recurrent stroke after transient ischemic attack: results from the Korean Transient Ischemic Attack Expression Registry. JAMA Neurol. 2015;72(3):301-308. doi: 10.1001/jamaneurol.2014.3958 [DOI] [PubMed] [Google Scholar]
- 29.Melkas S, Sibolt G, Oksala NK, et al. Extensive white matter changes predict stroke recurrence up to 5 years after a first-ever ischemic stroke. Cerebrovasc Dis. 2012;34(3):191-198. doi: 10.1159/000341404 [DOI] [PubMed] [Google Scholar]
- 30.Mok VC, Lau AY, Wong A, et al. Long-term prognosis of Chinese patients with a lacunar infarct associated with small vessel disease: a five-year longitudinal study. Int J Stroke. 2009;4(2):81-88. doi: 10.1111/j.1747-4949.2009.00262.x [DOI] [PubMed] [Google Scholar]
- 31.Naka H, Nomura E, Takahashi T, et al. Combinations of the presence or absence of cerebral microbleeds and advanced white matter hyperintensity as predictors of subsequent stroke types. AJNR Am J Neuroradiol. 2006;27(4):830-835. [PMC free article] [PubMed] [Google Scholar]
- 32.Nam KW, Kwon HM, Lim JS, Han MK, Nam H, Lee YS. The presence and severity of cerebral small vessel disease increases the frequency of stroke in a cohort of patients with large artery occlusive disease. PLoS One. 2017;12(10):e0184944. doi: 10.1371/journal.pone.0184944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Putaala J, Haapaniemi E, Kurkinen M, Salonen O, Kaste M, Tatlisumak T. Silent brain infarcts, leukoaraiosis, and long-term prognosis in young ischemic stroke patients. Neurology. 2011;76(20):1742-1749. doi: 10.1212/WNL.0b013e31821a44ad [DOI] [PubMed] [Google Scholar]
- 34.Smith EE, Gurol ME, Eng JA, et al. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology. 2004;63(9):1606-1612. doi: 10.1212/01.WNL.0000142966.22886.20 [DOI] [PubMed] [Google Scholar]
- 35.Weinstein G, Wolf PA, Beiser AS, Au R, Seshadri S. Risk estimations, risk factors, and genetic variants associated with Alzheimer’s disease in selected publications from the Framingham Heart Study. J Alzheimers Dis. 2013;33(suppl 1):S439-S445. doi: 10.3233/JAD-2012-129040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Windham BG, Deere B, Griswold ME, et al. Small brain lesions and incident stroke and mortality: a cohort study. Ann Intern Med. 2015;163(1):22-31. doi: 10.7326/M14-2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamauchi H, Fukuda H, Oyanagi C. Significance of white matter high intensity lesions as a predictor of stroke from arteriolosclerosis. J Neurol Neurosurg Psychiatry. 2002;72(5):576-582. doi: 10.1136/jnnp.72.5.576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bombois S, Debette S, Bruandet A, et al. Vascular subcortical hyperintensities predict conversion to vascular and mixed dementia in MCI patients. Stroke. 2008;39(7):2046-2051. doi: 10.1161/STROKEAHA.107.505206 [DOI] [PubMed] [Google Scholar]
- 39.Debette S, Beiser A, DeCarli C, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke. 2010;41(4):600-606. doi: 10.1161/STROKEAHA.109.570044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeCarli C, Mungas D, Harvey D, et al. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63(2):220-227. doi: 10.1212/01.WNL.0000130531.90205.EF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eckerström C, Olsson E, Klasson N, et al. Multimodal prediction of dementia with up to 10 years follow up: the Gothenburg MCI study. J Alzheimers Dis. 2015;44(1):205-214. doi: 10.3233/JAD-141053 [DOI] [PubMed] [Google Scholar]
- 42.Firbank MJ, Burton EJ, Barber R, et al. Medial temporal atrophy rather than white matter hyperintensities predict cognitive decline in stroke survivors. Neurobiol Aging. 2007;28(11):1664-1669. doi: 10.1016/j.neurobiolaging.2006.07.009 [DOI] [PubMed] [Google Scholar]
- 43.Geroldi C, Rossi R, Calvagna C, et al. Medial temporal atrophy but not memory deficit predicts progression to dementia in patients with mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2006;77(11):1219-1222. doi: 10.1136/jnnp.2005.082651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jokinen H, Kalska H, Ylikoski R, et al. ; LADIS group . Longitudinal cognitive decline in subcortical ischemic vascular disease: the LADIS Study. Cerebrovasc Dis. 2009;27(4):384-391. doi: 10.1159/000207442 [DOI] [PubMed] [Google Scholar]
- 45.Kantarci K, Weigand SD, Przybelski SA, et al. Risk of dementia in MCI: combined effect of cerebrovascular disease, volumetric MRI, and 1H MRS. Neurology. 2009;72(17):1519-1525. doi: 10.1212/WNL.0b013e3181a2e864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim S, Choi SH, Lee YM, et al. Periventricular white matter hyperintensities and the risk of dementia: a CREDOS study. Int Psychogeriatr. 2015;27(12):2069-2077. doi: 10.1017/S1041610215001076 [DOI] [PubMed] [Google Scholar]
- 47.Korf ES, Wahlund LO, Visser PJ, Scheltens P. Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology. 2004;63(1):94-100. doi: 10.1212/01.WNL.0000133114.92694.93 [DOI] [PubMed] [Google Scholar]
- 48.Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22(1):13-22. doi: 10.1159/000067109 [DOI] [PubMed] [Google Scholar]
- 49.Miwa K, Tanaka M, Okazaki S, et al. Increased total homocysteine levels predict the risk of incident dementia independent of cerebral small-vessel diseases and vascular risk factors. J Alzheimers Dis. 2016;49(2):503-513. doi: 10.3233/JAD-150458 [DOI] [PubMed] [Google Scholar]
- 50.Prasad K, Wiryasaputra L, Ng A, Kandiah N. White matter disease independently predicts progression from mild cognitive impairment to Alzheimer’s disease in a clinic cohort. Dement Geriatr Cogn Disord. 2011;31(6):431-434. doi: 10.1159/000330019 [DOI] [PubMed] [Google Scholar]
- 51.Prins ND, van der Flier WM, Brashear HR, et al. Predictors of progression from mild cognitive impairment to dementia in the placebo-arm of a clinical trial population. J Alzheimers Dis. 2013;36(1):79-85. doi: 10.3233/JAD-122233 [DOI] [PubMed] [Google Scholar]
- 52.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61(10):1531-1534. doi: 10.1001/archneur.61.10.1531 [DOI] [PubMed] [Google Scholar]
- 53.Smith EE, Egorova S, Blacker D, et al. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch Neurol. 2008;65(1):94-100. doi: 10.1001/archneurol.2007.23 [DOI] [PubMed] [Google Scholar]
- 54.Staekenborg SS, Koedam EL, Henneman WJ, et al. Progression of mild cognitive impairment to dementia: contribution of cerebrovascular disease compared with medial temporal lobe atrophy. Stroke. 2009;40(4):1269-1274. doi: 10.1161/STROKEAHA.108.531343 [DOI] [PubMed] [Google Scholar]
- 55.Tapiola T, Pennanen C, Tapiola M, et al. MRI of hippocampus and entorhinal cortex in mild cognitive impairment: a follow-up study. Neurobiol Aging. 2008;29(1):31-38. doi: 10.1016/j.neurobiolaging.2006.09.007 [DOI] [PubMed] [Google Scholar]
- 56.van Straaten EC, Harvey D, Scheltens P, et al. ; Alzheimer’s Disease Cooperative Study Group . Periventricular white matter hyperintensities increase the likelihood of progression from amnestic mild cognitive impairment to dementia. J Neurol. 2008;255(9):1302-1308. doi: 10.1007/s00415-008-0874-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Uden IW, van der Holst HM, Tuladhar AM, et al. White matter and hippocampal volume predict the risk of dementia in patients with cerebral small vessel disease: the RUN DMC study. J Alzheimers Dis. 2016;49(3):863-873. doi: 10.3233/JAD-150573 [DOI] [PubMed] [Google Scholar]
- 58.Henneman WJ, Sluimer JD, Cordonnier C, et al. MRI biomarkers of vascular damage and atrophy predicting mortality in a memory clinic population. Stroke. 2009;40(2):492-498. doi: 10.1161/STROKEAHA.108.516286 [DOI] [PubMed] [Google Scholar]
- 59.Ikram MA, Vernooij MW, Vrooman HA, Hofman A, Breteler MM. Brain tissue volumes and small vessel disease in relation to the risk of mortality. Neurobiol Aging. 2009;30(3):450-456. doi: 10.1016/j.neurobiolaging.2007.07.009 [DOI] [PubMed] [Google Scholar]
- 60.Kerber KA, Whitman GT, Brown DL, Baloh RW. Increased risk of death in community-dwelling older people with white matter hyperintensities on MRI. J Neurol Sci. 2006;250(1-2):33-38. doi: 10.1016/j.jns.2006.06.022 [DOI] [PubMed] [Google Scholar]
- 61.Kuller LH, Arnold AM, Longstreth WT Jr, et al. White matter grade and ventricular volume on brain MRI as markers of longevity in the cardiovascular health study. Neurobiol Aging. 2007;28(9):1307-1315. doi: 10.1016/j.neurobiolaging.2006.06.010 [DOI] [PubMed] [Google Scholar]
- 62.Levy RM, Steffens DC, McQuoid DR, Provenzale JM, MacFall JR, Krishnan KR. MRI lesion severity and mortality in geriatric depression. Am J Geriatr Psychiatry. 2003;11(6):678-682. doi: 10.1097/00019442-200311000-00013 [DOI] [PubMed] [Google Scholar]
- 63.Oksala NK, Oksala A, Pohjasvaara T, et al. Age related white matter changes predict stroke death in long term follow-up. J Neurol Neurosurg Psychiatry. 2009;80(7):762-766. doi: 10.1136/jnnp.2008.154104 [DOI] [PubMed] [Google Scholar]
- 64.van der Holst HM, van Uden IW, Tuladhar AM, et al. Factors associated with 8-year mortality in older patients with cerebral small vessel disease: the Radboud University Nijmegen Diffusion Tensor and Magnetic Resonance Cohort (RUN DMC) Study. JAMA Neurol. 2016;73(4):402-409. doi: 10.1001/jamaneurol.2015.4560 [DOI] [PubMed] [Google Scholar]
- 65.Bernick C, Kuller L, Dulberg C, et al. ; Cardiovascular Health Study Collaborative Research Group . Silent MRI infarcts and the risk of future stroke: the Cardiovascular Health Study. Neurology. 2001;57(7):1222-1229. doi: 10.1212/WNL.57.7.1222 [DOI] [PubMed] [Google Scholar]
- 66.Di Tullio MR, Jin Z, Russo C, et al. Patent foramen ovale, subclinical cerebrovascular disease, and ischemic stroke in a population-based cohort. J Am Coll Cardiol. 2013;62(1):35-41. doi: 10.1016/j.jacc.2013.03.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kario K, Shimada K, Schwartz JE, Matsuo T, Hoshide S, Pickering TG. Silent and clinically overt stroke in older Japanese subjects with white-coat and sustained hypertension. J Am Coll Cardiol. 2001;38(1):238-245. doi: 10.1016/S0735-1097(01)01325-0 [DOI] [PubMed] [Google Scholar]
- 68.Miwa K, Hoshi T, Hougaku H, et al. Silent cerebral infarction is associated with incident stroke and TIA independent of carotid intima-media thickness. Intern Med. 2010;49(9):817-822. doi: 10.2169/internalmedicine.49.3211 [DOI] [PubMed] [Google Scholar]
- 69.Poels MM, Steyerberg EW, Wieberdink RG, et al. Assessment of cerebral small vessel disease predicts individual stroke risk. J Neurol Neurosurg Psychiatry. 2012;83(12):1174-1179. doi: 10.1136/jnnp-2012-302381 [DOI] [PubMed] [Google Scholar]
- 70.Umemura T, Kawamura T, Umegaki H, et al. Endothelial and inflammatory markers in relation to progression of ischaemic cerebral small-vessel disease and cognitive impairment: a 6-year longitudinal study in patients with type 2 diabetes mellitus. J Neurol Neurosurg Psychiatry. 2011;82(11):1186-1194. doi: 10.1136/jnnp.2010.217380 [DOI] [PubMed] [Google Scholar]
- 71.Weber R, Weimar C, Wanke I, et al. ; PRoFESS Imaging Substudy Group . Risk of recurrent stroke in patients with silent brain infarction in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) imaging substudy. Stroke. 2012;43(2):350-355. doi: 10.1161/STROKEAHA.111.631739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sigurdsson S, Aspelund T, Kjartansson O, et al. Incidence of brain infarcts, cognitive change, and risk of dementia in the general population: the AGES-Reykjavik Study (Age Gene/Environment Susceptibility-Reykjavik Study). Stroke. 2017;48(9):2353-2360. doi: 10.1161/STROKEAHA.117.017357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamashita H, Fujikawa T, Takami H, et al. Long-term prognosis of patients with major depression and silent cerebral infarction. Neuropsychobiology. 2010;62(3):177-181. doi: 10.1159/000319359 [DOI] [PubMed] [Google Scholar]
- 74.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215-1222. doi: 10.1056/NEJMoa022066 [DOI] [PubMed] [Google Scholar]
- 75.Akoudad S, Portegies ML, Koudstaal PJ, et al. Cerebral microbleeds are associated with an increased risk of stroke: the Rotterdam Study. Circulation. 2015;132(6):509-516. doi: 10.1161/CIRCULATIONAHA.115.016261 [DOI] [PubMed] [Google Scholar]
- 76.Benedictus MR, Prins ND, Goos JD, Scheltens P, Barkhof F, van der Flier WM. Microbleeds, mortality, and stroke in Alzheimer disease: the MISTRAL Study. JAMA Neurol. 2015;72(5):539-545. doi: 10.1001/jamaneurol.2015.14 [DOI] [PubMed] [Google Scholar]
- 77.Boulanger JM, Coutts SB, Eliasziw M, et al. ; VISION Study Group . Cerebral microhemorrhages predict new disabling or fatal strokes in patients with acute ischemic stroke or transient ischemic attack. Stroke. 2006;37(3):911-914. doi: 10.1161/01.STR.0000204237.66466.5f [DOI] [PubMed] [Google Scholar]
- 78.Wilson D, Charidimou A, Ambler G, et al. Recurrent stroke risk and cerebral microbleed burden in ischemic stroke and TIA: a meta-analysis. Neurology. 2016;87(14):1501-1510. doi: 10.1212/WNL.0000000000003183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fluri F, Jax F, Amort M, et al. Significance of microbleeds in patients with transient ischaemic attack. Eur J Neurol. 2012;19(3):522-524. doi: 10.1111/j.1468-1331.2011.03522.x [DOI] [PubMed] [Google Scholar]
- 80.Huang Y, Cheng Y, Wu J, et al. ; Cilostazol versus Aspirin for Secondary Ischaemic Stroke Prevention Cooperation Investigators . Cilostazol as an alternative to aspirin after ischaemic stroke: a randomised, double-blind, pilot study. Lancet Neurol. 2008;7(6):494-499. doi: 10.1016/S1474-4422(08)70094-2 [DOI] [PubMed] [Google Scholar]
- 81.Jeon SB, Kang DW, Cho AH, et al. Initial microbleeds at MR imaging can predict recurrent intracerebral hemorrhage. J Neurol. 2007;254(4):508-512. doi: 10.1007/s00415-006-0406-6 [DOI] [PubMed] [Google Scholar]
- 82.Kang DW, Han MK, Kim HJ, et al. New ischemic lesions coexisting with acute intracerebral hemorrhage. Neurology. 2012;79(9):848-855. doi: 10.1212/WNL.0b013e3182648a79 [DOI] [PubMed] [Google Scholar]
- 83.Kwa VI, Algra A, Brundel M, Bouvy W, Kappelle LJ; MICRO Study Group . Microbleeds as a predictor of intracerebral haemorrhage and ischaemic stroke after a TIA or minor ischaemic stroke: a cohort study. BMJ Open. 2013;3(5):e002575. doi: 10.1136/bmjopen-2013-002575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nishikawa T, Ueba T, Kajiwara M, Fujisawa I, Miyamatsu N, Yamashita K. Cerebral microbleeds predict first-ever symptomatic cerebrovascular events. Clin Neurol Neurosurg. 2009;111(10):825-828. doi: 10.1016/j.clineuro.2009.08.011 [DOI] [PubMed] [Google Scholar]
- 85.Orken DN, Uysal E, Timer E, Kuloglu-Pazarcı N, Mumcu S, Forta H. New cerebral microbleeds in ischemic stroke patients on warfarin treatment: two-year follow-up. Clin Neurol Neurosurg. 2013;115(9):1682-1685. doi: 10.1016/j.clineuro.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 86.Pasquini M, Benedictus MR, Boulouis G, Rossi C, Dequatre-Ponchelle N, Cordonnier C. Incident cerebral microbleeds in a cohort of intracerebral hemorrhage. Stroke. 2016;47(3):689-694. [DOI] [PubMed] [Google Scholar]
- 87.Samarasekera N, Fonville A, Lerpiniere C, et al. ; Lothian Audit of the Treatment of Cerebral Haemorrhage Collaborators . Influence of intracerebral hemorrhage location on incidence, characteristics, and outcome: population-based study. Stroke. 2015;46(2):361-368. doi: 10.1161/STROKEAHA.114.007953 [DOI] [PubMed] [Google Scholar]
- 88.Shoamanesh A, Pearce LA, Bazan C, et al. ; SPS3 Trial Investigators . Microbleeds in the Secondary Prevention of Small Subcortical Strokes Trial: stroke, mortality, and treatment interactions. Ann Neurol. 2017;82(2):196-207. doi: 10.1002/ana.24988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Song TJ, Kim J, Lee HS, et al. The frequency of cerebral microbleeds increases with CHADS(2) scores in stroke patients with non-valvular atrial fibrillation. Eur J Neurol. 2013;20(3):502-508. doi: 10.1111/ene.12003 [DOI] [PubMed] [Google Scholar]
- 90.Fan YH, Zhang L, Lam WW, Mok VC, Wong KS. Cerebral microbleeds as a risk factor for subsequent intracerebral hemorrhages among patients with acute ischemic stroke. Stroke. 2003;34(10):2459-2462. doi: 10.1161/01.STR.0000090841.90286.81 [DOI] [PubMed] [Google Scholar]
- 91.Soo YO, Yang SR, Lam WW, et al. Risk vs benefit of anti-thrombotic therapy in ischaemic stroke patients with cerebral microbleeds. J Neurol. 2008;255(11):1679-1686. doi: 10.1007/s00415-008-0967-7 [DOI] [PubMed] [Google Scholar]
- 92.Thijs V, Lemmens R, Schoofs C, et al. Microbleeds and the risk of recurrent stroke. Stroke. 2010;41(9):2005-2009. doi: 10.1161/STROKEAHA.110.588020 [DOI] [PubMed] [Google Scholar]
- 93.Akoudad S, Wolters FJ, Viswanathan A, et al. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. 2016;73(8):934-943. doi: 10.1001/jamaneurol.2016.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miwa K, Tanaka M, Okazaki S, et al. Multiple or mixed cerebral microbleeds and dementia in patients with vascular risk factors. Neurology. 2014;83(7):646-653. doi: 10.1212/WNL.0000000000000692 [DOI] [PubMed] [Google Scholar]
- 95.Romero JR, Beiser A, Himali JJ, Shoamanesh A, DeCarli C, Seshadri S. Cerebral microbleeds and risk of incident dementia: the Framingham Heart Study. Neurobiol Aging. 2017;54:94-99. doi: 10.1016/j.neurobiolaging.2017.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ding J, Sigurðsson S, Jónsson PV, et al. Space and location of cerebral microbleeds, cognitive decline, and dementia in the community. Neurology. 2017;88(22):2089-2097. doi: 10.1212/WNL.0000000000003983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Akoudad S, Ikram MA, Koudstaal PJ, Hofman A, van der Lugt A, Vernooij MW. Cerebral microbleeds and the risk of mortality in the general population. Eur J Epidemiol. 2013;28(10):815-821. doi: 10.1007/s10654-013-9854-3 [DOI] [PubMed] [Google Scholar]
- 98.Altmann-Schneider I, Trompet S, de Craen AJ, et al. Cerebral microbleeds are predictive of mortality in the elderly. Stroke. 2011;42(3):638-644. doi: 10.1161/STROKEAHA.110.595611 [DOI] [PubMed] [Google Scholar]
- 99.Romero JR, Preis SR, Beiser A, et al. Cerebral microbleeds as predictors of mortality: the Framingham Heart Study. Stroke. 2017;48(3):781-783. doi: 10.1161/STROKEAHA.116.015354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Charidimou A, Imaizumi T, Moulin S, et al. Brain hemorrhage recurrence, small vessel disease type, and cerebral microbleeds: a meta-analysis. Neurology. 2017;89(8):820-829. doi: 10.1212/WNL.0000000000004259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Charidimou A, Karayiannis C, Song TJ, et al. ; International META-MICROBLEEDS Initiative . Brain microbleeds, anticoagulation, and hemorrhage risk: meta-analysis in stroke patients with AF. Neurology. 2017;89(23):2317-2326. doi: 10.1212/WNL.0000000000004704 [DOI] [PubMed] [Google Scholar]
- 102.Gutierrez J, Elkind MSV, Dong C, et al. Brain perivascular spaces as biomarkers of vascular risk: results from the Northern Manhattan Study. AJNR Am J Neuroradiol. 2017;38(5):862-867. doi: 10.3174/ajnr.A5129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lau KK, Li L, Lovelock CE, et al. Clinical correlates, ethnic differences, and prognostic implications of perivascular spaces in transient ischemic attack and ischemic stroke. Stroke. 2017;48(6):1470-1477. doi: 10.1161/STROKEAHA.117.016694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ding J, Sigurðsson S, Jónsson PV, et al. Large perivascular spaces visible on magnetic resonance imaging, cerebral small vessel disease progression, and risk of dementia: the Age, Gene/Environment Susceptibility-Reykjavik Study. JAMA Neurol. 2017;74(9):1105-1112. doi: 10.1001/jamaneurol.2017.1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhu YC, Dufouil C, Soumaré A, Mazoyer B, Chabriat H, Tzourio C. High degree of dilated Virchow-Robin spaces on MRI is associated with increased risk of dementia. J Alzheimers Dis. 2010;22(2):663-672. doi: 10.3233/JAD-2010-100378 [DOI] [PubMed] [Google Scholar]
- 106.Gupta A, Giambrone AE, Gialdini G, et al. Silent brain infarction and risk of future stroke: a systematic review and meta-analysis. Stroke. 2016;47(3):719-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Charidimou A, Kakar P, Fox Z, Werring DJ. Cerebral microbleeds and recurrent stroke risk: systematic review and meta-analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke. 2013;44(4):995-1001. doi: 10.1161/STROKEAHA.111.000038 [DOI] [PubMed] [Google Scholar]
- 108.Debette S, Bis JC, Fornage M, et al. Genome-wide association studies of MRI-defined brain infarcts: meta-analysis from the CHARGE Consortium. Stroke. 2010;41(2):210-217. doi: 10.1161/STROKEAHA.109.569194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Poels MM, Vernooij MW, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. 2010;41(10, suppl):S103-S106. doi: 10.1161/STROKEAHA.110.595181 [DOI] [PubMed] [Google Scholar]
- 110.Zhu YC, Dufouil C, Mazoyer B, et al. Frequency and location of dilated Virchow-Robin spaces in elderly people: a population-based 3D MR imaging study. AJNR Am J Neuroradiol. 2011;32(4):709-713. doi: 10.3174/ajnr.A2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsushima Y, Tanizaki Y, Aoki J, Endo K. MR detection of microhemorrhages in neurologically healthy adults. Neuroradiology. 2002;44(1):31-36. doi: 10.1007/s002340100649 [DOI] [PubMed] [Google Scholar]
- 112.Zhu YC, Tzourio C, Soumaré A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke. 2010;41(11):2483-2490. doi: 10.1161/STROKEAHA.110.591586 [DOI] [PubMed] [Google Scholar]
- 113.Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010;41(3):450-454. doi: 10.1161/STROKEAHA.109.564914 [DOI] [PubMed] [Google Scholar]
- 114.Potter GM, Doubal FN, Jackson CA, et al. Enlarged perivascular spaces and cerebral small vessel disease. Int J Stroke. 2015;10(3):376-381. doi: 10.1111/ijs.12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maclullich AM, Wardlaw JM, Ferguson KJ, Starr JM, Seckl JR, Deary IJ. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry. 2004;75(11):1519-1523. doi: 10.1136/jnnp.2003.030858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huijts M, Duits A, Staals J, Kroon AA, de Leeuw PW, van Oostenbrugge RJ. Basal ganglia enlarged perivascular spaces are linked to cognitive function in patients with cerebral small vessel disease. Curr Neurovasc Res. 2014;11(2):136-141. doi: 10.2174/1567202611666140310102248 [DOI] [PubMed] [Google Scholar]
- 117.Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA; SPS3 Investigators . Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367(9):817-825. doi: 10.1056/NEJMoa1204133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tzourio C, Laurent S, Debette S. Is hypertension associated with an accelerated aging of the brain? Hypertension. 2014;63(5):894-903. doi: 10.1161/HYPERTENSIONAHA.113.00147 [DOI] [PubMed] [Google Scholar]
- 119.Godin O, Tzourio C, Maillard P, Mazoyer B, Dufouil C. Antihypertensive treatment and change in blood pressure are associated with the progression of white matter lesion volumes: the Three-City (3C)-Dijon Magnetic Resonance Imaging Study. Circulation. 2011;123(3):266-273. doi: 10.1161/CIRCULATIONAHA.110.961052 [DOI] [PubMed] [Google Scholar]
- 120.Dufouil C, Chalmers J, Coskun O, et al. ; PROGRESS MRI Substudy Investigators . Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) magnetic resonance imaging substudy. Circulation. 2005;112(11):1644-1650. doi: 10.1161/CIRCULATIONAHA.104.501163 [DOI] [PubMed] [Google Scholar]
- 121.Tzourio C, Anderson C, Chapman N, et al. ; PROGRESS Collaborative Group . Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163(9):1069-1075. doi: 10.1001/archinte.163.9.1069 [DOI] [PubMed] [Google Scholar]
- 122.Croall ID, Lohner V, Moynihan B, et al. Using DTI to assess white matter microstructure in cerebral small vessel disease (SVD) in multicentre studies. Clin Sci (Lond). 2017;131(12):1361-1373. doi: 10.1042/CS20170146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Benavente OR, Coffey CS, Conwit R, et al. ; SPS3 Study Group . Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial [published correction appears in Lancet. 2013;382(9891):506]. Lancet. 2013;382(9891):507-515. doi: 10.1016/S0140-6736(13)60852-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rannikmäe K, Sivakumaran V, Millar H, et al. ; Stroke Genetics Network (SiGN), METASTROKE Collaboration, and International Stroke Genetics Consortium (ISGC) . COL4A2 is associated with lacunar ischemic stroke and deep ICH: meta-analyses among 21,500 cases and 40,600 controls. Neurology. 2017;89(17):1829-1839. doi: 10.1212/WNL.0000000000004560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lawrence AJ, Patel B, Morris RG, et al. Mechanisms of cognitive impairment in cerebral small vessel disease: multimodal MRI results from the St George’s Cognition and Neuroimaging in Stroke (SCANS) study. PLoS One. 2013;8(4):106014. doi: 10.1371/journal.pone.0061014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Knopman DS, Griswold ME, Lirette ST, et al. ; ARIC Neurocognitive Investigators . Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: Atherosclerosis Risk in Communities-Neurocognitive Study. Stroke. 2015;46(2):433-440. doi: 10.1161/STROKEAHA.114.007847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Patel B, Lawrence AJ, Chung AW, et al. Cerebral microbleeds and cognition in patients with symptomatic small vessel disease. Stroke. 2013;44(2):356-361. doi: 10.1161/STROKEAHA.112.670216 [DOI] [PubMed] [Google Scholar]
- 128.Poels MM, Ikram MA, van der Lugt A, et al. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology. 2012;78(5):326-333. doi: 10.1212/WNL.0b013e3182452928 [DOI] [PubMed] [Google Scholar]
- 129.Hilal S, Saini M, Tan CS, et al. Cerebral microbleeds and cognition: the epidemiology of dementia in Singapore study. Alzheimer Dis Assoc Disord. 2014;28(2):106-112. doi: 10.1097/WAD.0000000000000015 [DOI] [PubMed] [Google Scholar]
- 130.Molad J, Kliper E, Korczyn AD, et al. Only white matter hyperintensities predicts post-stroke cognitive performances among cerebral small vessel disease markers: results from the TABASCO Study. J Alzheimers Dis. 2017;56(4):1293-1299. doi: 10.3233/JAD-160939 [DOI] [PubMed] [Google Scholar]
- 131.Benjamin P, Lawrence AJ, Lambert C, et al. Strategic lacunes and their relationship to cognitive impairment in cerebral small vessel disease. Neuroimage Clin. 2014;4:828-837. doi: 10.1016/j.nicl.2014.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.O’Sullivan M, Morris RG, Huckstep B, Jones DK, Williams SC, Markus HS. Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J Neurol Neurosurg Psychiatry. 2004;75(3):441-447. doi: 10.1136/jnnp.2003.014910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lawrence AJ, Chung AW, Morris RG, Markus HS, Barrick TR. Structural network efficiency is associated with cognitive impairment in small-vessel disease. Neurology. 2014;83(4):304-311. doi: 10.1212/WNL.0000000000000612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tuladhar AM, van Uden IW, Rutten-Jacobs LC, et al. Structural network efficiency predicts conversion to dementia. Neurology. 2016;86(12):1112-1119. doi: 10.1212/WNL.0000000000002502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, Schneider JA. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol. 2011;69(2):320-327. doi: 10.1002/ana.22112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Boyle PA, Yu L, Nag S, et al. Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology. 2015;85(22):1930-1936. doi: 10.1212/WNL.0000000000002175 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Search terms and variables definition.
eResults. Additional results regarding cerebral microbleeds locations.
eTable 1. Quality criteria of included studies.
eTable 2. Assessment of quality of included studies using the Newcastle-Ottawa scale.
eTable 3. Summary of meta-analysis results for the association of magnetic resonance imaging (MRI) markers of vascular brain injury (VBI) with incident stroke, dementia, and death in the general population and in high-risk populations.
eTable 4. Studies testing the association of burden of white matter hyperintensity (WMH) of presumed vascular origin with incident stroke.
eTable 5. Studies testing the association of burden of white matter hyperintensity (WMH) of presumed vascular origin with incident dementia.
eTable 6. Studies testing the association of burden of white matter hyperintensity (WMH) of presumed vascular origin with mortality.
eTable 7. Studies testing the association of magnetic resonance imaging (MRI)–defined covert brain infarcts (BI) with incident stroke.
eTable 8. Studies testing the association of magnetic resonance imaging (MRI)–defined covert brain infarcts (BI) with incident dementia.
eTable 9. Studies testing the association of magnetic resonance imaging (MRI)–defined covert brain infarcts (BI) with mortality.
eTable 10. Studies testing the association of cerebral microbleed (CMB) with incident stroke.
eTable 11. Studies testing the association of cerebral microbleed (CMB) with incident dementia.
eTable 12. Studies testing the association of cerebral microbleed (CMB) with mortality.
eTable 13. Studies testing the association of perivascular spaces (PVS) with incident stroke.
eTable 14. Studies testing the association of perivascular spaces (PVS) with incident dementia.
eTable 15. Studies testing the association of perivascular spaces (PVS) with mortality.
eTable 16. Sensitivity analyses after exclusion of studies with medium-quality to low-quality scores on the Newcastle-Ottawa Scale (NOS) or studies reporting odds ratios only.
eTable 17. Meta-regression showing the P value for the regression coefficient.
eFigure 1. Flow chart of article selection for the systematic review and meta-analyses.
eFigure 2. Association of extensive white matter hyperintensity (WMH) of presumed vascular origin with incident stroke and dementia subtypes.
eFigure 3. Association of magnetic resonance imaging (MRI)–defined covert brain infarcts (BI) with incident stroke subtypes.
eFigure 4. Association of cerebral microbleeds with incident stroke and dementia subtypes.
eFigure 5. Funnels plots of the association of extensive white matter hyperintensity (WMH) burden with incident stroke, dementia, and mortality.
eFigure 6. Funnels plots of the association of extensive white matter hyperintensity (WMH) burden with incident stroke and dementia subtypes.
eFigure 7. Funnels plots of the association of magnetic resonance imaging (MRI)–defined brain infarct (BI) with incident stroke, dementia, and mortality.
eFigure 8. Funnels plots of the association of magnetic resonance imaging (MRI)–defined brain infarct (BI) with incident stroke subtypes.
eFigure 9. Funnels plots of the association of cerebral microbleeds (CMB) with incident stroke, dementia, and mortality.
eFigure 10. Funnels plots of the association of cerebral microbleeds (CMB) with incident stroke and dementia subtypes.
eReferences.