Key Points
Question
How are diagnostic criteria associated with the reported prevalence of urinary tract infection (UTI) in bronchiolitis?
Findings
In this systematic review and meta-analysis, the estimated prevalence of UTI in bronchiolitis was 3.1%, using the individual study definitions of UTI. With the inclusion of a positive urinalysis result (pyuria or nitrites) as a diagnostic criterion, the UTI prevalence was 0.8%.
Meaning
When a positive urinalysis result is added as a diagnostic criterion for UTI, the estimated prevalence of concomitant UTI in bronchiolitis is less than the threshold for testing of 1% to 3% referenced in the 2011 American Academy of Pediatrics UTI guidelines.
Abstract
Importance
Concomitant urinary tract infection (UTI) is a frequent concern in febrile infants with bronchiolitis, with a prior meta-analysis suggesting a prevalence of 3.3%. However, the definition of UTI in these studies has generally not incorporated urinalysis (UA) results.
Objective
To conduct a systematic review and meta-analysis examining the prevalence of UTI in infants with bronchiolitis when positive UA results are incorporated into the UTI definition.
Data Sources
Medline (1946-2017) and Ovid EMBASE (1976-2017) through August 2017 and bibliographies of retrieved articles.
Study Selection
Studies reporting UTI prevalence in bronchiolitis.
Data Extraction
Data were extracted in accordance with meta-analysis of observational studies in epidemiology guidelines via independent abstraction by multiple investigators. Random-effects models generated a weighted pooled event rate with corresponding 95% confidence intervals.
Main Outcomes and Measures
Prevalence of UTI.
Results
We screened 477 unique articles by abstract, with full-text review of 30 studies. Eighteen bronchiolitis studies reported a UTI prevalence and 7 of these reported UA data for inclusion in the meta-analysis. The overall reported prevalence of UTI in bronchiolitis from these 18 studies was 3.1% (95% CI, 1.8%-4.6%). With the addition of positive UA results (defined as the presence of pyuria or nitrites) as a diagnostic criterion, the prevalence of UTI as reported in the 7 studies in bronchiolitis was 0.8% (95% CI, 0.3%-1.4%). Sensitivity analyses yielded similar results, including for infants younger than 90 days. Heterogeneous definitions of UTI and UA criteria introduced uncertainty into prevalence estimates.
Conclusions and Relevance
When a positive UA result is added as a diagnostic criterion, the estimated prevalence of concomitant UTI is less than recommended testing thresholds for bronchiolitis.
This systematic review and meta-analysis examines the prevalence of urinary tract infection in infants with bronchiolitis when positive urinalysis results are incorporated into the urinary tract infection definition.
Introduction
Multiple studies have reported an association between bronchiolitis and urinary tract infections (UTIs), with some studies suggesting that as many as 7% to 12% of infants with bronchiolitis have a concomitant UTI.1,2,3 A meta-analysis4 of UTI in infants younger than 3 months with bronchiolitis reported a prevalence of 3.3%.
However, because the sensitivity of the urinalysis (UA) historically has been considered suboptimal,5,6,7 the diagnosis of UTI in these studies and in most studies of febrile infants has been defined by a positive urine culture alone regardless of the UA results.8,9 However, there is increasing recognition that a positive urine culture in the absence of pyuria on a UA may reflect asymptomatic bacteriuria (AB) or contamination rather than a true UTI.10,11,12,13 In response to this concern, in 2011 the American Academy of Pediatrics (AAP) revised the diagnostic criteria for UTIs to include both an abnormal UA result (pyuria or bacteriuria) and a positive urine culture (>50 000 cfu/mL).
For a condition such as bronchiolitis, where the annual disease burden globally is 20 to 50 million children,14 the accurate diagnosis of a concomitant UTI is important given the associated exposure to antibiotic therapy, imaging, potential for hospital admission or prolonged courses of intravenous antibiotics in young infants,15 prophylactic antibiotics, and additional follow-up appointments. In the 2011 AAP UTI guidelines, pretest probability thresholds referenced a testing range from 1% to 3%,11 derived from a survey of directors of outpatient pediatric clinics and emergency departments.16 Consistent with this threshold range, a UTI risk calculator published in 201817 established a threshold for testing of 2%.
We hypothesized that by adding a positive UA result, defined as the presence of pyuria or nitrites, as a diagnostic criterion along with a positive urine culture for the diagnosis of UTI, the prevalence of concomitant UTI in bronchiolitis would be less than these recommended thresholds for testing. To evaluate this, we undertook a systematic review and meta-analysis to assess the scope of current definitions for the diagnosis of UTI within the bronchiolitis literature and to determine the prevalence of UTI in infants with bronchiolitis when positive UA results are included as a diagnostic criterion for UTI.
Methods
Search Strategy
This study was conducted in accordance with the consensus statement on reporting Meta-Analysis of Observational Studies in Epidemiology (MOOSE)18 (eTable in the Supplement) and is registered with PROSPERO International Prospective Register of Systematic Reviews (CRD42018088978).19 The initial search was conducted by a medical librarian in September 2017. The search included Medline and Medline-in-process from 1946 to August 2017 and Ovid EMBASE from 1976 to August 2017. Search terms were determined by diagnoses and Medical Subject Headings, limiting to articles in English, including infants from birth through age 23 months, and excluding variations of the word pregnancy to filter out maternal articles on UTI. The full search strategy is available in eAppendix 1 in the Supplement. Additional studies were identified through hand searching and a manual review of the bibliographies of qualifying studies and relevant other systematic reviews.
Study Selection
Studies were considered for inclusion if they reported a UTI prevalence rate in infants having a primary diagnosis of bronchiolitis and for subanalysis if they provided both urine culture and UA data. Studies were then excluded if they included patients with active immunodeficiency or oncologic processes or specifically included patients with known anatomically abnormal urinary tracts. Additionally, review articles, case reports or series, editorials, meta-analyses, and guidelines were excluded.
Studies identified from the search results underwent title and abstract review, conducted by 2 independent reviewers for inclusion (C.E.M. and S.R. or C.E.M. and A.R.S.). Any discrepancy regarding inclusion was discussed by the entire study team and consensus reached. For studies reporting a UTI prevalence in bronchiolitis but not reporting UA data, the corresponding authors were contacted via email to determine whether additional data were available.
Identification and Data Extraction
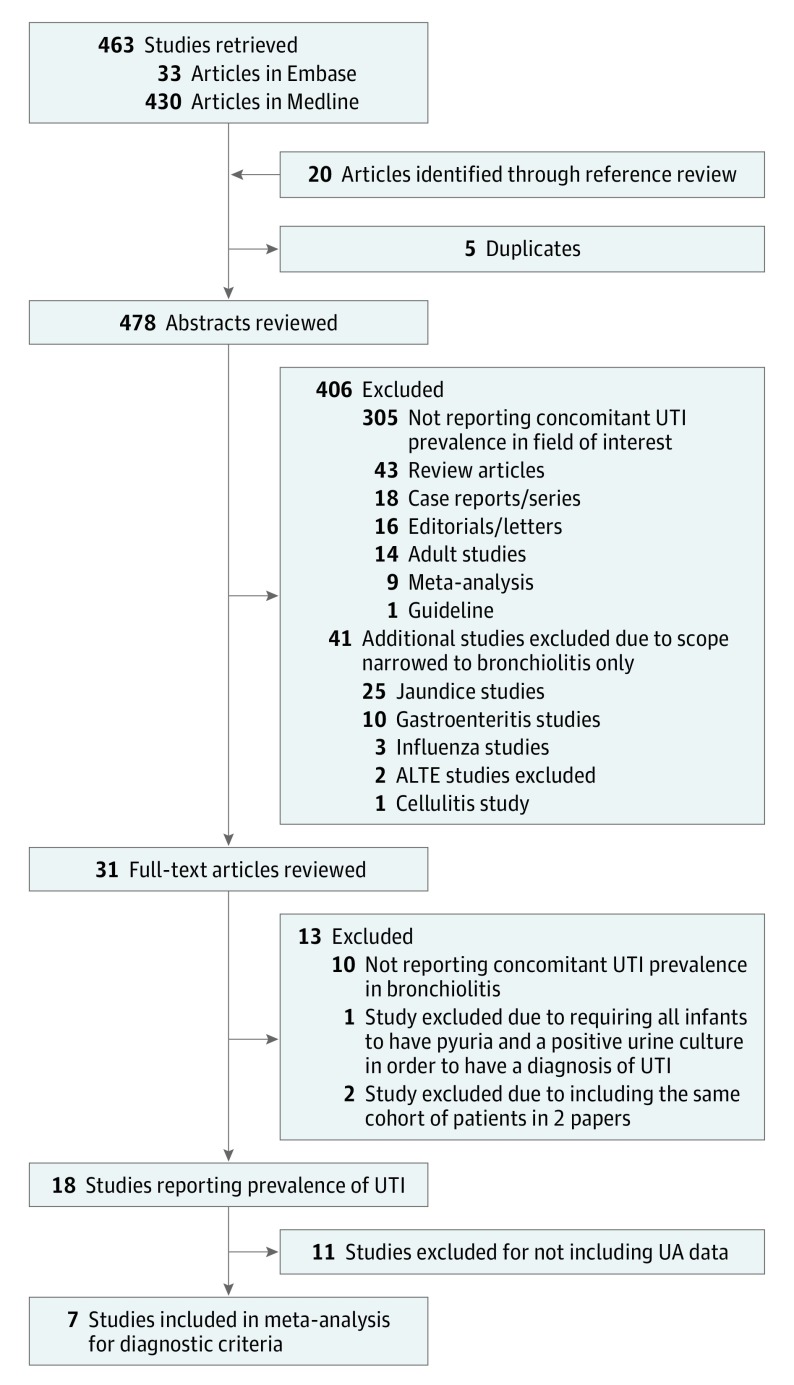
For full-text articles meeting inclusion criteria, data abstraction occurred independently by 2 study investigators (C.E.M. and S.R. and C.E.M. and A.R.S.). Using a standardized data extraction form piloted by 3 team members (C.E.M., S.R., and A.R.S.), the extraction was conducted by 2 reviewers, compared, and referred to the third for any disagreements. With discrepancies resolved by consensus, final data were extracted from 18 bronchiolitis studies (Figure 1).
Figure 1. Flow of Studies Through Database Search to Inclusion in the Meta-analysis.
ALTE indicates acute life-threatening event; UA, urinalysis; and UTI, urinary tract infection.
We defined the diagnosis of a UTI and the definition of a positive UA result using the individual study definition. If the study did not provide a formal definition, we defined a positive UA result based on reporting a positive leukocyte esterase, positive nitrite, or more than 5 white blood cells per high-powered field. We did not include bacteriuria in the definition of a positive UA result. Owing to suggestions from prior investigations that the sensitivity of the UA is lower for non–Escherichia coli organisms,20 the proportion of E coli UTIs were recorded for studies reporting urinary pathogen data.
Quality Assessment
To assess for study quality, we used a critical appraisal tool for prevalence studies, with modifications tailored to our study question.21 Specifically, 2 investigators independently evaluated the quality of all included full-text studies. The team modified the tool to address critical issues of validity for prevalence studies relevant to bronchiolitis, including ensuring a representative sample, appropriate recruitment, objective study definitions for UTI and bronchiolitis, appropriate accounting for confounding factors, and appropriate statistical analyses. Individual components for each study were rated as 0 (failing the measure), 1 (passing the measure), or not reported. The maximum score for a given study was 7.
Statistical Analysis
We used a κ statistic to report interobserver agreement on study inclusion and exclusion. To conduct a meta-analysis, we first transformed proportions with the Freeman-Tukey double arcsine transformation.22 For proportions of zero, we used a continuity correction. Point estimates and 95% confidence intervals were then computed using inverse-variance weighted random effects. For the 18 studies providing prevalence data, a baseline point estimate of UTI prevalence was calculated. From the 7 studies providing UA data, point estimates were calculated using the study definition for a UTI and then a second estimate based on the inclusion of positive UA criteria in addition to positive urine culture. Heterogeneity was assessed using the I2 statistic. Sensitivity analyses were performed removing studies identified as outliers on the study quality assessment tool. Lastly, a prevalence rate for E coli UTI with and without positive UA was calculated using the same analysis as discussed previously. All analyses were conducted with Stata, version 15.1 (StataCorp).
Results
A total of 463 studies were retrieved in our initial search and 5 were eliminated as duplicates. We identified an additional 20 studies through reference list review; thus, we reviewed 478 unique study abstracts and identified 31 studies for full-text review. Interobserver agreement on study inclusion/exclusion was high, with a κ of 0.89 (95% CI, 0.83-0.95). After full-text review, 13 studies were further excluded, with the most frequent reason for exclusion being studies not reporting UTI prevalence (10 of 12) (Figure 1). One study was excluded owing to using the same cohort of patients for 2 articles.23 Although corresponding authors with available contact information were emailed, no studies were able to provide additional UA data. Of the remaining 18 studies,1,2,3,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38 11 did not report UA data, leaving 7 for meta-analysis by reporting UA data and urine culture positivity (Tables 1 and 2).1,24,26,29,30,32,33
Table 1. Bronchiolitis Studies Reporting the Prevalence of Concomitant UTI That Do Not Report Urinalysis Data.
| Source, Year | Type of Study | Age | Inclusion Criteria | Fever Required | Exclusion Criteria | UTI Definition |
|---|---|---|---|---|---|---|
| Kuppermann et al,27 1997 | Prospective cohort | <90 d | Clinical bronchiolitis | Yes: temperature ≥38°C in ED | Infants with vaccination or antibiotics within 48 h or presentation, focal bacterial infection, identifiable viral infection, chronic illness or immunodeficiency, and immunosuppression | >10 000 cfu/mL of a single pathogen on catheterized specimen |
| Liebelt et al,28 1999 | Retrospective medical record review | <90 d | Clinical bronchiolitis | No: 42% documented febrile ≥38°C | History of bronchopulmonary dysplasia, immunodeficiency, complex congenital heart disease, and other bacterial or viral infections | >10 000 cfu/mL of a single pathogen on catheterized specimen or >1000 cfu/mL of gram-positive coccus on SPA or any cfu/mL of a gram-negative rod on SPA |
| Purcell and Fergie,31 2002 | Retrospective medical record review | All ages | RSV+ only | No: fever not specified | None stated | Positive urine culture |
| Titus and Wright,34 2003 | Retrospective cohort | <8 wk | RSV+ only | Yes: temperature ≥38°C documented in medical record | Infants with congenital heart disease, bronchopulmonary dysplasia, neurologic disorders, metabolic disorders, hematologic abnormalities, and significant medical history | Positive urine culture |
| Oray-Schrom et al,2 2003 | Retrospective medical record review | <90 d | RSV+ only | Yes: temperature ≥38°C | None stated | >10 000 cfu/mL of a single pathogen on catheterized specimen |
| Levine et al,37 2004 | Prospective cross-sectional study | <60 d | RSV+ only | Yes: rectal temperature ≥38°C in ED or by history | Infants who had received antibiotics within 48 h of presentation | >50 000 cfu/mL of a single pathogen or >10 000 cfu/mL of a single pathogen and a positive urinalysis on catheterized specimen or >1000 cfu/mL of a single pathogen by SPA |
| Purcell and Fergie,3 2004 | Retrospective medical record review | All ages | RSV+ only | No: temperature ≥38°C in 68% of cohort | None stated | Any cfu/mL on a SPA or >10 000 cfu/mL of a single pathogen on a catheterized specimen |
| Bilavsky et al,25 2008 | Prospective cohort | <90 d | Clinical bronchiolitis | Yes: rectal temperature ≥38°C in hospital | Infants with chronic disease, <32 wk gestation, afebrile, or who had received antibiotics within 48 h of presentation | Positive urine culture of any growth of a known pathogen obtained by SPA, catheterization, or midstream-collection |
| García et al,36 2010 | Retrospective medical record review | <2 y | Clinical bronchiolitis | No: fever not specified | None stated | Positive urine culture |
| Yarden-Bilavsky et al,35 2011 | Prospective observational | <90 d | Clinical bronchiolitis | Yes: rectal temperature ≥38°C | Infants with chronic disease, <32 wk gestation, or who had received antibiotics within 48 h of presentation | Positive urine culture of any growth of a known pathogen obtained by SPA, catheterization, or midstream-collection |
| Librizzi et al,38 2014 | Retrospective medical record review | <24 mo | Clinical bronchiolitis | No: 58% of cohort with fever at or during admission (temperature undefined) | ICU admission | >10 000 cfu/mL of a single pathogen on catheterized specimen |
Abbreviations: CFU, colony-forming unit; ED, emergency department; ICU, intensive care unit; RSV, respiratory syncytial virus; SPA, suprapubic aspiration; UTI, urinary tract infection.
Table 2. Bronchiolitis Studies Reporting the Prevalence of Concomitant UTI That Report Urinalysis Data.
| Source, Year | Type of Study | Age | Inclusion Criteria | Fever Required | Exclusion Criteria | UTI Definition | Positive UA Criteria |
|---|---|---|---|---|---|---|---|
| Antonow et al,24 1998 | Retrospective medical record review | <60 d | Clinical bronchiolitis | No: 72% fever documented | PICU admission and comorbidities including congenital heart disease, bronchopulmonary dysplasia, and immunodeficiencies | >10 000 cfu/mL of a single pathogen on catheterized specimen | None |
| Melendez and Harper,30 2003 | Retrospective medical record review | <90 d | Clinical bronchiolitis | Yes: rectal temperature ≥38°C in ED | No upper respiratory signs or symptoms, focal lung findings, bronchopulmonary dysplasia, congenital heart disease, focal bacterial infection, or identifiable viral infection | >10 000 cfu/mL of a single pathogen on catheterized specimen | None |
| Randolph et al,32 2004 | Retrospective medical record review | <36 mo | RSV+ only, ICU admission | No: fever not specified | <36 wks gestational age, immunodeficiency, cardiac disease, chronic lung disease, craniofacial anomalies, hematologic or oncologic disorders, renal disease, or neurologic dysfunction | Positive urine culture | None |
| Luginbuhl et al,29 2008 | Prospective cohort | <90 d | Clinical bronchiolitis | Yes: temperature ≥38°C at home or clinic | Any major comorbidity, including congenital anomalies, extreme prematurity, or organ failure | Positive urine culture | +LE, nitrite, or >5 WBC/HPF |
| Kaluarachchi et al,26 2014 | Retrospective medical record review | 2-12 mo | RSV+ only | Yes: rectal temperature ≥38°C documented | Gestational age <36 wks, previous history of UTI, any known immunodeficiency, systemic antibiotics within 72 h of presentation | A single pathogen: >1000 cfu/mL SPA, >50 000 cfu/mL catheterized specimen, or >10 000 cfu/mL catheterized specimen + positive UA | Trace or >LE, nitrite, or >5 WBC/HPF or >10 WBC if uncentrifuged |
| Elkhunovich and Wang,1 2015 | Prospective cohort | 2-12 mo | Clinical bronchiolitis | Yes: temperature ≥38°C at home or ED | Any known renal or urologic anomaly at time of ED evaluation, systemic antibiotics within 24 h of presentation, gestational age <35wks | >50 000 cfu/mL of a single pathogen on catheterized specimen | +LE, nitrite or >5 WBC/HPF |
| Schlechter Salinas et al,33 2017 | Retrospective medical record review | 2-12 mo | RSV+ only | Yes: temperature ≥38°C in ED | Any known renal or urologic anomaly at time of ED evaluation, systemic antibiotics within 24 h of presentation | >50 000 cfu/mL of a single pathogen on catheterized specimen | >5 WBC/HPF |
Abbreviations: cfu, colony-forming unit; ED, emergency department; HPF, high-powered field; ICU, intensive care unit; LE, leukocyte esterase; PICU, pediatric intensive care unit; RSV, respiratory syncytial virus; UA, urinalysis; UTI, urinary tract infection; WBC, white blood cell.
Quality Assessment
Of the 7 included studies with UA data, 3 defined bronchiolitis by a positive respiratory syncytial virus test result only,26,32,33 while the other 4 included children with clinical bronchiolitis as well.1,24,29,30 All of the studies excepting 2 required fever as part of the inclusion criteria.1,26,29,30,33 Three of the studies exclusively included infants younger than 90 days.24,29,30 Overall quality scores ranged from 5 to 7 on a 7-point scale. Scores within the individual categories are available in eAppendix 2 in the Supplement.
UTI Definitions
The criteria for diagnosing a UTI and the definition of a positive UA result varied between studies (Tables 1 and 2). Definitions of a positive urine culture ranged from any growth of a known pathogen35 to, most commonly, at least 10 000 cfu/mL on a catheterized specimen.2,24,27,30,38 Several studies used varying standards of colony-forming unit per milliliter for positivity based on method of obtaining the urine.3,26,28,37 Most studies included only cultures that grew a single organism, and 5 studies did not provide a definition for a positive culture.29,31,32,34,36 For the 7 studies that provided UA data, 2 of the studies used positive leukocyte esterase, positive nitrite, or more than 5 white blood cells per high-powered field.1,29 Several of the included studies presented UA data but did not define a positive UA result.24,30,32 There was no consistency in the definition of a UTI when stratified based on studies that provided UA data vs those that did not. There also was no consistency in the definition of UTI or of a positive UA data when stratified by year of manuscript publication.
Prevalence of Concomitant UTI in Bronchiolitis
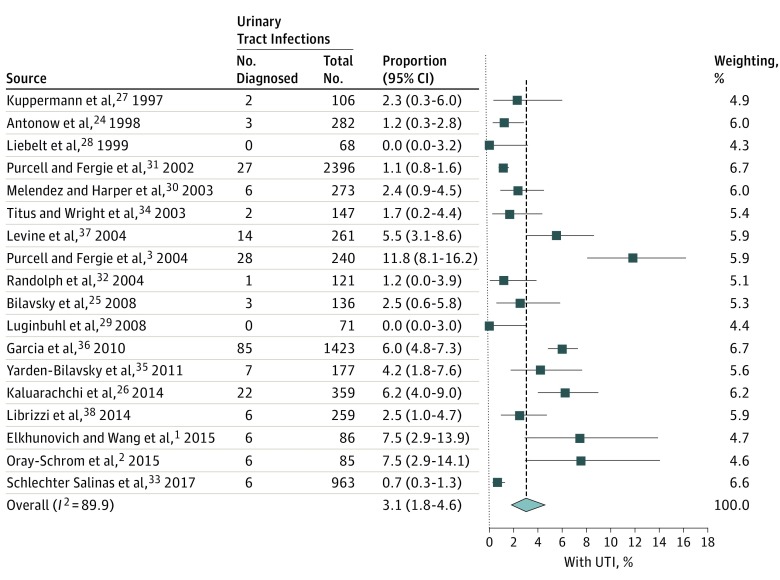
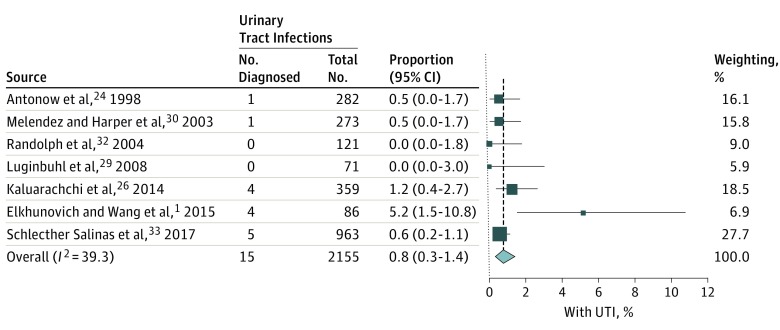
The overall reported prevalence from the 18 included studies of UTI in bronchiolitis was 3.1% (95% CI, 1.8-4.6; I2 = 89%) (Figure 2). When narrowed to the 7 studies that provided UA data, the overall prevalence of UTI without inclusion of this data was 2.2% (95% CI, 0.8-4.4; I2 = 85%). However, when a positive UA result was included as a diagnostic criterion, the prevalence of UTI was 0.8% (95% CI, 0.33-1.40; I2 = 39%) (Figure 3). From the 7 studies providing UA data, a sensitivity analysis removing 3 studies that defined bronchiolitis only by respiratory syncytial virus testing26,32,33 did not substantially alter the summary statistics, producing a point estimate for UTI of 1.0% (95% CI, 0.1%-2.7%; I2 = 61%) when a positive UA result was required. Similarly, for the studies exclusively including infants younger than 90 days,24,29,30 the point estimate for concomitant UTI with positive UA was 0.5% (95% CI, 0.1%-1.2%; I2 = 0%).
Figure 2. Weighted Pooled Prevalence of Urinary Tract Infections (UTIs) in Bronchiolitis.
Figure 3. Weighted Pooled Prevalence of Urinary Tract Infections (UTIs) in Bronchiolitis With the Addition of a Positive Urinalysis to a Positive Urine Culture for Diagnosis of a UTI.
Proportion of E coli UTI
In the 6 bronchiolitis studies with at least 1 positive urine culture (1 study had zero positive cultures29), all reported the specific causative UTI pathogens. In total, the weighted proportion of E coli UTIs was 63.9% in bronchiolitis.1,24,26,30,32,33 When applying the diagnostic criterion of a positive UA result, the weighted prevalence of E coli was 80.3%.1,24,26,30,33
Discussion
In studies examining the prevalence of UTI in bronchiolitis, inclusion of a positive UA result as a diagnostic criterion was significantly associated with the estimated prevalence of concomitant UTI. The overall prevalence of UA–positive UTI is 0.8%, less than the pretest probability threshold of 1% to 3% discussed in the 2011 AAP UTI guidelines.11 These results held consistent across sensitivity analyses.
Similar to the previously published meta-analysis of UTI in bronchiolitis,4 our study found an overall reported prevalence of 3.1%, with the addition of several newer studies published after the previous analysis. We also noted a low proportion (67%) of urine culture pathogens to be E coli compared with the proportion described in most febrile infant studies of 80% to 90%.39,40,41,42 Interestingly, when a positive UA result was required for diagnosis of a UTI, the E coli proportion increased to 80% in UA-positive UTIs. Non–E coli organisms have been associated with a lower rate of pyuria in several studies published in the last 5 years and may indicate that these organisms incite less of an inflammatory response when causing a UTI.20,43 Alternatively, these organisms may be more likely to associated with contamination or asymptomatic bacteriuria. Eliacik et al44 found that follow-up suprapubic aspirates performed on patients with untreated catheterized urine cultures positive for non–E coli organisms (most of with negative UA results) were predominantly negative.44 These findings suggest that most urine cultures that are positive for non–E coli organisms without pyuria either do not reflect true UTI or represent UTIs that spontaneously resolve and that the negative UA results found in these patients therefore reflect true negatives rather than false-negatives.
Within our study cohort, the definitions of UTI, a positive urine culture, and a positive UA result were highly variable, pointing to the need for more adoption of standardized definitions. In the 1999 AAP UTI Practice Parameter, the definition of UTI was based on urine culture results alone and varied by culture method (suprapubic aspiration, catheterization, or bagged specimen) and quantity of bacterial growth.45 The recommended diagnostic criteria were subsequently revised in 2011 to include both an abnormal UA result and a positive urine culture for infants older than 2 months.11 All 3 of the bronchiolitis studies1,26,33 published after the 2011 guidelines included subanalyses comparing diagnosis based on urine culture alone with urine culture plus a positive UA result; however, the definitions that they used to define a positive urine culture and UA were disparate.1,26,33
The revised AAP recommendations exclude infants younger than 2 months, the highest risk population for serious bacterial infections and where questions regarding the reliability of the UA still linger. Accordingly, none of the 3 studies in our meta-analysis that exclusively included infants younger than 90 days required a positive UA result for the primary reporting of a concomitant diagnosis of a UTI, regardless of year of study enrollment. Interestingly, both the baseline reported pooled prevalence (1.4%) and the pooled prevalence of UA-positive UTI (0.5%) was lower in this younger age group.
Since the publication of the revised diagnostic criteria for UTI, the utility of an abnormal UA in young infants as a necessary component for the diagnosis of a UTI has been re-evaluated. Two investigations46,47 published within the past 5 years have reported UA sensitivities in bacteremic UTI in young infants nearing or reaching 100%. These findings are relevant given that simultaneously positive urine and blood cultures with the same organism cannot be explained by asymptomatic bacteriuria or contamination and therefore, in contrast to a positive urine culture alone, bacteremic UTI represents true infection. Additionally, Hoberman et al10 found that evidence of acute pyelonephritis was not present on dimercaptosuccinic acid renal scan in any patients with positive urine cultures and less than 10 leukocytes/mm3 on UA,48 and Wettergren et al13 demonstrated that asymptomatic bacteriuria (detected by suprapubic aspirate) is surprisingly common in healthy infants, with a period prevalence of 0.9% in girls and 2.5% in boys during the first year of life and a point prevalence as high as 1.5% in boys younger than 2 months. These studies suggest that the paired finding of a positive urine culture without pyuria is unlikely to reflect true UTI.
Misdiagnosis of UTI in infants with bronchiolitis has significant implications for patient care. Early exposure to antibiotics has been associated with obesity, asthma, anaphylaxis, allergies, and development of inflammatory bowel disease49,50,51,52 and may lead to unnecessary laboratory workup, imaging and radiation, antibiotic prophylaxis, and hospitalization. Additionally, inflated prevalence estimates are likely to drive initial urine testing, which is often invasive. While we are unable to conclude that all reported UTIs with unremarkable UA results were misdiagnosed, our findings do suggest that prior estimates of the prevalence of UTI in bronchiolitis may have been exaggerated. Given that most UTI investigations have not required a positive UA to define UTI, investigations similar to this that reassess UTI risk using UA criteria in other clinical conditions, such as fever without source, jaundice, influenza, or brief resolved unexplained events, would be informative.
Under the premise that UA results should indeed be incorporated into the definition of UTI, the estimated prevalence of UTI in bronchiolitis of 0.8% translates to 125 infants being tested to detect 1 UTI. This number needed to test is high enough that routine urine testing in infants with bronchiolitis and fever seems unjustified. However, even within bronchiolitis, the probability of UTI is likely affected by known risk factors described for UTI in general,17 such that a 2-month-old uncircumcised boy with bronchiolitis may have a higher probability of UTI than a 14-month-old circumcised boy with bronchiolitis. Ultimately, clinicians can incorporate pretest probability, severity of illness, and parent preferences to determine when urine testing may be appropriate. For those infants in whom testing may be desired, a screening UA by a urine bag may help to further reduce the trauma of urethral catheterization.53
Limitations
In this cohort, the definitions of UTI and positive UA result were highly heterogeneous between studies. Owing to these variations, we were forced to define a UTI based on the individual study’s definition rather than a standard applied to all included studies. Because many of the studies include urine culture results for growth less than 50 000 cfu/mL and the significant variability within the UA positivity definition, the exact prevalence of concomitant UTI in bronchiolitis remains elusive. Several studies in their definition of a positive UA included nitrites, which are indicators of a positive urine culture but may not help distinguish a UTI from asymptomatic bacteriuria. As such, the inclusion of nitrites in these studies may further inflate the calculated prevalence of UTI greater than the actual prevalence. There was a difference in overall prevalence of UTI between the calculations based on all 18 included studies vs the 7 that provided UA data (3.1% vs 2.2%). Qualitatively, there are no apparent differences in inclusion or exclusion criteria or consistency in UTI definitions between the 7 studies and the larger cohort that would explain this difference, so the reason for this small difference remains unclear. Lastly, there were 2 studies that did not require fever as an inclusion criteria,24,32 which introduces potential threats to generalizability given that practitioners are less apt to worry about UTI in afebrile patients with bronchiolitis. In one of these studies, 72% of infants had a documented fever,24 and in the other, presence of fever was not documented.32 However, the prevalence of UTI in these 2 studies (1.2% and 1.2%, respectively) was similar to the studies that mandated fever.
Conclusions
The prevalence of UTI in infants with bronchiolitis is affected by UTI diagnostic criteria. With the incorporation of a positive UA result into the disease definition, the estimated prevalence of concomitant UTI is less than proposed thresholds for testing. Applying a positive UA result as a diagnostic criterion for UTI may have the potential to decrease the misdiagnosis of UTI in infants with other recognizable clinical syndromes as well.
eAppendix 1. Search Strategy
eAppendix 2. Quality Assessment
eTable. MOOSE Checklist: Impact of Diagnostic Criteria on UTI Prevalence in Bronchiolitis: a Meta-Analysis
References
- 1.Elkhunovich MA, Wang VJ. Assessing the utility of urine testing in febrile infants aged 2 to 12 months with bronchiolitis. Pediatr Emerg Care. 2015;31(9):616-620. doi: 10.1097/PEC.0000000000000359 [DOI] [PubMed] [Google Scholar]
- 2.Oray-Schrom P, Phoenix C, St Martin D, Amoateng-Adjepong Y. Sepsis workup in febrile infants 0-90 days of age with respiratory syncytial virus infection. Pediatr Emerg Care. 2003;19(5):314-319. doi: 10.1097/01.pec.0000092576.40174.28 [DOI] [PubMed] [Google Scholar]
- 3.Purcell K, Fergie J. Concurrent serious bacterial infections in 912 infants and children hospitalized for treatment of respiratory syncytial virus lower respiratory tract infection. Pediatr Infect Dis J. 2004;23(3):267-269. doi: 10.1097/01.inf.0000116759.21252.29 [DOI] [PubMed] [Google Scholar]
- 4.Ralston S, Hill V, Waters A. Occult serious bacterial infection in infants younger than 60 to 90 days with bronchiolitis: a systematic review. Arch Pediatr Adolesc Med. 2011;165(10):951-956. doi: 10.1001/archpediatrics.2011.155 [DOI] [PubMed] [Google Scholar]
- 5.Bachur R, Harper MB. Reliability of the urinalysis for predicting urinary tract infections in young febrile children. Arch Pediatr Adolesc Med. 2001;155(1):60-65. doi: 10.1001/archpedi.155.1.60 [DOI] [PubMed] [Google Scholar]
- 6.Bonadio W, Maida G. Urinary tract infection in outpatient febrile infants younger than 30 days of age: a 10-year evaluation. Pediatr Infect Dis J. 2014;33(4):342-344. doi: 10.1097/INF.0000000000000110 [DOI] [PubMed] [Google Scholar]
- 7.Shaw KN, Gorelick M, McGowan KL, Yakscoe NM, Schwartz JS. Prevalence of urinary tract infection in febrile young children in the emergency department. Pediatrics. 1998;102(2):e16. doi: 10.1542/peds.102.2.e16 [DOI] [PubMed] [Google Scholar]
- 8.Glissmeyer EW, Korgenski EK, Wilkes J, et al. . Dipstick screening for urinary tract infection in febrile infants. Pediatrics. 2014;133(5):e1121-e1127. doi: 10.1542/peds.2013-3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin DS, Huang SH, Lin CC, et al. . Urinary tract infection in febrile infants younger than eight weeks of age. Pediatrics. 2000;105(2):E20. doi: 10.1542/peds.105.2.e20 [DOI] [PubMed] [Google Scholar]
- 10.Hoberman A, Wald ER, Reynolds EA, Penchansky L, Charron M. Is urine culture necessary to rule out urinary tract infection in young febrile children? Pediatr Infect Dis J. 1996;15(4):304-309. doi: 10.1097/00006454-199604000-00005 [DOI] [PubMed] [Google Scholar]
- 11.Roberts KB; Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management . Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595-610. doi: 10.1542/peds.2011-1330 [DOI] [PubMed] [Google Scholar]
- 12.Wettergren B, Jodal U. Spontaneous clearance of asymptomatic bacteriuria in infants. Acta Paediatr Scand. 1990;79(3):300-304. doi: 10.1111/j.1651-2227.1990.tb11460.x [DOI] [PubMed] [Google Scholar]
- 13.Wettergren B, Jodal U, Jonasson G. Epidemiology of bacteriuria during the first year of life. Acta Paediatr Scand. 1985;74(6):925-933. doi: 10.1111/j.1651-2227.1985.tb10059.x [DOI] [PubMed] [Google Scholar]
- 14.Shi T, McAllister DA, O’Brien KL, et al. ; RSV Global Epidemiology Network . Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390(10098):946-958. doi: 10.1016/S0140-6736(17)30938-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi NS, Lucas BP, Schroeder AR. Physician preferences surrounding urinary tract infection management in neonates. Hosp Pediatr. 2018;8(1):21-27. doi: 10.1542/hpeds.2017-0082 [DOI] [PubMed] [Google Scholar]
- 16.Roberts KB, Charney E, Sweren RJ, et al. . Urinary tract infection in infants with unexplained fever: a collaborative study. J Pediatr. 1983;103(6):864-867. doi: 10.1016/S0022-3476(83)80702-1 [DOI] [PubMed] [Google Scholar]
- 17.Shaikh N, Hoberman A, Hum SW, et al. . Development and validation of a calculator for estimating the probability of urinary tract infection in young febrile children. JAMA Pediatr. 2018;172(6):550-556. doi: 10.1001/jamapediatrics.2018.0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC, et al. . Meta-analysis of observational studies in epidemiology: a proposal for reporting: Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 19.Reviews PIPRoS The impact of diagnostic criteria on reported UTI prevalence in neonatal jaundice and bronchiolitis: systematic review and meta-analysis. https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=88978. Accessed February 16, 2018.
- 20.Shaikh N, Shope TR, Hoberman A, Vigliotti A, Kurs-Lasky M, Martin JM. Association between uropathogen and pyuria. Pediatrics. 2016;138(1):e20160087. doi: 10.1542/peds.2016-0087 [DOI] [PubMed] [Google Scholar]
- 21.Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3(3):123-128. doi: 10.15171/ijhpm.2014.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974-978. doi: 10.1136/jech-2013-203104 [DOI] [PubMed] [Google Scholar]
- 23.Kaluarachchi D, Kaldas V, Erickson E, Nunez R, Mendez M. When to perform urine cultures in respiratory syncytial virus-positive febrile older infants? Pediatr Emerg Care. 2014;30(9):598-601. doi: 10.1097/PEC.0000000000000203 [DOI] [PubMed] [Google Scholar]
- 24.Antonow JA, Hansen K, McKinstry CA, Byington CL. Sepsis evaluations in hospitalized infants with bronchiolitis. Pediatr Infect Dis J. 1998;17(3):231-236. doi: 10.1097/00006454-199803000-00011 [DOI] [PubMed] [Google Scholar]
- 25.Bilavsky E, Shouval DS, Yarden-Bilavsky H, Fisch N, Ashkenazi S, Amir J. A prospective study of the risk for serious bacterial infections in hospitalized febrile infants with or without bronchiolitis. Pediatr Infect Dis J. 2008;27(3):269-270. doi: 10.1097/INF.0b013e31815e85b1 [DOI] [PubMed] [Google Scholar]
- 26.Kaluarachchi D, Kaldas V, Roques E, Nunez R, Mendez M. Comparison of urinary tract infection rates among 2- to 12-month-old febrile infants with RSV infections using 1999 and 2011 AAP diagnostic criteria. Clin Pediatr (Phila). 2014;53(8):742-746. doi: 10.1177/0009922814529015 [DOI] [PubMed] [Google Scholar]
- 27.Kuppermann N, Bank DE, Walton EA, Senac MO Jr, McCaslin I. Risks for bacteremia and urinary tract infections in young febrile children with bronchiolitis. Arch Pediatr Adolesc Med. 1997;151(12):1207-1214. doi: 10.1001/archpedi.1997.02170490033006 [DOI] [PubMed] [Google Scholar]
- 28.Liebelt EL, Qi K, Harvey K. Diagnostic testing for serious bacterial infections in infants aged 90 days or younger with bronchiolitis. Arch Pediatr Adolesc Med. 1999;153(5):525-530. doi: 10.1001/archpedi.153.5.525 [DOI] [PubMed] [Google Scholar]
- 29.Luginbuhl LM, Newman TB, Pantell RH, Finch SA, Wasserman RC. Office-based treatment and outcomes for febrile infants with clinically diagnosed bronchiolitis. Pediatrics. 2008;122(5):947-954. doi: 10.1542/peds.2007-3206 [DOI] [PubMed] [Google Scholar]
- 30.Melendez E, Harper MB. Utility of sepsis evaluation in infants 90 days of age or younger with fever and clinical bronchiolitis. Pediatr Infect Dis J. 2003;22(12):1053-1056. doi: 10.1097/01.inf.0000101296.68993.4d [DOI] [PubMed] [Google Scholar]
- 31.Purcell K, Fergie J. Concurrent serious bacterial infections in 2396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156(4):322-324. doi: 10.1001/archpedi.156.4.322 [DOI] [PubMed] [Google Scholar]
- 32.Randolph AG, Reder L, Englund JA. Risk of bacterial infection in previously healthy respiratory syncytial virus-infected young children admitted to the intensive care unit. Pediatr Infect Dis J. 2004;23(11):990-994. doi: 10.1097/01.inf.0000143647.88873.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlechter Salinas AK, Hains DS, Jones T, Harrell C, Meredith M. Testing for urinary tract infection in the influenza/respiratory syncytial virus-positive febrile infant aged 2 to 12 months. Pediatr Emerg Care. 2017. doi: 10.1097/PEC.0000000000001073 [DOI] [PubMed] [Google Scholar]
- 34.Titus MO, Wright SW. Prevalence of serious bacterial infections in febrile infants with respiratory syncytial virus infection. Pediatrics. 2003;112(2):282-284. doi: 10.1542/peds.112.2.282 [DOI] [PubMed] [Google Scholar]
- 35.Yarden-Bilavsky H, Ashkenazi-Hoffnung L, Livni G, Amir J, Bilavsky E. Month-by-month age analysis of the risk for serious bacterial infections in febrile infants with bronchiolitis. Clin Pediatr (Phila). 2011;50(11):1052-1056. doi: 10.1177/0009922811412949 [DOI] [PubMed] [Google Scholar]
- 36.García CG, Bhore R, Soriano-Fallas A, et al. . Risk factors in children hospitalized with RSV bronchiolitis versus non-RSV bronchiolitis. Pediatrics. 2010;126(6):e1453-e1460. doi: 10.1542/peds.2010-0507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine DA, Platt SL, Dayan PS, et al. ; Multicenter RSV-SBI Study Group of the Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics . Risk of serious bacterial infection in young febrile infants with respiratory syncytial virus infections. Pediatrics. 2004;113(6):1728-1734. doi: 10.1542/peds.113.6.1728 [DOI] [PubMed] [Google Scholar]
- 38.Librizzi J, McCulloh R, Koehn K, Alverson B. Appropriateness of testing for serious bacterial infection in children hospitalized with bronchiolitis. Hosp Pediatr. 2014;4(1):33-38. doi: 10.1542/hpeds.2013-0073 [DOI] [PubMed] [Google Scholar]
- 39.Greenhow TL, Hung YY, Herz AM, Losada E, Pantell RH. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33(6):595-599. doi: 10.1097/INF.0000000000000225 [DOI] [PubMed] [Google Scholar]
- 40.Hernández-Bou S, Trenchs V, Alarcón M, Luaces C. Afebrile very young infants with urinary tract infection and the risk for bacteremia. Pediatr Infect Dis J. 2014;33(3):244-247. doi: 10.1097/INF.0000000000000033 [DOI] [PubMed] [Google Scholar]
- 41.Roman HK, Chang PW, Schroeder AR. Diagnosis and management of bacteremic urinary tract infection in infants. Hosp Pediatr. 2015;5(1):1-8. doi: 10.1542/hpeds.2014-0051 [DOI] [PubMed] [Google Scholar]
- 42.Schroeder AR, Shen MW, Biondi EA, et al. . Bacteraemic urinary tract infection: management and outcomes in young infants. Arch Dis Child. 2016;101(2):125-130. doi: 10.1136/archdischild-2014-307997 [DOI] [PubMed] [Google Scholar]
- 43.Forster CS, Shaikh N, Hoberman A, Jackson E. Uropathogens and pyuria in children with neurogenic bladders. Pediatrics. 2018;141(5):e20173006. doi: 10.1542/peds.2017-3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eliacik K, Kanik A, Yavascan O, et al. . A comparison of bladder catheterization and suprapubic aspiration methods for urine sample collection from infants with a suspected urinary tract infection. Clin Pediatr (Phila). 2016;55(9):819-824. doi: 10.1177/0009922815608278 [DOI] [PubMed] [Google Scholar]
- 45.American Academy of Pediatrics, Committee on Quality Improvement, Subcommittee on Urinary Tract Infection Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics. 1999;103(4 Pt 1):843-852. [DOI] [PubMed] [Google Scholar]
- 46.Schroeder AR, Chang PW, Shen MW, Biondi EA, Greenhow TL. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics. 2015;135(6):965-971. doi: 10.1542/peds.2015-0012 [DOI] [PubMed] [Google Scholar]
- 47.Tzimenatos L, Mahajan P, Dayan PS, et al. ; Pediatric Emergency Care Applied Research Network (PECARN) . Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2):e20173068. doi: 10.1542/peds.2017-3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoberman A, Wald ER, Reynolds EA, Penchansky L, Charron M. Pyuria and bacteriuria in urine specimens obtained by catheter from young children with fever. J Pediatr. 1994;124(4):513-519. doi: 10.1016/S0022-3476(05)83127-0 [DOI] [PubMed] [Google Scholar]
- 49.Kronman MP, Zaoutis TE, Haynes K, Feng R, Coffin SE. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics. 2012;130(4):e794-e803. doi: 10.1542/peds.2011-3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marra F, Lynd L, Coombes M, et al. . Does antibiotic exposure during infancy lead to development of asthma?: a systematic review and metaanalysis. Chest. 2006;129(3):610-618. doi: 10.1378/chest.129.3.610 [DOI] [PubMed] [Google Scholar]
- 51.Shao X, Ding X, Wang B, et al. . Antibiotic exposure in early life increases risk of childhood obesity: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2017;8:170. doi: 10.3389/fendo.2017.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitre E, Susi A, Kropp LE, Schwartz DJ, Gorman GH, Nylund CM. Association between use of acid-suppressive medications and antibiotics during infancy and allergic diseases in early childhood. JAMA Pediatr. 2018;172(6):e180315. doi: 10.1001/jamapediatrics.2018.0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lavelle JM, Blackstone MM, Funari MK, et al. . Two-step process for ED UTI screening in febrile young children: reducing catheterization rates. Pediatrics. 2016;138(1):e20153023. doi: 10.1542/peds.2015-3023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Search Strategy
eAppendix 2. Quality Assessment
eTable. MOOSE Checklist: Impact of Diagnostic Criteria on UTI Prevalence in Bronchiolitis: a Meta-Analysis