Abstract
Brugada syndrome (BrS) and several cardiomyopathies, including dilated cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, left ventricular non-compaction (LVNC), and hypertrophic cardiomyopathy (HCM), share common genetic mutations and are associated with an arrhythmogenic substrate (AS) and increased risk of sudden cardiac death (SCD) due to malignant ventricular arrhythmias. We report a family in which a SCN5A mutation was found in both a father and daughter who presented with different phenotypes: the father with LVNC and the daughter with BrS, suggesting SCN5A may be important in cases of overlap between BrS and these various other cardiomyopathies and arrhythmias. Additionally, we report a family in which a MYBPC3 mutation was found in a father, daughter, and son, but they also presented with different phenotypes: the father with HCM and the daughter and son with BrS, suggesting patients with cardiomyopathies or BrS exhibiting sarcomeric mutations may have common genetic pathways that ultimately diverge into different phenotypes. Generally, prevention of SCD may involve the use of an implantable cardioverter-defibrillator and/or pharmaceutical therapy. However, patients continue to experience difficulties with this treatment. Epicardial mapping together with ajmaline challenge used to identify the AS in BrS patients can be used to identify and ablate the AS in cardiomyopathy patients, thus preventing the recurrence of ventricular tachycardia/fibrillation and reducing or eliminating the need for shock or pharmacological therapy. Future studies and longer follow-up times are warranted to understand the fullest duration of the therapeutic potential of this ajmaline and map-guided ablation therapy.
Keywords: Brugada syndrome, Catheter ablation, Implantable cardioverter-defibrillator, Mapping, Sudden cardiac death
Introduction
Sudden cardiac death (SCD) is the leading cause of death in western countries.1 In the general populations of the United States, Europe, and China, SCD occurs at an estimated rate of 50–100 per 100 000 people annually.1 The chance of SCD increases with age, with the incidence being higher for men than for women at every age. Brugada syndrome (BrS) and several cardiomyopathies, including dilated cardiomyopathy (DCM), arrhythmogenic right ventricular cardiomyopathy (ARVC), left ventricular non-compaction (LVNC), and hypertrophic cardiomyopathy (HCM), share common genetic mutations and are associated with an arrhythmogenic substrate (AS) and increased risk of sudden death due to malignant ventricular arrhythmias (VAs).
Generally, prevention of SCD in cardiomyopathy patients may involve the use of an implantable cardioverter-defibrillator (ICD) and/or pharmaceutical therapy. Implantable cardioverter-defibrillator is recommended for any patients who have survived a previous cardiac arrest, or who have documented ventricular tachycardia/fibrillation (VT/VF). Additionally, ICD is also recommended for DCM patients with an ejection fraction ≤35% despite ≥3 months of optimal pharmacological therapy,2 DCM patients with a confirmed LMNA mutation and clinical risk factors, ARVC patients with haemodynamically well-tolerated sustained VT, LVNC patients with Chagas cardiomyopathy and a left ventricular ejection fraction <40%, or HCM patients with spontaneous sustained VT causing syncope or haemodynamic compromise.2
Identifying ‘high-risk’ patients to recommend for pharmacological therapy, ICD, and/or ablation, can be difficult. In DCM, autonomic tests are not significant predictors of risk.2 Guidelines for use of ICDs in LVNC patients are generally the same for DCM, meaning there is a difficulty in distinguishing ‘low-risk’ and ‘high-risk’ patients. Pharmaceutical intervention may result in intolerable side effects that are especially difficult for young people to endure over a lifetime. ICDs are all too often associated with inappropriate shocks, lead fractures/failure, and device infections, not to mention the need for battery replacement, again exposing the patient to risk of infection. An increase in shock delivery is associated with increased mortality, and it is debated whether this is indicative of disease progression or effects of the ICD itself. Studies have suggested that myocardial stunning may occur as a result of shock delivery, which may in turn lead to pulseless electrical activity and sudden death.3 Here, the question emerges: how do we predict which patients will actually suffer a lethal VT/VF, and thus would overall benefit from medical intervention, despite all the undesirable consequences of that intervention? Epicardial mapping used for BrS patients may provide the answer to this for cardiomyopathy patients. The AS identified may then be ablated to prevent the recurrence of VT/VF, reducing or eliminating the need for shock therapy.
Genetic cardiomyopathies and Brugada syndrome
ARVC, LVNC, HCM, and BrS are frequently inherited as an autosomal dominant genetic trait. Hypertrophic cardiomyopathy and DCM are more common in men than in women. In North America, Europe, Asia, and Africa, unexplained left ventricular hypertrophy in adults occurs in about 0.02–0.23% of the population, while the rates are lower for people under 25 years old.2 ARVC occurs in about one in 1000–5000 of the general population, while DCM occurs overall in about one in 2500. HCM occurs similarly between racial groups. In adults, familial disease with isolated LVNC occurs at a rate of 18–50%.2
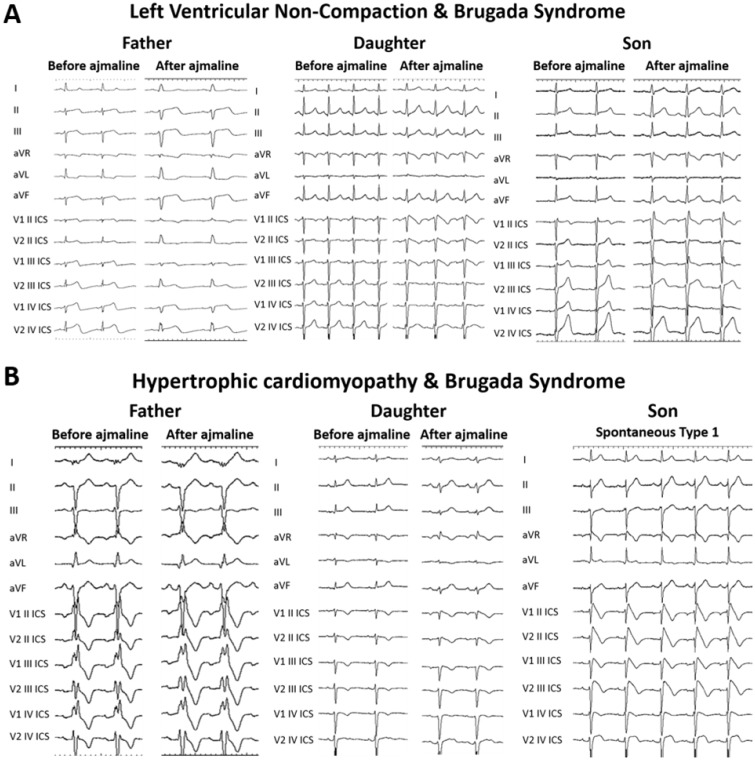
The BrS is characterized by an elevated ST-segment in the right precordial leads (V1-3) on the electrocardiogram (ECG) and an increased risk of VT/VF and SCD. The most common BrS genotype is a sodium channel loss-of-function SCN5A mutation, which accounts for 15–30% of BrS cases.4 SCN5A mutations can also be found in DCM, ARVC, and LVNC, in addition to many other pathologies, such as early repolarization syndrome, atrial standstill type 1, atrial fibrillation, long QT syndrome, sick sinus syndrome type 2, idiopathic VF, and heart block type 1A patients.5 Several individual mutations in the SCN5A gene have been associated with both a cardiomyopathy phenotype, such as ARVC or DCM, and VTs.5 Furthermore, we report here previously unpublished data from a family in which a SCN5A mutation was found in both a father and daughter, but these two family members presented with different phenotypes: the father with LVNC and the daughter with BrS (Table 1, Figure 1A). The son was negative for the SNC5A mutation and presented with neither LVNC nor BrS. These observations indicate that SCN5A may be important in cases of overlap between BrS and these various other cardiomyopathies and arrhythmias.
Table 1.
Patients with left ventricular non-compaction (LVNC) and Brugada syndrome (BrS) share SCN5A mutation
| Father | Daughter | Son | |
|---|---|---|---|
| Age (years) | 61 | 37 | 33 |
| Symptoms | Asymptomatic | Asymptomatic | |
| Phenotype | LVNC with ↓EF | BrS | Early repolarization on ECG |
| SCN5A mutation | Yes | Yes | No |
| Ajmaline test | Negative | Positive | Negative |
| EPS test for VT/VF inducibility | Positive | Negative | |
| ICD | Yes | Yes | No |
A family in which a SCN5A mutation was found in both a father and daughter, but these two family members presented with different phenotypes: the father with LVNC and the daughter with BrS. The son was negative for the SNC5A mutation and presented with neither LVNC nor BrS.
Figure 1.
(A) Family members with shared SCN5A mutation exhibit different ECG phenotypes. A father and daughter both positive for a SCN5A mutation exhibit different phenotypes during ajmaline challenge. The father is negative, whereas the daughter is affected by BrS. The son, negative for the SCN5A mutation, is also showing a negative ajmaline test. (B) Family members with shared MYBPC3 mutation exhibit different ECG phenotypes. A father and daughter both positive for a MYBPC3 mutation exhibit different ECG phenotypes during ajmaline challenge. The father is negative, whereas the daughter shows a mild positive response to ajmaline. The son, also positive for the MYBPC3 mutation, exhibits a spontaneous BrS type 1 pattern.
Arrhythmogenic right ventricular cardiomyopathy, LVNC, HCM, and DCM have all been associated with genetic mutations of sarcomeric proteins.6 Arrhythmogenic right ventricular cardiomyopathy is caused by mutations in genes encoding for desmosomal proteins (plakoglobin), desmoplakin, plakophilin-2, desmoglein-2, and desmocollin-2.2 Desmosomal protein gene mutations are the most common in DCM patients, although lamin A/C (LMNA) and desmin mutations are commonly found in those with conduction diseases.2 Hypertrophic cardiomyopathy has been associated with troponin C, troponin I, myosin heavy chain, and myosin regulatory light chain mutations, while troponin T mutations are associated with both HCM and DCM.7 Additionally, to the best of our knowledge, we report here, for the first time, a family in which a MYBPC3 mutation was found in a father, daughter, and son, but these family members presented with different phenotypes: the father with HCM and the daughter and son with BrS (Table 2, Figure 1B). Thus, patients with cardiomyopathies or BrS exhibiting sarcomeric mutations may have common genetic pathways that ultimately diverge into different phenotypes. Further analyses are needed to better understand the genetic alterations that occur in patients with each of these phenotypes and to better understand the relationship between genotype and phenotype.
Table 2.
Patients with hypertrophic cardiomyopathy (HCM) and Brugada syndrome (BrS) share MYBPC3 mutation
| Father | Daughter | Son | |
|---|---|---|---|
| Age (years) | 53 | 23 | 20 |
| Symptoms | Palpitation | Asymptomatic | Asymptomatic |
| Phenotype | HCM | BrS | BrS |
| MYBPC3 mutation | Yes | Yes | Yes |
| Ajmaline test | Negative | Mild Positive | (Spontaneous BrS type 1) |
| EPS test for VT/VF inducibility | Negative | Positive | Positive |
| ICD | Yes | Yes |
A family in which a MYBPC3 mutation was found in a father, daughter, and son, but these family members presented with different phenotypes: the father with HCM and the daughter and son with BrS.
Channelopathies, sarcomeropathies, or both?
The arrhythmic abnormality observed in BrS is considered to be a channelopathy characterized by ion channel dysfunction and has been linked to mutations in genes encoding for subunits of cardiac sodium, potassium, and calcium channels, as well as in genes involved in the trafficking or regulation of these channels, including ABCC9, CACNA1C, CACNA2D1, CACNB2, CACNB2b, FGF12, GPD1L, HCN4, KCND2, KCND3, KCNE5, KCNE3, KCNH2, KCNJ8, PKP2, RANGRF, SCN1B, SCN2B, SCN3B, SCN5A, SCN10A, SEMA3A, SLMAP, and TRPM4.8 Of these genes, SCN5A is currently the focus of most study, since it is the most common BrS genotype and accounts for 15–30% of BrS cases.4 However, of note, most BrS patients do not have the SCN5A mutation, so while it is the most common genetic mutation known to be associated with BrS, no single causal genetic factor appears to link all BrS patients.
Arrhythmogenic right ventricular cardiomyopathy, LVNC, and DCM have been associated with genetic mutations of ion channels,5 while these and HCM have been associated with sarcomeric protein mutations.6 While BrS is generally considered to be an ion channelopathy, an α-tropomyosin mutation appears to link HCM and BrS in one study,9 suggesting a role for sarcomeropathies in BrS. In fact, altered sarcomeric properties have been directly implicated in arrythmogenic sudden death.10 This indicates that phenotypic overlap of BrS and HCM may occur as a result of sarcomeropathies. Further studies are warranted to better understand what genetic commonalities of sarcomeric mutations may exist between BrS and various cardiomyopathies and how those mutations contribute to disease progression.
Epicardial mapping
While the phenotypes of the various cardiomyopathies presented herein are diverse, they share in common with each other and with BrS an identifiable electrophysiological AS, featured by areas exhibiting fragmented and prolonged electrograms (EGMs) also showing late and discrete activity. In some type of cardiomyopathies, such abnormal areas are located in regions of unhealthy tissue being characterized by low-voltage EGMs. However, this is not the case in patients affected by BrS, as the AS is generally characterized by EGMs with normal or near-normal voltage.11
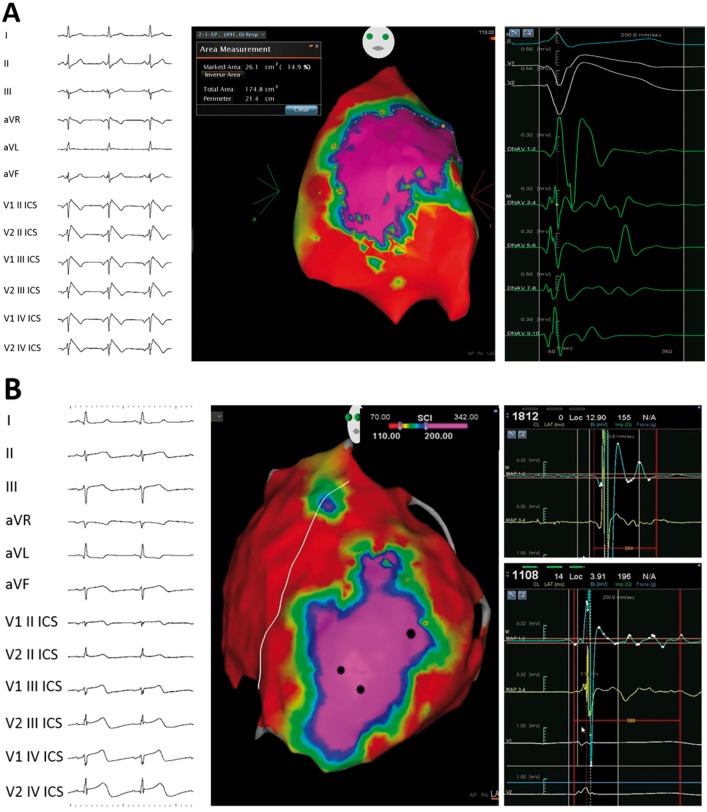
In BrS, the AS responsible for ECG abnormalities appears to be located in the epicardial surface of the right ventricular outflow tract. Therefore, recent clinical studies highlighted the value of epicardial radiofrequency catheter ablation of such regions, resulting in the normalization of the ECG pattern and reduction or elimination of life-threatening VA recurrences.11 In BrS and various cardiomyopathies, the exact anatomical position and extent of the AS can be determined using endo-epicardial mapping to indicate the pathological substrate that could be characterized by both EGM fragmentation with and without low voltage amplitude and late activity. The potential duration map is created by collecting the duration of each EGM. As a result, a colour-coded map is obtained showing the regions displaying the shortest (<110 ms cut-off, red colour) and the longest (>200 ms cut-off, purple colour) durations (Figure 2). The three-dimensional mapping used to localize the AS can then be used to guide ablation, aiming at abolition of abnormal potentials and elimination the recurrence of life-threatening VA preventing SCD. In fact, catheter ablation of the AS in patients with BrS at higher risk is an established procedure, performed routinely by our group.11 We have shown that ablation may normalize the ECG pattern and prevent ventricular malignant arrhythmias in BrS patients, relieving the patient from the consequences of frequent ICD discharges or storms.
Figure 2.
Potential duration maps (PDMs). (A) Coved-type Br-ECG pattern (on the left), epicardial right ventricular PDM with an area exhibiting EGMs with duration >200 ms (purple, in central panel), and example of abnormal potentials found in the purple area (on the right) is shown. (B) ECG (on the left), left and right ventricular epicardial PDM (centre), and examples of abnormal EGMs (on the right) of a patient affected by left ventricular non-compaction is shown. Epicardial PDM shows an area exhibiting abnormal potentials with duration >200 ms (purple) in the epicardial apex of the left ventricle.
Radiofrequency catheter ablation established to ablate the AS in BrS may be applied also to eliminate the AS in these aforementioned cardiomyopathies to reduce the incidence of VAs, limit ICD shocks, and prevent arrhythmogenic SCD. In other words, such as BrS patients, cardiomyopathy patients could benefit from the identification and elimination of the AS as a curative, rather than palliative, treatment of the disease. Current research is focused on improving the treatment of BrS patients by eliminating the AS. Thus, the treatment of this AS in BrS can serve as a model for the treatment of the AS in other diseases.
Ablation of the AS area in cardiomyopathies is important for patients typically deemed to be ‘high-risk’, with severe presentation of clinical signs or symptoms, or with a family history of SCD. However, in the future, it could be offered to many other patients presenting with an AS, as a potential alternative to an ICD. In fact, in the largest study to-date on the effects of substrate-based mapping/ablation in BrS,11 we have previously demonstrated the importance of AS mapping/ablation, even in patients previously thought to be at lower risk of having VT/VF, cardiac arrest, and SCD. In fact, many patients previously thought to be ‘lower-risk’ exhibited an AS provoked by ajmaline, suggesting that these ‘lower-risk’ patients could be at risk of VT/VF and SCD under certain circumstances known to provoke VT/VF, such as during fever or an increase in vagal tone, such as while resting or after a large meal. Since BrS and the many other phenotypes listed above are known to be inheritable, the epicardial mapping described herein can be useful in identifying family members who may be at risk of developing VT/VF, even in the absence of overt heart disease and spontaneous normal ECG patterns.
Conclusions
Identification and ablation of the AS area in cardiomyopathies, including ARVC, LVNC, HCM, and DCM, can be achieved using techniques currently available for the treatment of the AS in BrS patients, preventing the recurrence of VT/VF and reducing or eliminating the need for shock therapy. Three-dimensional electroanatomical mapping together with ajmaline challenge is important to reveal the extent of the AS to be ablated. We demonstrate for the first time a family with a shared sarcomeric mutation who present with different phenotypes—HCM or BrS. Future studies and longer follow-up times are warranted to understand the fullest duration of the therapeutic potential of this ajmaline and map-guided ablation therapy.
Conflict of interest: none declared.
References
- 1. Magi S, Lariccia V, Maiolino M, Amoroso S, Gratteri S.. Sudden cardiac death: focus on the genetics of channelopathies and cardiomyopathies. J Biomed Sci 2017;24: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ; ESC Scientific Document Group. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–2867.26320108 [Google Scholar]
- 3. Li A, Kaura A, Sunderland N, Dhillon PS, Scott PA.. The significance of shocks in implantable cardioverter defibrillator recipients. Arrhythm Electrophysiol Rev 2016;5:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kapplinger JD, Tester DJ, Alders M, Benito B, Berthet M, Brugada J, Brugada P, Fressart V, Guerchicoff A, Harris-Kerr C, Kamakura S, Kyndt F, Koopmann TT, Miyamoto Y, Pfeiffer R, Pollevick GD, Probst V, Zumhagen S, Vatta M, Towbin JA, Shimizu W, Schulze-Bahr E, Antzelevitch C, Salisbury BA, Guicheney P, Wilde AAM, Brugada R, Schott J-J, Ackerman MJ.. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm 2010;7:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zaklyazminskaya E, Dzemeshkevich S.. The role of mutations in the SCN5A gene in cardiomyopathies. Biochim Biophys Acta 2016;1863:1799–1805. [DOI] [PubMed] [Google Scholar]
- 6. Bang ML. Animal models of congenital cardiomyopathies associated with mutations in Z-line proteins. J Cell Physiol 2017;232:38–52. [DOI] [PubMed] [Google Scholar]
- 7. Chung JH, Biesiadecki BJ, Ziolo MT, Davis JP, Janssen PM.. Myofilament calcium sensitivity: role in regulation of in vivo cardiac contraction and relaxation. Front Physiol 2016;7:562.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brugada R, Campuzano O, Sarquella-Brugada G, Brugada P, Brugada J, Hong K.. Brugada syndrome In Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH. et al. , eds. GeneReviews(R). Seattle, WA; 1993. [Google Scholar]
- 9. Mango R, Luchetti A, Sangiuolo R, Ferradini V, Briglia N, Giardina E, Ferrè F, Helmer Citterich M, Romeo F, Novelli G, Sangiuolo F.. Next generation sequencing and linkage analysis for the molecular diagnosis of a novel overlapping syndrome characterized by hypertrophic cardiomyopathy and typical electrical instability of Brugada syndrome. Circ J 2016;80:938–949. [DOI] [PubMed] [Google Scholar]
- 10. Yar S, Monasky MM, Solaro RJ.. Maladaptive modifications in myofilament proteins and triggers in the progression to heart failure and sudden death. Pflugers Arch 2014;466:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pappone C, Brugada J, Vicedomini G, Ciconte G, Manguso F, Saviano M, Vitale R, Cuko A, Giannelli L, Calovic Z, Conti M, Pozzi P, Natalizia A, Crisà S, Borrelli V, Brugada R, Sarquella-Brugada G, Guazzi M, Frigiola A, Menicanti L, Santinelli V. Electrical substrate elimination in 135 consecutive patients with Brugada syndrome. Circ Arrhythm Electrophysiol 2017;10:e005053. [DOI] [PubMed] [Google Scholar]