Abstract
Myocarditis is an infectious–inflammatory disease often superimposed to individual genetic background which could favour or inhibit its progression into a chronic heart muscle disorder (most often dilated cardiomyopathy, rarely arrhythmogenic, or right-sided cardiomyopathy). Post-myocarditis cardiomyopathy is likely caused by a complex interaction between the viral infection and an individual predisposition. Some viruses are able to highlight a clinical phenotype replicating a model similar to the genetically determined conditions, while other can affect the resolution or the progressive remodelling of the left ventricle after the infectious process. The identification of specific individual genetic backgrounds, or genes favouring the progression of the disease, are important future research goals for precision medicine aiming at a specific and individualized treatment for patients affected with myocarditis.
Keywords: Myocarditis, Cardiomyopathy, Dilated cardiomyopathy, Genetics
Introduction
Since 25 April 1953, when James Watson and Francis Crick delineated the structure of Deoxyribonucleic Acid (DNA), researchers have outlined multiple and complex systems regulating the interaction between genes and proteins. The axiom ‘one gene-one protein’ has been progressively modified till becoming ‘one sequence-multiple effects’. DNA, in fact, does not only include protein producing information but is loaded with other fundamental components [from the histone interactions to the non-coding Ribonucleic Acid (RNA) sequences]. Accordingly the phenotypic expression of a disease stem from the interplay among a complex network of multiple genes, bio-molecular mechanisms, and their reaction to the offending agent. Therefore, the advance of precision medicine is representing also a clinical challenge.
The goal of precision medicine implies the interaction between the environmental factors and the genetic make-up of the individual patient as to optimize their outcomes. Myocarditis is an infectious–inflammatory disease often superimposed to individual genetic background which could favour or inhibit its progression into a chronic heart muscle disorder (most often dilated cardiomyopathy (DCM), rarely arrhythmogenic, or right-sided cardiomyopathy). The complex interactions between these two components are summarized in Table 1.
Table 1.
Key point in the interaction between offending agent and genetic background for the predisposition to myocarditis and its possible evolution towards cardiomyopathy
| 1. Myocarditis is an infectious/inflammatory condition but individual genetic background plays an important role in the predisposition to the infection/inflammation and in its possible evolution towards dilated cardiomyopathy. |
| 2. Some viruses release specific proteases able to degrade or integrate with proteins of cardiac myocytes thus reproducing condition similar to the genetically determined disorders. |
| 3. Some genotypes can affect either the resolution or, on the other hand, the progressive left ventricular remodelling |
Dilated cardiomyopathy: the importance of aetiology
Dilated cardiomyopathy is a myocardial condition characterized by dilatation and systolic dysfunction of the left ventricle, not caused by an identifiable pathological process such as ischaemia, valvular disease, or hypertension.1
Considering the wide pathogenic variability, aetiological characterization is a mainstay of clinical management of DCM. There are several potentially reversible causes of left ventricular dysfunction, such as myocarditis, but also tachycardia induced myopathy, or related to catecholamine or toxic substances (alcohol/cocaine), all of which are non-genetic/non-familial, and amenable to complete resolution, as the offending agent is removed, particularly if done in timely fashion. The new techniques of genetic sequencing, such as the next generation sequencing, expanded our knowledge, during the last few years, of the genetic causes of left ventricular dysfunction. Presently a genetic origin is recognized in up to 50% of the patients with DCM.2 To date over 50 genes have been identified, encoding for proteins responsible for several cellular structures, probably involved in common pathogenic pathways (Table 2).3–6 Albeit the number has been progressively decreasing, there still remain a significant number of idiopathic DCM, representing almost 20% of non-ischaemic left ventricular dysfunctions. This number embodies our inability to provide a complete aetiological characterization of the condition, although the vast majority of the cases are likely connected to not yet identified genetic mutations, or predominantly chronic inflammatory processes. In this contest myocarditis, with its possible evolution towards DCM, constitute the area of most complex interaction among genetic-immune-environmental causes.
Table 2.
Genes most frequently implicated in genetic dilated cardiomyopathy
| Gene | Protein | Percentage of cases with DCM | Possible association/overlap |
|---|---|---|---|
| Autosomal dominant transmission | |||
| TTN 3 | Titin | 10–25 | Limb girdle muscular dystrophy |
| Myopathy with cardiac and respiratory muscular involvement | |||
| Tibial muscle dystrophy | |||
| LMNA | Pre-lamin-A/C | 6 | Type 2 Charcot–Marie–Tooth neuropathy |
| Emery-Dreifuss muscular dystrophy | |||
| Hutchinson–Gilford Progeria | |||
| Werner syndrome | |||
| MYH7 | Myosin-7 | 4.2 | Laing myopathy |
| Hypertrophic cardiomyopathy | |||
| Non-compacted ventricle | |||
| MYH7 associated myopathy | |||
| MYH6 | Myosin-6 | 3–4 | Hypertrophic cardiomyopathy |
| SCN5A | Sodium channel subunit α Type 5 | 2–4 | Long QT syndrome—Type 3 |
| Brugada syndrome | |||
| Idiopathic ventricular fibrillation | |||
| Sinus node disease | |||
| Conduction system disorders | |||
| MYBPC3 | Myosin-binding protein | 2–4 | Hypertrophic cardiomyopathy |
| TNNT2 | Troponin T | 2.9 | Hypertrophic cardiomyopathy |
| Non-compacted ventricle | |||
| Restrictive cardiomyopathy TTNT2 related | |||
| BAG3 | Chaperon regulating protein 3 | 2.5 | Progressive myofibrillar myopathy |
| ANKRD1 | Cardiac ankyrin | 2.2 | |
| RBM20 | RNA binding motif protein | 1.9 | |
| TMPO | Thymopoietin | 1.1 | |
| LDB3 | Proteina Enigma 3 | 1 | Myofibrillar myopathy |
| TCAP | Telethonin | 1 | Hypertrophic cardiomyopathy |
| Limb girdle muscular dystrophy | |||
| VCL | Vinculin | 1 | |
| TPM1 | Tropomyosin 1 | <2 | Hypertrophic cardiomyopathy |
| TNNI3 | Troponin I | 1.3 | Hypertrophic cardiomyopathy |
| Restrictive cardiomyopathy | |||
| TNNC1 | Troponin C | <1.3 | Hypertrophic cardiomyopathy |
| ACTC1 | Actin | <1 | Hypertrophic cardiomyopathy |
| ACTN2 | α-actin | <1 | Hypertrophic cardiomyopathy |
| DES | Desmin | <1 | Desminopathy |
| Myofibrillar myopathy | |||
| NEXN | Nexilin | <1 | Hypertrophic cardiomyopathy |
| PSEN | Presenilin-1 e−2 | <1 | Alzheimer syndrome |
| SGCD | Sarcoglycan delta | <1 | Limb girdle muscular dystrophy |
| PLN | Phospholamban | ||
| DSG2 | Desmoglein-2 | ||
| X-linked transmission | |||
| DMD | Dystrophin | Duchenne and Becker muscular dystrophy | |
| TAZ | Tafazzin | Barth syndrome | |
| Type 2 endocardial fibroelastosis | |||
| Non-compacted ventricle | |||
| Autosomal recessive transmission | |||
| TNNI3 | Troponin I | <1 | |
Modified from Hershberger e Morales, Gene Review, 2015. Dilated Cardiomyopathy Overview.
Myocarditis as a cause of dilated cardiomyopathy
Myocarditis constitutes a specific subgroup of myocardial diseases, sometimes involving the pericardium, with inflammatory aetiology. The diagnosis of myocarditis is based on histological, immunological, and immune-histochemical criteria.7 Although the definition of myocarditis is well-described, the disease is heterogeneous and, at times, undetermined as far as pathophysiology, aetiology, clinical presentation, and natural history.8 In fact potential causes of myocarditis are infectious (bacteria, fungi, and protozoans), as well as non-infectious agents (toxins, drugs, and physical agents), and auto-immune conditions, hypersensitivity, and hyper-catecholamines states.9 Nonetheless 95% of myocarditis are secondary to a viral infection, and its consequent immune response determining a pattern of lymphocytic myocarditis (Table 3).9–11 The pathophysiological mechanisms of lymphocytic myocarditis are the result of the interaction between the offending agent and the host immune response,12 and include three well-recognized phases8:
Table 3.
Main cardiotrophic viruses and prevalence of the viral genome persistence in patients with biopsy-proven myocarditis
| Cardiotrophic virus | Percentage |
|---|---|
| Coxsackievirus B3 (CVB3) | 14–32.6 |
| Parvovirus B19 (PB19) | <1–36.6 |
| Human herpesvirus 6 (HHV6) | 10.5 |
| Adenovirus | 8.1–23 |
| Multiple Infections (60% of the cases = PB19 + HHV6) | 12.2 |
| Rare cases | |
| Citomegalovirus | <3 |
| Epstein–Barr virus | <1 |
| Herpes simplex virus | <1 |
| Influenza virus | <2 |
Acute phase (1–7 days). Direct infection with resulting cytolysis and apoptosis of the myocytes.
Subacute phase (7 days–1 month). The immune response is specific, and the myocardium is infiltrated with T lymphocytes and macrophages which create tissue damage despite a decrease in viral titration.
Chronic phase (months–years). The immune response attenuates and the condition evolves either towards complete healing ‘restitutio ad integrum’ or post-inflammatory cardiomyopathy.
Myocarditis can occur without symptoms, or resemble a myocardial infarction, or various stage of heart failure, or with a wide range of ventricular and supraventricular arrhythmias, and not infrequently manifest itself with sudden death. The natural history is highly variable spanning from a complete uneventful resolution to the development of post-inflammatory DCM.10 Some forms of myocarditis manifest a favourable response to standard heart failure treatment and immunosuppression and have a satisfactory outcome, others, on the other hand, are characterized by a persistent left ventricular dysfunction.10 The post-myocarditis (or inflammatory) DCM is typified by a mid-term (about 5 years) natural history similar to the classic forms of genetic or idiopathic DCM.13 The early distinction between the two behaviours of myocarditis is the challenge for the future research. Furthermore would be important to evaluate, in the long term, whether post-myocarditis DCM maintains a progressive myocardial remodelling process fuelled by a still active inflammatory process.13
Myocarditis and post-myocarditis dilated cardiomyopathy: the role of genetics
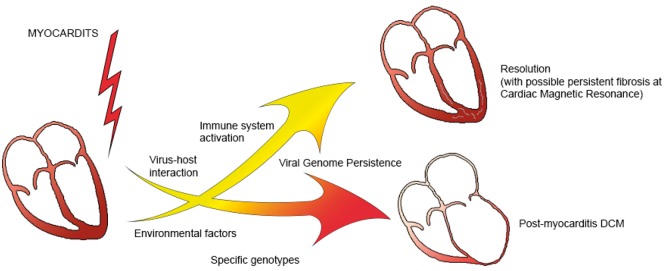
There are four key moment in which the interaction genetics-inflammatory process develops (Figure 1).
Figure 1.
Schematic representation of the processes involved in the progression form myocarditis to dilated cardiomyopathy or its resolution.
Myocarditis as a critical environmental factor in the transition genotype/phenotype in dilated cardiomyopathy
In post-myocarditis DCM, the origin of the condition is probably not homogenous.
There is, in fact, a complex interaction between genetic and non-genetic causes, to the extent that DCM is the final common pathway linking the offending agent and the individual genetic make-up. Even though this general assertion is widely acknowledged, the scientific evidences supporting it are scarce. Presently, for instance, the persistence of viral genome inside the cardio-myocytes is debated, and genetic variants and potentially pathogenic mutations could favour the progression from myocarditis to DCM, or on the other hand, portend to a favourable outcome.10
Genetic predisposition to infection/inflammation
Notwithstanding the high prevalence of infections in the general population, is not completely clear why only a small portion of them develops a myocarditis, and only a portion of them will go on to DCM, emphasizing the role of genetic predisposition in this setting. Our understanding of this problem stems mainly from animal models.
Animal models
Despite being infected with the same virus, only some groups of mice, with definite genetic background, develop myocarditis. The genetic predisposition is characterized by specific polymorphism both of the major histocompatibility complex, in particular human leucocyte antigen-DR4, and of the proteins involved in the activation and intracellular transduction of the signalling following infection.14 Knock-out (KO) mice for genes codifying for proteins involved in the anti-viral immune response [interferon-B, Toll-like receptor (TLR)-3], are more susceptible to both the Coxsackievirus B3 (CVB3) myocarditis and its chronic progression.15 Furthermore the selective removal from the cardiac myocytes of the receptor for Coxsackievirus and adenovirus, or KO mice for the receptor activated by protease-2 (PAR2) seems to characterize phenotypes more resistant to myocarditis despite an high viral load.16 Lastly the complex interaction virus-cardiac myocytes-immune system has a crucial role in the progression of the disease.
Evidences in humans
The evidences derived from human studies are less solid.
The role of genetic mutations pertaining to the innate immunity in the susceptibility to myocarditis in humans has been recently investigated.17 Alike the inferences from the animal models, in human, inflammatory activation and elevated levels of Interleukin (IL)-17, IL-1, tumour necrosis factor (TNF)-α and dysregulation between metalloproteinase and inhibitor of metalloproteinase, seems able to predict the progression towards DCM. Furthermore Casanova group studied the role of immune system mutations in the development of myocarditis by using cardiac myocytes derived from induced Pluripotent Stem Cells (iPSCs) from human fibroblasts. Cardiac myocytes with mutation of the genes codifying for proteins for the interferon-mediated immune response [TLR-3 and Signal Transducer and Activator of Transcription (STAT-1)], were not susceptible to myocarditis. These results are somewhat restricted by the fact that the study contemplates only the response of cardiac myocytes to the infection, disregarding the impact of the circulating factors, as well as the innate and the cell-mediated immune response, crucial for the development of myocarditis in vivo.17 Additionally this model is based on cardiac myocytes not completely mature, producing results which could be at variance from the mature cells.18
The interference of some viruses with proteins reproducing model similar to the genetically determined forms
Enterovirus-produced specific proteases (2A and 3C, critical in the replication and successive cytolysis of cardiac myocytes) could be responsible for a dilatative phenotype in transgenic murine models, because of the selective expression, in the cardiac myocytes of these proteins.19 The 2A and 3C proteases are able to cleavage, among other proteins, dystrophin and dysferlin, nuclear transcription factors and several caspase (cysteine-aspartic acid protease), causing on one hand increased cytoplasmic membrane permeability, and on the other the dysregulation of the reparative processes, and of the transmission of mechanical force, as well as apoptosis and autophagia.16,20,21 More recently, the role of microRNA (miRNA) has been highlighted in the development of the infectious process. Patients with myocarditis have an altered miRNA profile, even though the specific dysregulation pattern has not yet been identified.19 All those mechanisms concur to the predisposition to myocarditis, as well as to its progression towards DCM.
Specific genotypes affect the resolution or the left ventricular remodelling in post-myocarditis dilated cardiomyopathy
The presence of potentially harmful polymorphism of the genes responsible for genetically determined cardiomyopathies appears to be also correlated with acute myocarditis and the development of post-myocarditis DCM.17 A myocardial viral infection can trigger the progression of hypertrophic cardiomyopathy or DCM secondary to Duchenne muscular dystrophy,22 likely through an interaction between viral proteases and cytoskeleton proteins. Furthermore the association between arrhythmogenic cardiomyopathy and myocarditis is well known. Akin to the animal models of myocarditis,23 mutations affecting the genes codifying for cytoskeleton proteins increase the myocardial susceptibility to viral infection as well as the development of DCM. Some mutation affecting cytoskeleton proteins, such as dystrophin, predispose these proteins to cleavage by viral proteases, magnifying the vicious circle leading to unfavourable evolutions.24 Several potentially harmful genetic variants, such as desmoplakin (DSP), prokineticin 2 (PK2), and cardiac troponin (TNNI3), are associated with progression of post-myocarditis DCM, whether other genes associated with cardiomyopathies, SCN5A (sodium channels) and BAG3 (Bcl-2–associated athanogene 3), seems to portend a less severe progression.17
In summary, in humans as well, structural genetic modifications would favour the persistence and progression of myocarditis, whether mutations affecting genes codifying for non-structural proteins portend to a more favourable progression.17,18 It is still not clear if the viral infection is directly responsible for the development of DCM, or, instead, it represents a trigger for the manifestation of a genetically determined DCM (Figure 1). Accordingly the complete understanding of the mechanisms leading to post-myocarditis DCM, and an improved stratification of the patients, necessitates a strict interaction between clinical and basic research. The new genetic sequencing techniques as well the opportunity to test the impact of the inflammatory insult both in vivo and in vitro will help identifying genes involved in this process, leading to ever more individualized treatments.
Conclusions and future perspectives
The key question still remains: is myocarditis the offending cause which uncover the myocardial genetic anomalies, or the genetic alterations render the myocardium prone to myocarditis and its evolution towards DCM?
The answer to this question will necessitate genetic investigation on wide panels of genes of patients with myocarditis both in the regression and in the progressive phase. The identification of genetic mutation or specific genes responsible for the complex interaction between bio-molecular mechanism and offending agents will represent the challenge of the future research aiming at refining precision medicine which goal is to individualize the treatment of patients with myocarditis and preventing its progression towards chronic cardiomyopathy.
Conflict of interest: none declared.
References
- 1. Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kuhl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A.. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2007;29:270–276. [DOI] [PubMed] [Google Scholar]
- 2.Mestroni L, Maisch B, McKenna WJ, Schwartz K, Charron P, Rocco C, Tesson F, Richter A, Wilke A, Komajda M, on behalf of the Collaborative Research Group of the European Human and Capital Mobility Project on Familial Dilated Cardiomyopathy. Guidelines for the study of familial dilated cardiomyopathies. Collaborative Research Group of the European Human and Capital Mobility Project on familial dilated cardiomyopathy. Eur Heart J 1999;20:93–102. [DOI] [PubMed] [Google Scholar]
- 3. Bowles NE, Bowles KR, Towbin JA.. The “final common pathway” hypothesis and inherited cardiovascular disease. The role of cytoskeletal proteins in dilated cardiomyopathy. Herz 2000;25:168–175. [DOI] [PubMed] [Google Scholar]
- 4. Hershberger RE, Hedges DJ, Morales A.. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol 2013;10:531–547. [DOI] [PubMed] [Google Scholar]
- 5. Herman DS, Lam L, Taylor MRG, Wang L, Teekakirikul P, Christodoulou D, Conner L, DePalma SR, McDonough B, Sparks E, Teodorescu DL, Cirino AL, Banner NR, Pennell DJ, Graw S, Merlo M, Di Lenarda A, Sinagra G, Bos JM, Ackerman MJ, Mitchell RN, Murry CE, Lakdawala NK, Ho CY, Barton PJR, Cook SA, Mestroni L, Seidman JG, Seidman CE.. Truncations of titin causing dilated cardiomyopathy. N Engl J Med 2012;366:619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McNair WP, Sinagra G, Taylor MRG, Di Lenarda A, Ferguson DA, Salcedo EE, Slavov D, Zhu X, Caldwell JH, Mestroni L.. SCN5A mutations associate with arrhythmic dilated cardiomyopathy and commonly localize to the voltage-sensing mechanism. J Am Coll Cardiol 2011;57:2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648, 2648a–2648d. [DOI] [PubMed] [Google Scholar]
- 8. Sinagra G, Anzini M, Pereira NL, Bussani R, Finocchiaro G, Bartunek J, Merlo M.. Myocarditis in clinical practice. Mayo Clin Proc 2016;91:1256–1266. [DOI] [PubMed] [Google Scholar]
- 9. Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski M, Yilmaz A, Klingel K, Kandolf R, Sechtem U, Cooper LT, Böhm M.. Update on myocarditis. J Am Coll Cardiol 2012;59:779–792. [DOI] [PubMed] [Google Scholar]
- 10. Anzini M, Merlo M, Sabbadini G, Barbati G, Finocchiaro G, Pinamonti B, Salvi A, Perkan A, Di Lenarda A, Bussani R, Bartunek J, Sinagra G.. Long-term evolution and prognostic stratification of biopsy-proven active myocarditis. Circulation 2013;128:2384–2394. [DOI] [PubMed] [Google Scholar]
- 11. Kühl U, Pauschinger M, Noutsias M, Seeberg B, Bock T, Lassner D, Poller W, Kandolf R, Schultheiss H-P.. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation 2005;111:887–893. [DOI] [PubMed] [Google Scholar]
- 12. Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation 1999;99:1091–1100. [DOI] [PubMed] [Google Scholar]
- 13. Merlo M, Anzini M, Bussani R, Artico J, Barbati G, Stolfo D, Gigli M, Muça M, Naso P, Ramani F, Di Lenarda A, Pinamonti B, Sinagra G.. Characterization and long-term prognosis of postmyocarditic dilated cardiomyopathy compared with idiopathic dilated cardiomyopathy. Am J Cardiol 2016;118:895–900. [DOI] [PubMed] [Google Scholar]
- 14. Li HS, Ligons DL, Rose NR.. Genetic complexity of autoimmune myocarditis. Autoimmun Rev 2008;7:168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Negishi H, Osawa T, Ogami K, Ouyang X, Sakaguchi S, Koshiba R, Yanai H, Seko Y, Shitara H, Bishop K, Yonekawa H, Tamura T, Kaisho T, Taya C, Taniguchi T, Honda K.. A critical link between Toll-like receptor 3 and type II interferon signaling pathways in antiviral innate immunity. Proc Natl Acad Sci U S A 2008;105:20446–20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weithauser A, Bobbert P, Antoniak S, Böhm A, Rauch BH, Klingel K, Savvatis K, Kroemer HK, Tschope C, Stroux A, Zeichhardt H, Poller W, Mackman N, Schultheiss H-P, Rauch U.. Protease-activated receptor-2 regulates the innate immune response to viral infection in a Coxsackie virus B3-induced myocarditis. J Am Coll Cardiol 2013;62:1737–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Belkaya S, Kontorovich AR, Byun M, Mulero-Navarro S, Bajolle F, Cobat A, Josowitz R, Itan Y, Quint R, Lorenzo L, Boucherit S, Stoven C, Di Filippo S, Abel L, Zhang S-Y, Bonnet D, Gelb BD, Casanova J-L.. Autosomal recessive cardiomyopathy presenting as acute myocarditis. J Am Coll Cardiol 2017;69:1653–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knowlton KU. Myocarditis: an intersection between genetic and acquired causes of human cardiomyopathy. J Am Coll Cardiol 2017;69:1666–1668. [DOI] [PubMed] [Google Scholar]
- 19. Fung G, Luo H, Qiu Y, Yang D, McManus B.. Myocarditis. Circ Res 2016;118:496–514. [DOI] [PubMed] [Google Scholar]
- 20. Xiong D, Yajima T, Lim B-K, Stenbit A, Dublin A, Dalton ND, Summers-Torres D, Molkentin JD, Duplain H, Wessely R, Chen J, Knowlton KU.. Inducible cardiac-restricted expression of enteroviral protease 2A is sufficient to induce dilated cardiomyopathy. Circulation 2007;115:94–102. [DOI] [PubMed] [Google Scholar]
- 21. Lamphear BJ, Yan R, Yang F, Waters D. Mapping the cleavage site in protein synthesis initiation factor eIF-4 gamma of the 2A proteases from human Coxsackievirus and rhinovirus. J Biol Chem 1993;268:19200–19203. [PubMed] [Google Scholar]
- 22. Mavrogeni S, Markousis MG, Papavasiliou A, Kolovou G.. Cardiac involvement in Duchenne and Becker muscular dystrophy. World J Cardiol 2015;7:410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gavillet B, Rougier J-S, Domenighetti AA, Behar R, Boixel C, Ruchat P, Lehr H-A, Pedrazzini T, Abriel H.. Cardiac sodium channel Nav1.5 is regulated by a multiprotein complex composed of syntrophins and dystrophin. Circ Res 2006;99:407–414. [DOI] [PubMed] [Google Scholar]
- 24. Xiong D, Lee G-H, Badorff C, Dorner A, Lee S, Wolf P, Knowlton KU.. Dystrophin deficiency markedly increases enterovirus-induced cardiomyopathy: a genetic predisposition to viral heart disease. Nat Med 2002;8:872–877. [DOI] [PubMed] [Google Scholar]