Abstract
Atherosclerosis is a chronic degenerative disease, with a significant inflammatory component, characterized by phases of rapid activation leading to important clinical events, such as myocardial infarction. One of the major challenges of modern cardiology is limiting the progression of atherosclerotic disease and anticipating the phases of instability as to limit its consequences. In this contest modern techniques of intra-coronary imaging, such as optical coherence tomography, could have a pivotal role in identifying patients at higher risk of acute events in the short term. The purpose of the CLIMA study is to identify and map the vulnerability criteria of atherosclerotic coronary plaques in the individual patient, and provide a personalized risk score for coronary events.
Keywords: Vulnerable plaque, Intravascular imaging, Acute myocardial infarction
Atherosclerosis as inflammatory disease
Atherosclerosis is customary referred to as a chronically progressive degenerative vascular disease affecting the intima of the medium and large size arteries.1 The condition is characterized by the progressive buildup and oxidation, at endothelial level, of lipoproteins, and consequent development of chronic inflammatory state, which, in time, leads to rearrangement of the vascular wall with formation of lesions known as atheroma or atherosclerotic plaques.2 In practical terms atherosclerosis affects the function of the vascular endothelium, with a progressive thickening of the intima generated by the accrual of lipids (fats), and proliferation of fibrous tissue. The endothelial dysfunction favours the progression of atherosclerosis, thus creating a vicious circle, shaping a dynamic and progressive disease.3 The progression of atherosclerosis is usually slow and clinically asymptomatic during the first decades of life. The deleterious effects of the disease start appearing during the 40–50 years of age, particularly in susceptible individuals with cardiovascular risk factors (e.g. cigarettes smoking, diabetes), who manifest acute ischaemic events affecting the brain, the heart, or the limbs.1 The acute events are usually consequent to a rupture plaque which, in turn, activates a thrombotic event of variable intensity. In practical terms, endothelial dysfunction and the related inflammatory state, enhance the growth of the lipid component inside the vessel wall, thus thinning the surrounding collagen fibrous cap. These events determine an instability of the atheroma, which becomes more vulnerable to the circulatory haemodynamic stress (e.g. shear stress, variation of arterial blood pressure). The end result is the erosion or the ulceration of the lipid plaque, and the exposure, on the flow side of the vessel, of highly thrombogenic material, such as tissue factors and collagen; what ensues is platelet and coagulation cascade activation, intended to repair and re-establish the integrity of the vessel wall.4 The reparative process could have a favourable effect, and lead to chronic progression of the atherosclerotic process, or establish a thrombus formation causing a critical primary reduction of the blood flow, or its complete blockage (vascular occlusion). For the cardiologist, the sudden critical reduction of the blood flow translates, clinically, in the acute coronary syndrome (ACS), with ST elevation myocardial infarction (STEMI) or sudden cardiac death its most ominous manifestations. Epidemiologic data on atherosclerosis concern mainly cardiovascular mortality, which in the western countries, represent the first cause of death in both sexes. Considering the multifactorial aetiology and the irreversibility of the vessel wall lesions, the therapeutic intervention should aim, initially, at primary prevention (prevention and/or control of progression of atherosclerosis), with the use of behavioural and/or pharmacologic measure. While the behavioural measure could, and should, be implemented for the population in general, the extensive use of drugs in primary prevention settings is neither recommended by the guidelines, nor is economically sustainable. Hence the importance of identifying patients at higher cardiovascular risk, by accurately staging the atherosclerotic disease, also during its clinically silent phase.5
Intra-coronary imaging with optical coherence tomography in the assessment of atherosclerosis
Optical coherence tomography (OCT) is an imaging technique employing retro-reflection of light at near infrared frequency from the optical interface of the tissue to generate high resolution images (10–15 µm).6,7 Coronary application of OCT allows for atherosclerotic plaque visualization and its functional characterization8–10 with high degree of specificity and sensibility. In particular, as compared to other imaging techniques (e.g. intravascular ultrasound), OCT allows for identification of the cellular components of the plaque10,11: it is possible to measure the density of inflammatory cells utilizing algorithms exploiting tissues acoustic properties12; it is reliable enough as to measure the small vascular structures feeding the coronary lesions (vasa vasorum)13; it is the sole presently available technology able to quantify the component of the atherosclerotic plaque, among which the extension of the lipid pool and the thickness of the fibrous cap in vivo.7,14 The major shortcoming of this technique is its limited tissue penetration, such that an optimal plaque definition is possible only in its superficial layer, not deeper than 500 µm. This limitation is the reason why OCT doesn’t have the same accuracy for all the various vascular districts. Optical coherence tomography is then the imaging technique with the highest potential for identification of vulnerable plaques, regardless their extension/severity (e.g. angiographically non-critical lesions) (Figure 1). Post-mortem studies revealed that vulnerable plaques are rich in inflammatory cells (mostly macrophages and lymphocytes, and lesser amount of mastocytes), producing lytic enzymes (metalloprotease) able to breakdown the collagen of the fibrous cap, which then becomes vulnerable to the haemodynamic stress. The density of the neo-formed vessels has also been considered a factor in the progression/instability of the plaque, in that it provide for the influx of monocytes/macrophages in the lesions, as well as the intra-plaque haemorrhage, originating from the rupture of the neo-formed vessels. Furthermore, OCT offers a better visualization of intra-plaque calcifications, which according to some researchers, illustrate the stage of the atheroma, and represent a point of less resistance to the mechanical solicitations.15
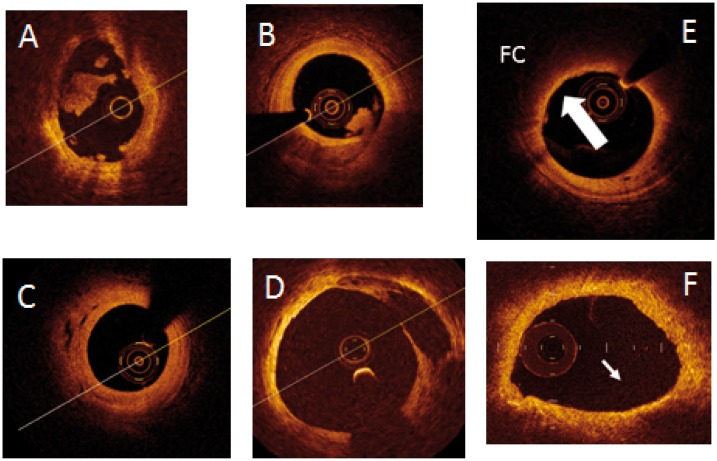
Figure 1.
Ulcerated lesions responsible for coronary thrombosis in the acute phase (A and B) and not (C and D). (A) Acute erosion with thrombosis; (B) acute ulceration with thrombosis; (C) evolution of previously eroded plaque; (D) evolution of previously ulcerated plaque. Examples of vulnerable plaques (E and F); (E) lesion with high lipid component and thin fibrous cap (arrow); (F) lesion with high inflammatory component (arrow).
Rationale for a novel use of intra-coronary optical coherence tomography
During the last decade several groups utilized intracoronary OCT with the purpose to validate, in vivo, the findings of post-mortem studies, and to understand new pathophysiologic scenarios. Serial studies from our group outlined that the evolution/healing mechanisms of the culprit plaques, responsible for ACS, are heterogeneous and not always predictable.16 The opportunity to employ OCT, in vivo, in serial studies, is of significant advantage over the post-mortem studies.17,18 It is, in fact possible to evaluate the evolution of vulnerable plaques both from the morphologic and functional aspect (variation of the thickness of the fibrous cap, and reduction of the inflammatory component), as well as the efficacy of the lipid lowering therapy on the progression/regression of coronary atherosclerosis.
The CLIMA study
The CLIMA study (clinical trial.gov NCT02883088) was conceived as a prospective observational registry to investigate the correlation between the morphology of atherosclerotic plaques, as assessed by OCT analysis, and the risk of future cardiovascular events in the mid (months), and long-term follow-up (years). This is a multicentre study, involving high volume units with significant experience in intravascular imaging, aiming at collecting data on all patients who underwent OCT evaluation of the left anterior descending coronary artery in its mid-proximal portion (at least 30 mm), which was not revascularized (percutaneous and/or surgical approach). The enrolled patients will be regularly followed both with telephone and office visits, for a 10 years period. During the follow-up all new cardiovascular events and cardiology related hospitalizations will be recorded; the clinical documentation will be carefully collected and transferred in a dedicated database which will be evaluated by a specific committee. The comprehensiveness and the accuracy of the data in the registry will be constantly verified, in an effort to limit incomplete data (<5% for each variable recorded).
Study endpoints
Primary endpoint of the study is the correlation between OCT criteria of plaque vulnerability (derived from post-mortem studies), and the actual incidence of cardiac events attributable to the atherosclerotic plaques. A map of the coronary lesions will be constructed for each single patient, and the tendency toward instability over time will be assessed, according to the presence of OCT vulnerability criteria. In detail, will be evaluated the effectiveness of a risk scoring system based on several OCT instability criteria present concurrently in the same plaque. For the study, only patients with non-critical atherosclerotic plaques in the mid-proximal portion of the left anterior descending coronary artery will be enrolled, and only major cardiac events (cardiac death and anterior myocardial infarction [STEMI/NSTEMI]) immediately attributable to such plaques will be considered.
Optical coherence tomography criteria for vulnerability
Based on post-mortem studies and the present pathophysiologic understanding the following OCT criteria were selected for the definition of vulnerable atherosclerotic plaque:
Minimal luminal area at the level of the plaque <3.5 mm2
Lipid pool extension inside the plaque >180°
Fibrous cap thickness <75 µm
Presence of macrophage inflammatory infiltrate
Presence of calcific nodules
Presence of neo-vascularization (vasa-vasorum) and/or intra-plaque haemorrhage
Presence of cholesterol crystals (within the lipid pool)
Optical coherence tomography images will be evaluated by two independent specialists.
Study population and preliminary results
After a first initial phase which included 500 patients, necessary to test the research hypothesis and to calculated the necessary statistical sample, a total of 1003 patients (1776 lipid plaques) have been enrolled after OCT evaluation in the setting of ACS or stable ischaemic cardiomyopathy. The enrolment process started in January 2013 and was concluded in December 2016, and presently all the enrolled patients reached, at least, the minimum follow-up of 1 year; the rate of major cardiac events has been 4%. The ability of OCT to study plaque morphological features with unprecedented detail, including the accurate assessment of fibrous cap thickness and signs of local inflammation enabled the identification of a population subgroup with a high risk of developing hard events. Indeed, the CLIMA registry applied for the first time in a prospective study a novel OCT grading based on the simultaneous presence of four adopted vulnerability criteria including the presence of macrophages.
Conflict of interest: none declared.
References
- 1.Lee Goldman e Andrew Shafer. Chapter 70: Atherosclerosis, Thrombosis, and Vascular Biology in Glodman's Cecil Medicine. 24a ed.Philadelphia: Elsevier; 2012. [Google Scholar]
- 2. Erling F, Valentin F.. Chapter 32: Atherothrombosis: Disease Burden, Activity, and Vulnerability in Hurst's the Heart. 14a ed Philadelphia, PA, USA: McGraw-Hill Education; 2017. [Google Scholar]
- 3. De Caterina R. Endothelial dysfunctions: common denominators in vascular disease. Curr Opin Clin Nutr Metab Care 2000;3:453–467. [DOI] [PubMed] [Google Scholar]
- 4. Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R.. Update on acute coronary syndromes: the pathologists' view. Eur. Heart J 2013;34:719–728. [DOI] [PubMed] [Google Scholar]
- 5. Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Juhani Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang I-K, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT.. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation 2003;108:1664–1672. [DOI] [PubMed] [Google Scholar]
- 6. Prati F, Regar E, Mintz GS, Arbustini E, Di Mario C, Jang I-K, Akasaka T, Costa M, Guagliumi G, Grube E, Ozaki Y, Pinto F, Serruys PWJ.. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J 2010;31:401–415. [DOI] [PubMed] [Google Scholar]
- 7. Prati F, Guagliumi G, Mintz GS, Costa M, Regar E, Akasaka T, Barlis P, Tearney GJ, Jang I-K, Arbustini E, Bezerra HG, Ozaki Y, Bruining N, Dudek D, Radu M, Erglis A, Motreff P, Alfonso F, Toutouzas K, Gonzalo N, Tamburino C, Adriaenssens T, Pinto F, Serruys PWJ, Di Mario C.. Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur Heart J 2012;33:2513–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suter MJ, Nadkarni SK, Weisz G, Tanaka A, Jaffer FA, Bouma BE, Tearney GJ.. Intravascular optical imaging technology for investigating the coronary artery. J Am Coll Cardiol Cardiovasc Imaging 2011;4:1022–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gonzalo N, Escaned J, Alfonso F, Nolte C, Rodriguez V, Jimenez-Quevedo P, Bañuelos C, Fernández-Ortiz A, Garcia E, Hernandez-Antolin R, Macaya C.. Morphometric assessment of coronary stenosis relevance with optical coherence tomography: a comparison with fractional flow reserve and intravascular ultrasound. J Am Coll Cardiol 2012;59:1080–1089. [DOI] [PubMed] [Google Scholar]
- 10. Jang I-K, Bouma BE, Kang D-H, Park S-J, Park S-W, Seung K-B, Choi K-B, Shishkov M, Schlendorf K, Pomerantsev E, Houser SL, Aretz HT, Tearney GJ.. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol 2002;39:604–609. [DOI] [PubMed] [Google Scholar]
- 11. Kawasaki M, Bouma BE, Bressner J, Houser SL, Nadkarni SK, MacNeill BD, Jang I-K, Fujiwara H, Tearney GJ.. Diagnostic accuracy of optical coherence tomography and integrated backscatter intravascular ultrasound images for tissue characterization of human coronary plaques. J Am Coll Cardiol 2006;48:81–88. [DOI] [PubMed] [Google Scholar]
- 12. Di Vito L, Agozzino M, Marco V, Ricciardi A, Concardi M, Romagnoli E, Gatto L, Calogero G, Tavazzi L, Arbustini E, Prati F.. Identification and quantification of macrophage presence in coronary atherosclerotic plaques by optical coherence tomography. Eur Heart J Cardiovasc Imaging 2015;16:807–813. [DOI] [PubMed] [Google Scholar]
- 13. Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho J-M, Chowdhary S, Costa MA, de Silva R, Dijkstra J, Di Mario C, Dudeck D, Falk E, Feldman MD, Fitzgerald P, Garcia H, Gonzalo N, Granada JF, Guagliumi G, Holm NR, Honda Y, Ikeno F, Kawasaki M, Kochman J, Koltowski L, Kubo T, Kume T, Kyono H, Lam CCS, Lamouche G, Lee DP, Leon MB, Maehara A, Manfrini O, Mintz GS, Mizuno K, Morel M-A, Nadkarni S, Okura H, Otake H, Pietrasik A, Prati F, Räber L, Radu MD, Rieber J, Riga M, Rollins A, Rosenberg M, Sirbu V, Serruys PWJC, Shimada K, Shinke T, Shite J, Siegel E, Sonada S, Suter M, Takarada S, Tanaka A, Terashima M, Troels T, Uemura S, Ughi GJ, van Beusekom HMM, van der Steen AFW, van Es G-A, van Soest G, Virmani R, Waxman S, Weissman NJ, Weisz G.. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol 2012;59:1058–1072. [DOI] [PubMed] [Google Scholar]
- 14. Mulligan-Keohe MJ. The vasa vasorum in diseased and non-diseased arteries. Am J Physiol 2010;298:H295–H305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Virmani R, Burke AP, Farb A, Kolodgie FD.. Pathology of the vulnerable plaque. J Am Coll Cardiol 2006;47:C13–C18. [DOI] [PubMed] [Google Scholar]
- 16. Souteyrand G, Arbustini E, Motreff P, Gatto L, Di Vito L, Marco V, Amabile N, Chisari A, Kodama T, Romagnoli E, Tavazzi L, Crea F, Narula J, Prati F.. Serial optical coherence tomography imaging of ACS-causing culprit plaques. EuroIntervention 2015;11:319–324. [DOI] [PubMed] [Google Scholar]
- 17. Gatto L, Marco V, Contarini M, Prati F.. Atherosclerosis to predict cardiac events: where and how to look for it. J Cardiovasc Med 2017;18:e154–e156. [DOI] [PubMed] [Google Scholar]
- 18. Gatto LM, Albertucci PF.. Primary prevention of coronary artery disease: let’s start with calcium score. J Cardiovasc Med 2018;19:e103–e106. [DOI] [PubMed] [Google Scholar]