Abstract
Background
Obstructive sleep apnea (OSA) is often reported in connection with interstitial lung disease. As yet, there is insufficient data on the association of OSA severity parameters with lung involvement. We purposed to assess the frequency of OSA in our study group and to investigate the relationship between radiological involvement and OSA severity parameters.
Material/Methods
The study included 79 patients with interstitial lung disease who underwent spirometry, a carbon monoxide diffusion test (DLCO), high-resolution computed tomography, and polysomnography. The data were analyzed using SPSS 22 software.
Results
Of the 79 patients, 53 patients (67.1%) had OSA, and there was a negative correlation between DLCO and the mean time spent with oxygen saturation levels below 90% (r=−0.686, P=0.001). The Warrick score was used as an indicator of the extent and severity of pulmonary involvement and was positively correlated with the apnea-hypopnea index, oxygen desaturation index, and the mean time spent with oxygen saturation below 90% (r=0.275, P=0.014; r=0.264 P=0.019; r=0.235, P=0.038).
Conclusions
In our study, a significant relationship was found between the Warrick score and the OSA severity parameters, as determined by polysomnography. Polysomnographic examinations might be useful, especially in patients with a Warrick score greater than 15, to avoid possible complications.
MeSH Keywords: Lung Diseases, Interstitial; Polysomnography; Sleep Apnea, Obstructive
Background
Obstructive sleep apnea (OSA) is often reported in connection with interstitial lung disease (ILD) [1,2]. OSA has been reported in 60–90% of patients with idiopathic pulmonary fibrosis (IPF) [3,4], 52% of patients with sarcoidosis, and 66% of patients with scleroderma with pulmonary involvement [5,6]. Two different mechanisms have been described. In the presence of ILD, decreased lung volume results in impaired upper respiratory airway stability, as well as increased pharyngeal collapsibility, by reducing the downward expanding force (caudal traction) on the pharynx [7]. A further mechanism for the association of sleep-disordered breathing in patients with ILD, is obesity, which develops in relation to the corticosteroids used for the treatment of ILD [8]. The apnea-hypopnea index (AHI) has been found to be higher in patients with diffuse radiological involvement [3,6]. It is notable that there are several reports regarding the frequency of OSA in ILD patients, but as far as we know, there are no studies that have evaluated the potential relationship between obstructive sleep apnea syndrome (OSAS) severity with radiological disease involvement, and the changes in lung function seen in clinical practice. In our study, we purposed to assess OSA frequency in ILD patients by using polysomnography. The secondary aim of our study was to evaluate the relationship between indicators, such as radiologic involvement, the changes of lung function, diffusion capacity, and OSA severity, that can be determined by polysomnography.
Material and Methods
Patients
In this study, 110 patients who were referred to our chest diseases education and research hospital from different medical centers between January 2015 and June 2017 were screened. The patients’ selection criteria were as follows. Inclusion criteria were: aged 18 years and older; provided consent for participation in this study; and clinically and radiologically diagnosed as ILD. Exclusion criteria were: any upper airway pathology that may cause OSAS such as apparent nasal deviation, nasal polyp and tonsil hypertrophy; a non-stable disease period during the clinical follow-up; admission to the intensive care unit within the last 3 months; any change in therapy within the 3 months prior to referral to the study; evidence of any severe neurological or psychiatric disorder; chronic lung disease other than ILD; on-compliance with spirometry requirements; use of drugs that modulate sleep architecture such as selective serotonin reuptake inhibitors (SSRI), benzodiazepine, or narcotics; and total sleep duration of less than 4 hours per night based on polysomnography (low sleep efficiency).
The study received approval from the local ethics committee. All patients were informed about the study and then written consent was obtained. All patients were screened, and 31 patients were excluded. The excluded patients included 14 who refused to be in the study. The other 17 patients were excluded from the study for several different reasons such as upper airway lesion causing OSA (n=6), non-compliance with spirometry (n=8), and low-sleep efficiency (n=3). After detailed anamnesis, physical examination of the patients was performed. The body weight and height of each patient was measured by the same researcher, and body mass index (BMI) was calculated. The study was completed with the remaining 79 patients. If not available, a new high-resolution computed tomography (HRCT) was performed. Rheumatologically markers that were HRCT findings for all the patients in the last 6 months of the study were evaluated, measured, and rheumatology consultations were held. All cases were evaluated by a multidisciplinary team comprising radiologists, chest physicians, thoracic surgeons, and pathologists. If necessary, a surgical biopsy was performed.
The study was ultimately conducted on a final sample of 79 patients (41 males and 38 females) of which 16 patients had idiopathic pulmonary fibrosis (IPF), and 21 patients had sarcoidosis. Patients with early IPF and sarcoidosis received a histopathological diagnosis. Of the remaining patients, 14 patients had scleroderma, 12 patients had non-specific interstitial pneumonia, 8 patients had rheumatoid arthritis, 6 patients had systemic lupus erythematosus, and 2 patients had bronchiolitis obliterans organized pneumonia. This group was evaluated separately (Group 3) (Figure 1). While the diagnoses of rheumatoid arthritis, scleroderma, and systemic lupus erythematosus were made based on the consensus of the American/European Rheumatology Society [9–11], the diagnoses for IPF, non-specific interstitial pneumonia, and bronchiolitis obliterans organized pneumonia were made in accordance with the American Thoracic Society/European Respiratory Society (ATS/ERS) consensus [12]. The IPF patients did not use antifibrotic drugs. None of the systemic lupus erythematosus and rheumatoid arthritis patients were using immunomodulatory drugs during the study. Some of sarcoidosis and bronchiolitis obliterans organized pneumonia patients were using steroids. The scleroderma patients did not use cyclophosphamide or methotrexate.
Figure 1.
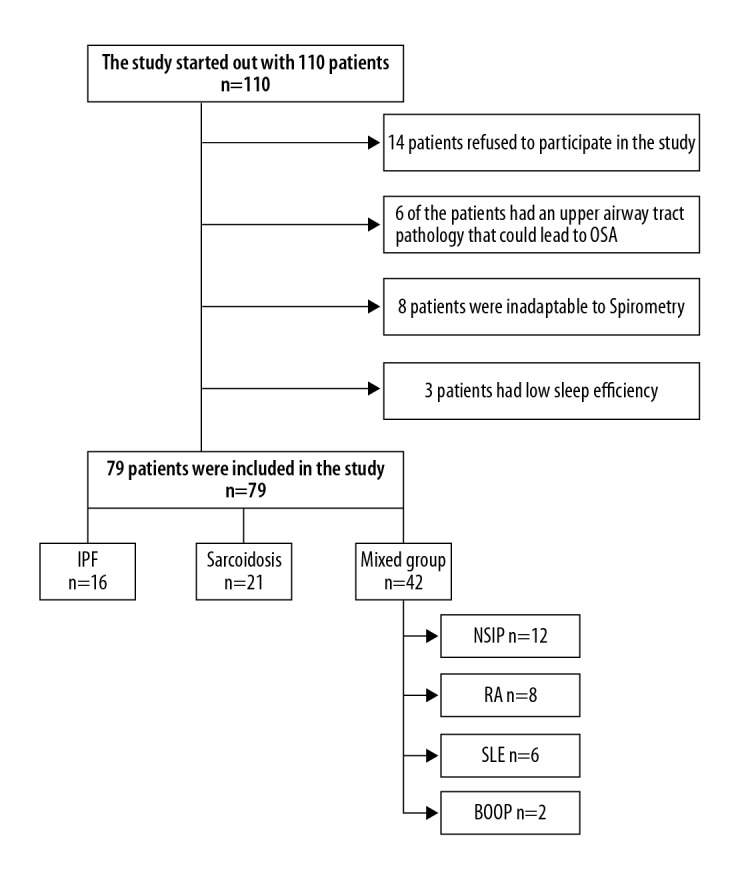
Patient disposition. IPF – idiopathic pulmonary fibrosis; NSIP – non-specific interstitial pneumonia; RA – rheumatoid arthritis; SLE – systemic lupus erythematosus; BOOP – bronchiolitis obliterans organized pneumonia.
Pulmonary function tests
All patients underwent spirometry (using a Zan 74) and carbon monoxide diffusion tests (DLCOs) [13], in accordance with ATS/ERS standards. DLCOs were carried out on all patients using the diffusion single breath method (using Jaeger) for 5 minutes, while the patients were in a sitting position and wearing a nose clip. Pre-procedural hemoglobin levels were considered. Women were examined out of their menstruation period. Results were recorded in mL/minute/mmHg unit.
Radiological evaluation
Findings of HRCT screenings obtained within the last 6 months were evaluated.
Patients underwent chest HRCT with a 16-detector scanner (Alexion; Toshiba Medical Systems, Tokyo, Japan) in the supine position with full inspiration and without contrast enhancement. Thin-section scans with 1-mm collimation were obtained at 10-mm intervals through the chest. The scanning parameters included 120 kVp, 150 mA, slice thickness 1 mm, and 1-second scanning time. All images were viewed with a window level of −550 HU and a width of 1600 HU. Scans were reconstructed with a high-frequency reconstruction algorithm.
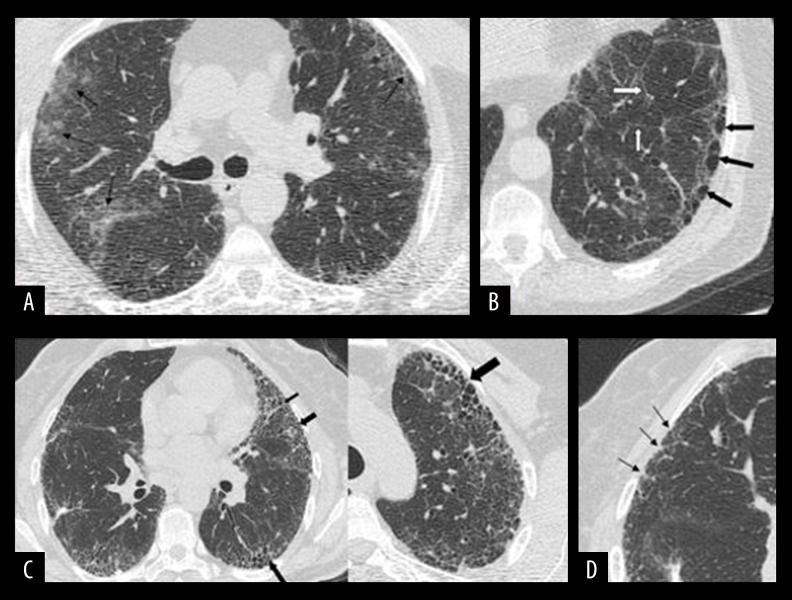
The Warrick score was scored based on observed parenchymal pathology and extent of lesions. The total score was calculated based on the radiological appearance and extent, which can vary from alveolitis to fibrosis. The total score can vary between 0 and 30 where the higher score indicates a higher degree of radiological change. The alveolitis index was calculated based on the presence and extent of ground glass opacities (between 0 and 4). The fibrosis index was calculated based on irregularities in pleural margins, septal/sub pleural lines, honeycombing appearance, and sub pleural cysts and their extent (between 0 and 26) (Figure 2).
Figure 2.
High-resolution computed tomography (HRCT) images of interstitial lung Disease (ILD) patients that present examples for Warrick scoring. (A) HRCT images that indicate patchy ground glass opacities in the peripheral regions of the left upper lobes in both lung parenchyma (black arrows). (B) Hypodense, thin-walled and single-layered cystic structures at the bases of the lungs are consistent with subpleural cysts (black arrows) and interlobular septal thickening in the parenchyma (white arrows). (C) Images on the left and right show thick-walled, multi-layered honeycombing with peripheral localization in 2 cases (black arrows). (D) Marked irregularities in the pleural margins in the right superior lung are consistent with early fibrotic changes (black arrows).
Alveolitis index and fibrosis index were calculated separately for each case. Two values were collected with each other. A value ranging from 0 to 30 was obtained. This value was named as the Warrick score [14].
These parameters were scored by 2 radiologists. If there was discrepancy, another radiologist who was unaware of the study decided on score. Patients were divided into 2 groups according to the Warrick score of higher-equal 15 and less than 15.15 (≥15 and <15).
Polysomnography
All patients underwent a standard diagnostic overnight PSG (Comet, Grass; Astro Med, Inc., West Warwick, RI, USA). For the 2-channel electrooculogram and 3-channel electroencephalogram, a submental electrode was used to determine sleep stages during polysomnography records; nasal airflow catheter and thermistor for intranasal pressure monitoring. Two tibia electromyography were used for leg movement records; oxygen saturation measurement probe and chest and abdomen belts were used for the presence of ventilatory effort during respiration. Scoring was based on the 2012 criteria of the American Academy of Sleep Medicine (AASM) [15]. Apnea was defined as 90% or more decrease in airflow for at least 10 seconds. Hypopnea was scored as a decrease in airflow of at least 30% for ≥10 seconds accompanied by a SaO2 (oxygen saturation) decrease ≥3%. AHI was considered as the number of apnea-hypopnea observed in every hour of sleep. If AHI score was 5 or higher, the patient was deemed to have OSA, and the severity of OSA was categorized as mild (AHI: 5.0–14.9 events/hour), moderate (AHI: 15.0–29.9 events/hour), or severe (AHI >30.0 events/hour) [16].
Statistical analyses
Continuous variables were analyzed for normality using the Shapiro-Wilk test. Normally distributed variables were analyzed with the t-test and variance of analysis, and were presented as the mean ± standard deviation. The Mann-Whitney U test and Kruskal-Wallis test were used to analyze abnormally distributed variables, and these were shown as medians (25–75%). The Spearman correlation analysis was carried out to evaluate the potential relationship between variables. Statistical analyses were performed using the SPSS 22.0 (IBM Corp., Chicago, IL, USA) software. A P value <0.05 was considered to be statistically significant.
Results
The mean age of the overall patient population was 55.4±11.3 years (n=79); and 48.1% were women (n=38). The mean BMI was 28.7±4.7 kg/m2.
Pulmonary function characteristics
Forced expiratory volume at 1 second (FEV1) and forced vital capacity (FVC) were estimated as 83 mL% (range, 69–100 mL%) and 81 mL% (range, 68–99 mL%), respectively; and FEV1/FVC was 104% (range 96–112%). The mean DLCO was 67 mmHg/dL (range, 51–81 mmHg/dL), 66.4±20.4 mmHg/dL. Of the all patients, 30 patients had a DLCO of less than 60 mmHg/dL.
Radiological characteristics
The mean Warrick score, as a marker of the extent and severity of pulmonary invasion, was 12 (7–21) (n=79). The Warrick scores of 35 patients were 15 and above, and 44 patients had a score below 15. Based on these Warrick scores, patients were defined as mild to moderate with respect to pulmonary invasion.
Polysomnographic characteristics
The frequency of OSAS among patients included in this study was 67% (n=53). The sleep structure characteristics showed that total sleep time (TST) was 380.5 minutes (range, 317.8–423.3 minutes), rapid eye movement (REM) was decreased by 8.3% (range, 2.9–16.1%,) and the number of sleep interruptions was 11.3±8.6. The AHI was 21.9 (range, 10.9–31.4) and the apnea index was 3 (range, 0.8–8.5). The mean hypopnea index was 17.9±12.2. The majority of patients had moderate and severe OSAS. Of the total study population, 10 patients (18.8%) had mild OSAS, 30 patients (56.6%) had moderate OSAS, and 13 patients (24.5%) had severe OSAS. The majority of respiratory events were hypopnea, and their frequency increased in the supine position (Table 1).
Table 1.
The outcomes of polysomnography.
| AHI index*,events/hours | 21.9 (10.9–31.4) |
| A index,* events/hours | 3.0 (0.8–8.5) |
| H index*, events/hours | 17.9±12.2 |
| REM AHI*, events/hours | 13.6 (0.0–30.6) |
| Supine AHI*, events/hours | 35.4 (17.7–51.2) |
| Nonsupine AHI,* events/hours | 8.81 (3.6–18.1) |
| TST*, minutes, | 380.5 (317.8–423.3) |
| Sleep EFF*% | 86.2 (68–93) |
| N1%* | 2.7 (1.7–4.6) |
| N2%* | 55.2±14.1 |
| N3%* | 13.7 (9.9–25.5) |
| REM%* | 8.3 (2.9–16.1) |
| Average SAT*% | 93.0 (90.0–95.0) |
| Min SAT*% | 80 (72–85) |
| ODI* events/hours | 14 (3–26) |
| SPO2 <90% | 11.5 (1–53.2) |
| Number of sleep interruption | 11.3±8.6 |
AHI – apnea-hypopnea index; A – apnea index; H – hypopnea; TST – total sleep time; ODI – oxygen desaturation index; Min sat – minimum saturation; SPO2 <90 – time spent while oxygen saturation was <9 0%, N1%, N2%, N3%; REM% – percentage of sleep stages.
Median (25–75%).
Comparison of interstitial lung disease (ILD) groups
The frequency of OSAS in the IPF, sarcoidosis, and mixed groups was 75%, 61.9%, and 66.7%, respectively. Among the different types of ILDs, the highest frequency of OSAS was noted in IPF patients (75%), although the difference was not significant when compared to the other disease groups. The mean age and Warrick scores of IPF patients were higher than the other groups (P=0.001) (Table 2).
Table 2.
Comparison of interstitial lung disease groups.
| IPF (n=16) | Sarcoidosis (n=21) | Mixed group (n=42) | P value | |
|---|---|---|---|---|
| Age, years | 62.0±9.4 | 48.7±8.4 | 56.1±11.7 | <0.001 |
| Female, number % | 5 (31.3%) | 12 (57.1%) | 21 (50.0%) | 0.277 |
| BMI kg/m2 | 27.9±4.8 | 30.2±4.4 | 28.1±4.6 | 0.215 |
| Warrick score | 23.5 (19–27.7) | 9 (6.5–12) | 11 (6–19.5) | <0.001 |
| FEV1 mL% | 78 (62–91.5) | 81 (70–102.5) | 83.5 (69–103.2) | 0.293 |
| FVC mL% | 71 (62–85.25) | 78 (66–98) | 85.5 (73.2–103.1) | 0.061 |
| FEV1/FVC% | 107 (100–114) | 102 (94–105) | 103 (95.5–113) | 0.268 |
| DLCO mL/Hg/min | 59.5 (47–78.7) | 72 (47.5–82) | 71 (55–81) | 0.393 |
| OSA(+) | 12 (75%) | 13 (61%) | 28 (66%) | 0.700 |
IPF – idiopathic pulmonary fibrosis; BMI – body mass index; FEV1 – forced expiratory volume at 1 second; FVC – forced vital capacity; DLCO – carbon monoxide diffusion test; OSA – obstructive sleep apnea.
Correlations between parameters
The Warrick score was positively correlated the with AHI, oxygen desaturation index (ODI), total time spent with an oxygen saturation less than 90%, and the number of sleep interruptions (r=0.275, P=0.014; r=0.264, P=0.019; r=0.235, P=0.038; r=0.312, P=0.015, respectively). The Warrick score was negatively correlated with minimum oxygen saturation and mean oxygen saturation (r=−0.225, P=0.046; r=−0.112, P=0.038, respectively). (Table 3). Figure 3 shows the correlation between AHI index and Warrick score. In addition, apnea-hypopnea and oxygen desaturation indices were significantly higher in patients whose mean Warrick score was above 15 compared with patients whose Warrick scores were below 15 (P=0.010, P=0.008, respectively). A positive correlation was found between DLCO and minimum oxygen saturation, mean oxygen saturation (r=0.474, P=0.001; r=0.364, P=0.001, respectively) (Table 3). DLCO was negatively correlated with the time spent while oxygen saturation was lower than 90% (r=−0.686 P=0.001) (Figure 4).
Table 3.
Correlation between Warrick score, carbon monoxide diffusion test and polysomnographic parameters.
| AHI events/hours | ODI events/hours | MinSat% | Average Sat% | SPO2 <90% | |
|---|---|---|---|---|---|
| Warrick score (r; P) | 0.28; 0.014 | 0.264; 0.019 | −0.225; 0.046 | −0.11; 0.33 | 0.235; 0.038 |
| Carbon monoxide diffusion test (r; P) | 0.04; 0.75 | 0.01; 0.97 | 0.474; 0.001 | 0.364; 0.001 | −0.686; 0.001 |
AHI – apnea-hypopnea index; ODI – oxygen desaturation index; Min sat – minimum saturation; SPO2 <90 – total sleep time during which oxygen saturation was lower than 90%.
Figure 3.
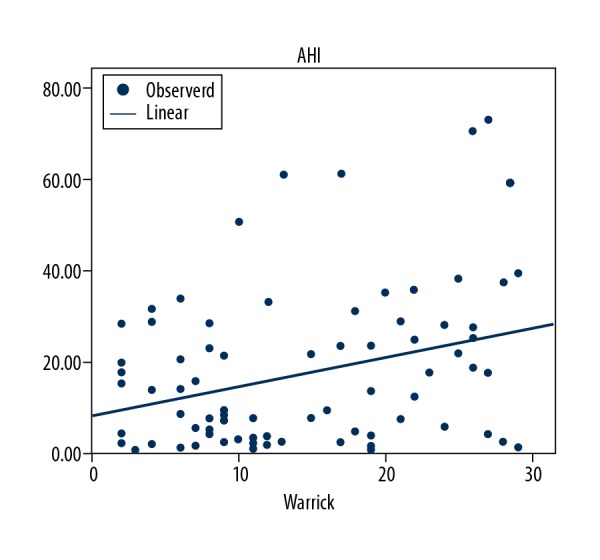
Graph showing the relation between apnea-hypopnea index and Warrick score (r=0.275; P=0.014).
Figure 4.
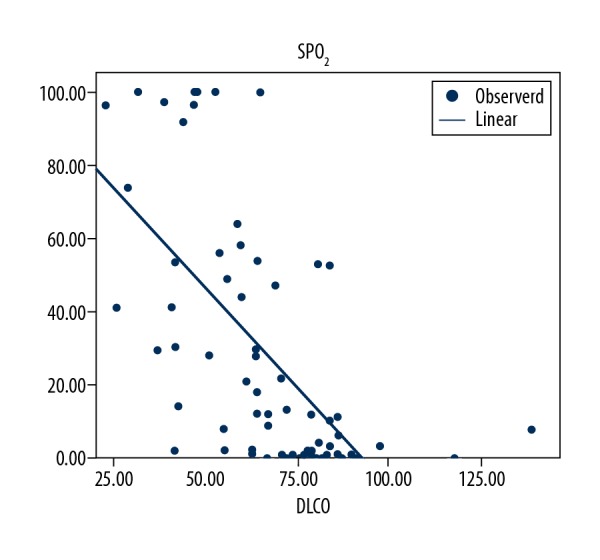
0Graph showing the relation between carbon monoxide diffusion test (DLCO) and SPO2 (r=−0.686, P<0.001).
A multivariate regression analysis was performed between OSA known predictors such as age, sex, BMI, hypertension, diabetes, and Warrick score. Regression analysis was found significant only for gender among all these factors P=0.006 (Table 4)
Table 4.
Regression analysis of obstructive sleep apnea predictors.
| OR | 95%CI | P value | |
|---|---|---|---|
| Age (years) | 1.019 | 0.970, 1.071 | 0.447 |
| Gender | 4.514 | 1.526, 13.357 | 0.006 |
| BMI (kg/m2) | 0.997 | 0.892, 1.115 | 0.958 |
| Diabetes | 0.685 | 0.118, 3.991 | 0.674 |
| Hypertension | 0.679 | 0.233, 1.980 | 0.478 |
| Warrick score | 1.005 | 0.938, 1.076 | 0.897 |
BMI – body mass index.
Discussion
In the present study, the frequency of OSA was found to be 67.1% in cases with ILD. A significant correlation was found between the Warrick score (a semi-quantitative scoring method that shows the prevalence of pulmonary involvement in HRCT and the known OSA severity parameters shown by polysomnography.
The OSA frequency in our study was higher than described in the literature for the general population. The worldwide OSA frequency is reported to be 10% in men aged 30–49 years, 17% in men between 50–70 years, 3% in women between 30–49 years, and 9% in women between 50–70 years [17]. Previous studies have reported frequencies of OSAS of between 17–88% among patients with ILD [3–6] and 60%–90% in IPF patients [1,2], 66% in scleroderma patients [6], and 52% in sarcoidosis patients [5,6]. In the present study, the rate of OSAS in patients with IPF, sarcoidosis, and scleroderma was 75%, 61%, and 57%, respectively, consistent with the literature. In a previous study performed in Turkey by Pihtili et al. [6], the frequency of OSAS in patients with IPF, sarcoidosis, and scleroderma was reported as 82.3%, 66.6%, and 55.5%, respectively. In our mixed-disease group, OSAS was diagnosed in 62.5% of patients with rheumatoid arthritis, 66.2% of patients with systemic lupus erythematosus, 75% of patients with non-specific interstitial pneumonia, and 50% of patients with bronchiolitis obliterans organized pneumonia. As previously demonstrated, the frequency of OSAS in IPF patients was higher compared to the other interstitial disease groups, and the findings of the present study were consistent with these results.
The polysomnography examinations of our patients indicated that their total sleep and REM sleep times decreased, and the majority of respiratory events were hypopnea. This finding was consistent with those reported previously by Pihtili et al. [6]. However, Mermigikis et al. [3] and Pihtili et al. [6] detected REM-related OSAS in their studies, while the OSAS cases investigated here were not REM-related. In line with our findings, Lancaster et al. [4] found no relationship between moderate and severe OSAS cases and REM. Similar to the findings of Lancaster et al. [4], the OSAS cases in this study were moderate and severe, and the majority of our cases had positional OSAS. While sleeping in supine position, muscle tone reduces, additionally tongue retrograde displacement occurs which decrease the retroglossal space to the maximum extent, and ultimately results in increased airway resistance.
Among our patients with ILD, OSAS was more frequent among men, as has also been previously described for individuals without ILD [18]. While evaluating excessive daytime sleepiness, Milloli et al. [19] previously reported a significant correlation between the Epworth Sleep Scale (ESS) and the AHI, while Lancaster et al. [4] reported a weak correlation between these two variables, and Pihtili et al. [6] and Fong et al. [20] found no correlation. In the present study, we similarly found no significant correlation between the ESS and AHI. We also found no correlation between OSAS and BMI in our study. Pihtili et al. [6] included patients with a BMI <30 kg/m2 in their study but still detected a high rate of OSAS among cases of ILD. Similarly, the frequency of OSAS in our study was high, but the mean BMI was relatively low. We concluded that ILD can contributes to OSAS, irrespective of the BMI. In the presence of restrictive lung diseases and the consequent increased airway resistance, decreases in lung volumes might result in upper airway collapse that is independent of the underlying upper airway pathology and AHI [21,22]. While it is unclear why such a relationship cannot be established, we believe that daytime sleepiness in patients with ILD might be suppressed due to dyspnea, and increased sympathetic activity associated with hypoxia and anxiety. In patients with ILD, Mermigikis et al. [3] reported a positive correlation between OSAS and BMI, while Lancaster et al. [4] found a weak correlation between these 2 parameters.
When we evaluated the relationship between OSAS parameters and functional parameters, such as spirometry and lung diffusion tests in patients with ILD, no significant correlations were noted between DLCO and the AHI or the ODI. However, DLCO was positively correlated with minimum oxygen saturation and mean oxygen saturation during sleep and was negatively correlated with the time spent while oxygen saturation was lower than 90%. Contrary to our findings, Mermigikis et al. [3] reported a positive correlation between total lung capacity in spirometry and the apnea-hypopnea index during REM, but in line with our findings, they also found a correlation between DLCO and mean oxygen saturation. Reoxygenation and hypoxemia episodes in OSAS increase oxidative stress and result in inflammation mediated by free oxygen radicals. This might contribute to the progression of ILD, although inflammatory cytokines released in the presence of ILD can indirectly cause OSAS by initiating upper airway edema and inflammation. Thus, we believe that the interaction between OSAS and ILD is mediated by mutual mechanisms; the steps between ODI contributing to pulmonary hypertension. Interestingly, in a study by Lee et al. comparing sleep and exercise conditions in IPF patients a higher level of cytokines was released during sleep, and these patients were more desaturated during sleep compared to exercise periods [23]. The concurrence of ILD and OSAS may result in a poorer prognosis by accelerating the development of pulmonary hypertension. Hence, the early detection and treatment of OSAS in these patients may be significant.
In the present study, the Warrick score was found to be positively correlated with markers of OSAS severity, including the AHI, ODI, total time spent with oxygen saturation less than 90%, and the number of sleep interruptions. However, it is notable that we found that OSAS severity was higher when the Warrick scores were above 15. Fibrosis might be adversely affected by the proliferation of oxidative stress-related inflammatory cells in the presence of OSA. In the regression analysis, Warrick score, as well as BMI, age, hypertension, and other parameters known as OSA predictors were not significant. This result was attributed to the inadequacy of our case number. The lack of sufficient cases for clinical epidemiological prevalence studies was the primary limiting factor in our study. Further factors, such as low sleep efficacy, the high number of sleep interruptions, and the effects of the first night spent in a sleep laboratory could also be noted as limitations of this study, given that we did not adjust our analyses to take these factors into account.
This study might be a starting point for future studies investigating the association between lung disease and its severity and involvement in relation to OSA
Conclusions
In our study, the prevalence of OSA in ILD patients was higher than that reported in the normal population. A significant correlation was observed between OSAS severity parameters and the Warrick scores. OSAS was more severe in cases with a Warrick score higher than 15. OSAS leads to serious consequences such as hypertension, coronary artery disease, and stroke. We recommend polysomnographic examinations in patients with a Warwick score of over 15 to avoid the aforementioned possible complications.
Acknowledgement
The authors thank Professor Dr. Fezan Sahin Mutlu for her kind support of statistical analyses.
Footnotes
Source of support: Departmental sources
References
- 1.Kim JS, Podolanczuk AJ, Boker P, et al. Obstructive sleep apnea and subclinical interstitial lung disease in the multi-ethnic study of atherosclerosis (MESA) Ann Am Thorac Soc. 2017;14(12):1786–95. doi: 10.1513/AnnalsATS.201701-091OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mavroudi M, Papakosta D, Kontakiotis T, et al. Sleep disorders and health-related quality of life in patients with interstitial lung disease. Sleep Breath. 2018;22(2):393–400. doi: 10.1007/s11325-017-1579-1. [DOI] [PubMed] [Google Scholar]
- 3.Mermigkis C, Stagaki E, Trayfon S, et al. How common is sleep disordered breathing in patients with idiopathic pulmonary fibrosis. Sleep Breath. 2010;14:387–90. doi: 10.1007/s11325-010-0336-5. [DOI] [PubMed] [Google Scholar]
- 4.Lancaster LH, Mason WR, Parnell J, et al. Obstructive sleep apnea is common in idiopathic pulmonary fibrosis. Chest. 2009;136:772–78. doi: 10.1378/chest.08-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bingöl Z, Pihtili A, Gülbaran Z, Kiyan E. Relationship between parenchymal involvement and obstructive sleep apnea in subjects with sarcoidosis. Clin Respir. 2015;J9:14–21. doi: 10.1111/crj.12098. [DOI] [PubMed] [Google Scholar]
- 6.Pihtili A, Bingöl Z, Kiyan E, et al. Obstructive sleep apnea is common in patients with interstitial lung diseases. Sleep Breath. 2013;17:1281–88. doi: 10.1007/s11325-013-0834-3. [DOI] [PubMed] [Google Scholar]
- 7.Tagaito Y, Isono S, Remmers JE, et al. Lung volume and collapsibility of the passive pharynx in patients with sleep-disordered breathing. J Appl Physiol. 2007;103:1379–85. doi: 10.1152/japplphysiol.00026.2007. [DOI] [PubMed] [Google Scholar]
- 8.Zammit C, Liddicoat H, Moonsie I, et al. Obesity and respiratory diseases. Int J Gen Med. 2010;3:335–43. doi: 10.2147/IJGM.S11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aletaha D, Neogi T, Silman AJ, et al. Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 10.Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–86. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoogen F, Khanna D, Fransen J, et al. Classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65:2737–47. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trawis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society Statement: Update of International Multidisciplinary Classification of the idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2013;188:733–48. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laszio G. Standardization of lung function testing: Helpful guidance from the ATS/ERS Task Force. Thorax. 2006;61:744–46. doi: 10.1136/thx.2006.061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warrick JH, Bhalla M, Schabel SI, et al. High-resolution computed tomography in early scleroderma lung disease. J Rheum. 1991;18:1520–28. [PubMed] [Google Scholar]
- 15.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. American Academy of Sleep Medicine; Darien, IL: 2014. [Google Scholar]
- 17.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(1):1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep disorders breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 19.Milloli G, Bosi M, Poletti V, et al. Sleep and respiratory disorders in idiopathic pulmonary fibrosis. Sleep Med Rev. 2015;26:57–63. doi: 10.1016/j.smrv.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Fong SY, Ho CK, Wing YK. Comparing MSLT and ESS in the measurement of excessive daytime sleepiness in obstructive sleep apnea syndrome. J Psychosom Res. 2005;58:55–60. doi: 10.1016/j.jpsychores.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Sériès F, Cormier Y, Lampron N, et al. Increasing the functional residual capacity may reverse obstructive sleep apnea. Sleep. 1988;11:349–53. [PubMed] [Google Scholar]
- 22.Heinzer RC, Stanchina ML, Malhotra A, et al. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:114–17. doi: 10.1164/rccm.200404-552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee RN, Kelly E, Nolan G, et al. Disordered breathing, during sleep and exercise, idiopathic pulmonary fibrosis. QJM. 2016;109:142. doi: 10.1093/qjmed/hcv159. [DOI] [PubMed] [Google Scholar]