Abstract
Background:
It was recently reported that, using Western blotting, some multiple sclerosis (MS) patients in the United States had antibodies against epsilon toxin (Etx) from Clostridium perfringens, suggesting that the toxin may play a role in the disease.
Objective:
We investigated for serum antibodies against Etx in UK patients with clinically definite multiple sclerosis (CDMS) or presenting with clinically isolated syndrome (CIS) or optic neuritis (ON) and in age- and gender-matched controls.
Methods:
We tested sera from CDMS, CIS or ON patients or controls by Western blotting. We also tested CDMS sera for reactivity with linear overlapping peptides spanning the amino acid sequence (Pepscan) of Etx.
Results:
Using Western blotting, 24% of sera in the combined CDMS, CIS and ON groups (n = 125) reacted with Etx. In the control group (n = 125), 10% of the samples reacted. Using Pepscan, 33% of sera tested reacted with at least one peptide, whereas in the control group only 16% of sera reacted. Out of 61 samples, 21 (43%) were positive to one or other testing methodology. Three samples were positive by Western blotting and Pepscan.
Conclusion:
Our results broadly support the previous findings and the role of Etx in the aetiology of MS warrants further investigation.
Keywords: Clostridium perfringens, epsilon toxin, multiple sclerosis
Introduction
Clostridium perfringens is found in the gastrointestinal tracts of many animals. The ability of different strains to cause a range of diseases in human and in animals is ascribed largely to the differential production of toxins.1 Epsilon toxin, produced by C. perfringens types B and D, is associated with dysentery and enterotoxaemia in ovines following the growth of bacteria in the intestine and the production of epsilon toxin.2 The toxin crosses the gut wall, accumulating in the kidneys and brain.3 In the brain, the toxin binds to the synaptosomal membranes,4 myelinated structures,5,6 glial cells7 and oligodendrocytes8 and causes demyelination.6 Peracute enterotoxaemia in ovines appears without clinical signs and results in sudden death while acute disease leads to convulsions and coma.9 There are only three reports of the isolation of C. perfringens producing epsilon toxin from humans,10,11 one from the stool of a patient who had presented with multiple sclerosis (MS) 3 months previously.12
Antibodies to epsilon toxin are reported to occur in 10% of patients with MS and in 1% of healthy individuals in the United States,12 and Cases et al.13 recently showed that epsilon toxin affects the propagation of action potentials in isolated optic nerves. As a result of these studies, and the similarities between the symptoms of ovine enterotoxaemia and humans suffering from MS, it has been suggested that epsilon toxin may contribute to the development of MS.
Here, we investigated whether antibodies to epsilon toxin are present in the sera of patients with clinically definite multiple sclerosis (CDMS) or presenting with clinically isolated syndrome (CIS) or optic neuritis (ON), the first demyelinating events suggestive of MS, alongside matched controls, using cohorts of individuals in the United Kingdom.
Material and methods
Patients and samples
Sera from patients with CDMS (n = 65), CIS (n = 20) or ON (n = 44) were obtained from UK centres. Patients were adults (age > 18 years) and disease assessment had been carried out by a MS specialist. McDonalds 2010 Criteria (revised) was used for the diagnosis of CIS and MS.14 Sera from CDMS, CIS or ON patients were obtained at University College London (UCL) and Sheffield from London-South East UK Research and Ethics Committee (ethics: 2011-003475-11) and Basildon NHS trusts collected under the ALS biomarkers study (ethics: 09/H0703/27) or at the Charing Cross Hospital in accordance with guidelines approved by 05/MRE12/8 NRES Committee South Central (Berkshire). ON patient samples were collected as part of the trial. Age- and gender-matched samples from control patients were collected as part of the Exeter 10,000 project (ethics: 09/H0106/75). Consent was obtained from subjects. Data on clinical subtypes, occurrence or absence of disease activity and/or progression, disease duration, the occurrence of magnetic resonance imaging (MRI) activity, use of disease-modifying therapy and use of high-dose steroids are summarised in Supplementary Tables S1–S3.
Toxins
Epsilon protoxin15 was activated with TPCK-treated trypsin from bovine pancreas (Sigma-Aldrich Company Ltd) for 1 hour at room temperature. Bacillus anthracis–protective antigen (PA83) was kindly provided by Dr ED Williamson (Dstl Porton Down).
Cell culture
Chinese hamster ovary (CHO) cells, CHO cells expressing green fluorescent protein (CHO-GFP) and CHO cells expressing human myelin and lymphocyte protein (CHO-hMAL) were cultured in Dulbecco’s Modified Eagle’s Medium/Ham’s F12 (DMEM/F12) medium (Life Technologies) supplemented with 10% foetal bovine serum at 37°C in 95% air:5% CO2.
Construction of CHO-stable cell line expressing hMAL
The hMAL gene (NCBI reference NP_002362.1) was synthesised (GeneArt, Thermo Fisher Scientific) and cloned into pEF1αAcGFP-N1 (Clontech). After sequencing, the plasmid was transfected into CHO cells using Turbofect (Thermo Fisher Scientific). Transfectants were selected in media containing 400 μg/mL G418 for 3 weeks. Individual clones were analysed under a fluorescence microscope (Olympus X81) to confirm membrane-associated MAL-GFP expression.
Neutralisation assay
Rabbit polyclonal antibody against epsilon toxin,16 pre-immune rabbit sera, MS patient sera or control sera were serially diluted in an equal volume or phosphate-buffered saline (PBS) containing 5× CT75 of activated epsilon toxin. After 1 hour, the mixtures were added to CHO-hMAL cells to a final toxin concentration of 1× CT75 of epsilon toxin. Control CHO-hMAL cells were treated with PBS, 1× CT75 of toxin and 0.1% Triton X-100. Following incubation for 3 hours at 37°C, the media was replaced with 100 μL of serum-free DMEM/F12 and 10 μL of WST-1 reagent (Abcam). Metabolic activity of cells was measured as the conversion of WST-1 into a coloured product. Absorbance at 420 nm was read following incubation for 1 hour at 37°C and normalised with respect to the Triton X-100-treated controls.
Competitive enzyme-linked immunosorbent assay
A competitive enzyme-linked immunosorbent assay (ELISA) to measure neutralising antibodies was carried out using a monoscreen ELISA kit (BioX Diagnostics, BIO K 222/2), according to the manufacturers’ instructions.
Western blotting
Toxins (3–6 µg) were separated using NuPAGE 4%–12% Bis-Tris gels and morpholineethanesulfonic acid (MES)-SDS running buffer (Life Technologies) and blotted onto nitrocellulose membranes which were blocked in phosphate-buffered saline–tween (PBST) containing 3% (w/v) dry milk powder. Toxin was detected after adding sera diluted 1000-fold in PBST with 3% (w/v) milk and incubated overnight at 4°C. This was followed by incubation with horseradish peroxide (HRP)-conjugated donkey anti-human IgG 1:10,000 (Jackson ImmunoResearch) in PBST with 3% (w/v) milk for 1 hour at room temperature followed by three 15-minute washes in PBST. Signals were detected using Pierce ECL Western Blotting substrate (Thermo Fisher Scientific) and a ChemiDoc imaging system equipped with Quantity One software (Bio-Rad Laboratories). Samples that were immunoreactive with epsilon toxin were Western blotted with a molar equivalent amount of B. anthracis–protective antigen (PA83). Serum samples reactive with epsilon toxin and PA83 were excluded from further analysis.
Epitope scanning
Overlapping peptides spanning epsilon toxin were synthesised and reacted with antisera by Pepscan (8243 RC Lelystad, The Netherlands). Antibody binding to peptides was tested using an ELISA. Peptide arrays were incubated with primary antibody (overnight at 4°C). After washing, the arrays were incubated with a 1/1000 dilution of an antibody peroxidase conjugate for 1 hour at 25°C. Colour development after adding 2,2′-azino-di-3-ethylbenzthiazoline sulphonate (ABTS) and 20 μL/mL of 3% (v/v) H2O2 1 hour was quantified with a charge-coupled device–camera and an image processing system. To verify the quality of the synthesised peptides, a separate set of positive and negative control peptides was synthesised in parallel. These were screened with antibody 57.9.17
Molecular modelling
Epsilon toxin (PDB ID: 1UYJ) and epitopes were visualised using PyMOL 42.
BLAST searches
We searched the US National Center for Biotechnology Information with the query epsilon toxin peptide ‘TGVSLTTSYSFANTN’ using BLASTP and TBLASTN algorithms with default values, but with the E-value set to 1000.
Results
Western blotting of sera
Western blotting was used to detect antibodies in human sera based on the method described by Rumah et al.,12 but we used native epsilon toxin in place of recombinant his-tagged protein and diluted sera 1000-fold, rather than 10,000-fold, before testing. Figure 1 shows a typical blot with human sera which reacted strongly to epsilon toxin, weakly to epsilon toxin or did not react with epsilon toxin under the test conditions.
Figure 1.
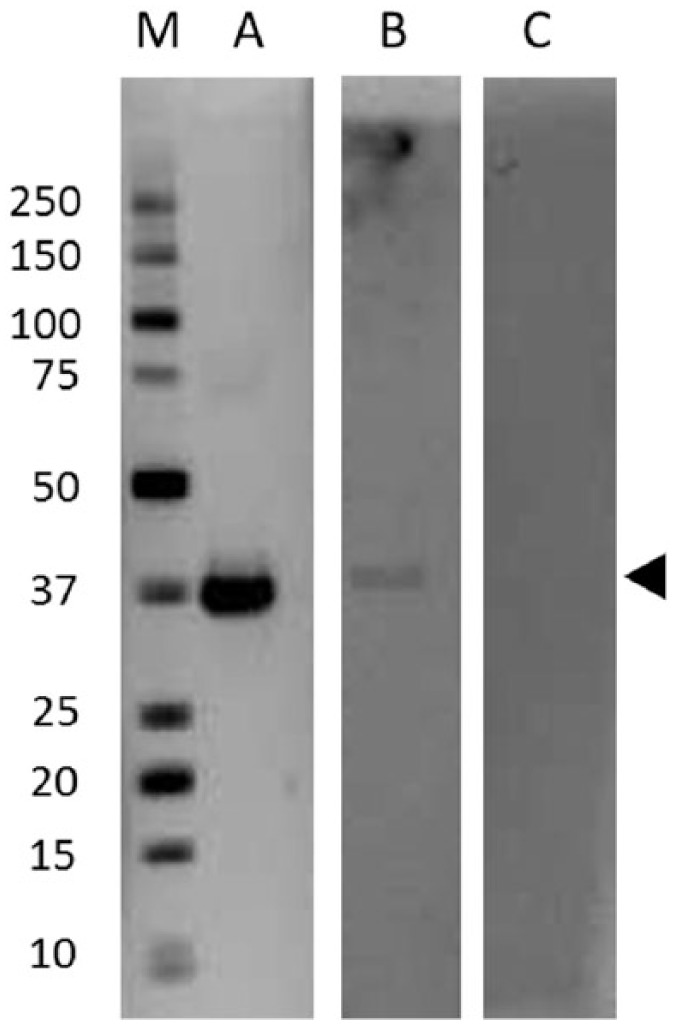
Western blot indicating immunoreactivity to epsilon toxin from different sera. Lane A shows epsilon toxin (arrowed) reacted with a strongly positive serum (BUH00226), lane B shows epsilon toxin reacted with a weakly positive serum (BLT00139) and Lane C shows an example of a serum (BUH00239) which did not react with epsilon toxin. Molecular size markers (kDa) in Lane M.
Samples that reacted with epsilon toxin were subsequently screened for reactivity with B. anthracis PA83, a pore-forming toxin which shares a hydrophobicity map similar to epsilon toxin.12 Seroconversion to PA83 is rare and would only occur in those individuals who were vaccinated against it or upon exposure to B. anthracis. Rumah et al.12 suggested that a positive PA83 result could suggest nonspecific antibody responses.
Antibodies to epsilon toxin identified by Western blotting
We assessed the prevalence of antibodies to epsilon toxin in patients with demyelination, the subgroups of which were CDMS, CIS or ON. The latter two groups may develop CDMS but are early in the disease course. Age- and gender-matched controls were then examined (Table 1 and Figure 2). We repeated the Western blotting at least three times for each sample. Supplementary Table S4 shows the demographic features of the different groups we tested in this study. In total, we identified 34 serum samples in the combined CDMS, CIS, ON groups (n = 129) that reacted with epsilon toxin, of which four also reacted with PA83 and therefore were excluded from further analysis. In the combined control groups (n = 129) we identified 17 serum samples that reacted with epsilon toxin, of which four also reacted with PA83 and were therefore excluded from further analysis outlined below.
Table 1.
Summary of sera tested for reactivity with epsilon toxin or PA83 by Western blotting.
| Test group | Total number of sera tested | Number of sera reactive with epsilon toxin | Number of sera reactive with PA83 | Number of sera which did not react with PA83 | Sera reactive with epsilon toxin but not with PA83 |
|
|---|---|---|---|---|---|---|
| n | % | |||||
| CDMS | 65 | 18 | 4 | 61 | 14 | 23 |
| CDMS control | 65 | 7 | 2 | 63 | 5 | 8 |
| CIS | 20 | 7 | 0 | 20 | 7 | 35 |
| CIS control | 20 | 2 | 0 | 20 | 2 | 10 |
| ON | 44 | 9 | 0 | 44 | 9 | 20 |
| ON control | 44 | 8 | 2 | 42 | 6 | 14 |
| Total CDMS/CIS/ON | 129 | 34 | 4 | 125 | 30 | 24 |
| Total controls | 129 | 17 | 4 | 125 | 13 | 10 |
CDMS: clinically definite multiple sclerosis; CIS: clinically isolated syndrome; ON: optic neuritis.
Figure 2.
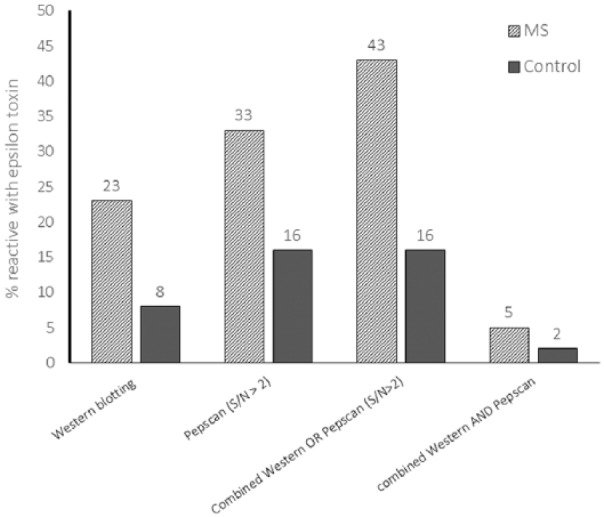
Proportion of CDMS and control sera that reacted with epsilon toxin by Western blotting or by Pepscan alone or in combination.
We found that 23% of the CDMS patients’ sera had antibodies to epsilon toxin while the matched control group for this cohort showed reactivity in 8% of samples (Table 1 and Figure 2). The proportion of patients diagnosed with CIS/MS, relapsing-remitting multiple sclerosis (RRMS), secondary progressive multiple sclerosis (SPMS) and showing reactivity with epsilon toxin was broadly similar (31%, 21% and 18%, respectively). None of the primary progressive multiple sclerosis (PPMS) samples we tested were reactive though the number tested (n = 5) may be too low to be representative of this group.
In CIS patients, we found that 35% of the sera had antibodies to epsilon toxin while in the matched control group 10% of sera was reactive. Follow-up patient data on the seven positive epsilon toxin samples from the CIS cohort showed that five of the seven patients were subsequently diagnosed with MS. We found that 20% of the sera from ON patients were positive for epsilon toxin antibodies while the matched control group showed that 14% of the sera samples had antibodies to epsilon toxin. All of the Western blotting positive sera from CDMS, CIS and ON patients reacted weakly with epsilon toxin by Western blotting (data not shown). One control serum sample reacted strongly with epsilon toxin by Western blotting (Figure 1, lane A), but the other control sera reacted weakly.
Epitope scanning of sera
Where sufficient sera from CDMS patients (n = 43) or control sera (n = 32) was available, we analysed them for reactivity with linear overlapping peptides spanning the amino acid sequence of epsilon toxin (Pepscan). We included negative- and positive-control sera from rabbits before and after immunisation with Y43A-Y209A, an epsilon toxoid vaccine.16 We used a stringent signal/noise cut-off of ≥2.0 to identify positive samples. Rabbit sera before immunisation did not react with the peptide array. Sera from rabbits immunised with the epsilon toxoid recognised several peptides (Figure 3 and Supplementary Table S5).
Figure 3.
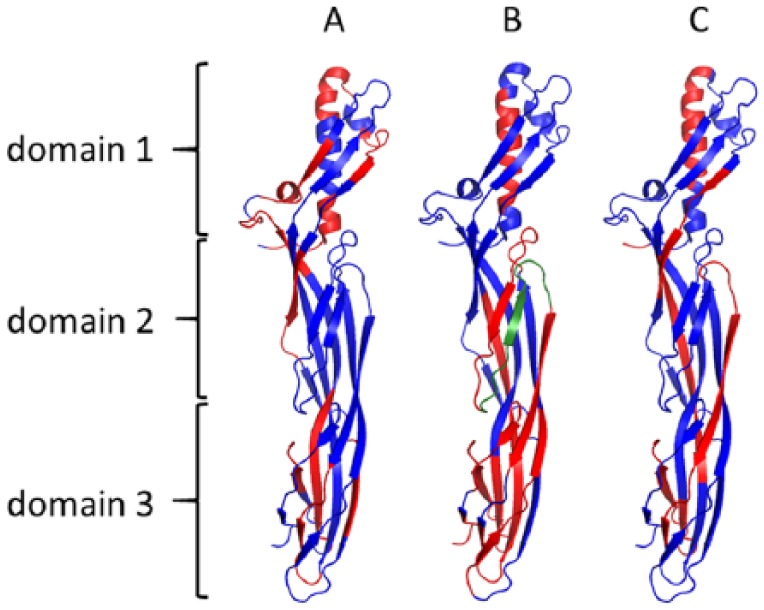
Peptides recognised by sera raised against epsilon toxoid in rabbits (n = 3; panel A), CDMS patients (n = 14; panel B) or control patient sera (n = 5; panel C). The peptides recognised are shown highlighted in red. Highlighted in green is the peptide TGVSLTTSYSFANTN.
Of 43 CDMS sera tested, 14 (33%) reacted with at least one peptide, whereas in the matched control group 5 of 32 (16%) reacted (Figure 2). Most sera recognised multiple peptides (Supplementary Table S2). Only three (5%) CDMS sera (309, BLT00139 and BLT00143) were positive by both Western blotting and Pepscan and one control (2%) was positive by both testing methods (Figure 2). Conversely, when the Western blotting and Pepscan results were considered together, 43% of CDMS samples and 16% of control sera were positive by at least one assay (Figure 2).
Most of the peptides recognised by CDMS sera and control sera were identical. However, one peptide (TGVSLTTSYSFANTN) was recognised by sera from CDMS patients that were positive by Western blotting but not by sera that were negative by Western blotting nor by control sera. When the National Center for Biotechnology Information (NCBI) database was searched using this peptide, the only complete matches identified were towards C. perfringens epsilon toxin. The next closest match, with a Mycobacterium heraklionense hypothetical protein, showed 11/15 residues matched. There were no complete or partial matches with human proteins.
We mapped the epitopes recognised by sera onto the molecular structure of epsilon toxin. The antisera raised against an epsilon toxoid in rabbits recognised a range of epitopes, mainly located in domain 1 and domain 3 of the protein (Figure 3). Control and CDMS sera also recognised domains 1 and 3, as well as additional peptides in domain 2. We found antibodies directed against the membrane insertion loop of domain 2 and especially against the TGVSLTTSYSFANTN peptide in CDMS patients but not in sera from controls.
Ability of sera to neutralise toxicity
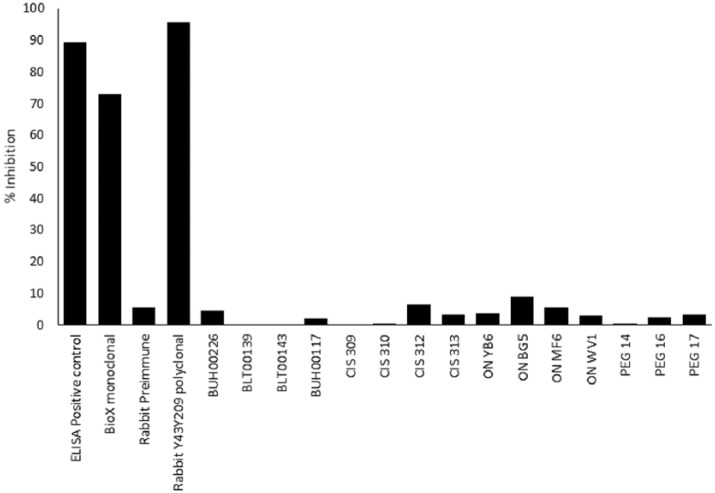
We tested serum neutralisation of epsilon toxin in two ways. First, using a competitive ELISA kit to measure competition between antisera and a neutralising monoclonal antibody for binding to C. perfringens epsilon toxin. The neutralising polyclonal serum and monoclonal antibody caused 90% and 75% signal inhibition, respectively (Figure 4). Rabbit sera against epsilon toxoid16 caused 95% inhibition of the signal, whereas pre-immune rabbit sera caused only 6% signal inhibition. None of the sera tested from CDMS, CIS or ON patients caused significant inhibition of the signal, even though the sera were reactive in Western blots or by Pepscan. None of the control sera, including the strongly positive BUH00226 sample, caused significant inhibition of the signal, even though these sera were reactive in Western blots or by Pepscan.
Figure 4.
Competition ELISA to measure neutralising antibodies. Sera BLT00139, BLT00143 and BUH00117 were obtained from CDMS patients, sera CIS 309, CIS 310, CIS 312 and CIS 313 from patients diagnosed with CIS and sera ON YB6, ON BG5, ON MF6 and ONWV1 from patients diagnosed with ON. PEG14, PEG16 and PEG17 are control sera.
We also tested the ability of sera to directly neutralise the cytotoxicity of epsilon toxin towards CHO-hMAL cells. Rabbit sera against an epsilon toxoid16 neutralised the toxin at 0.25 mg/mL antibody. Sera from non-immune rabbits or the strongly positive BUH00226 sample did not neutralise toxicity (data not shown).
Discussion
MS is a pro-inflammatory demyelinating disease of the central nervous system, the aetiology of which involves contribution from genetic and environmental factors. More recently, Rumah et al.12 showed that antibodies to epsilon toxin were more prevalent in MS patients than in healthy controls, suggesting a role for epsilon toxin in the development of MS.12 In support of this, many pathophysiological consequences of the exposure of animals to epsilon toxin are consistent with a role of the toxin in MS. Epsilon toxin targets synaptosomes,4 myelinic structures,5,6 glial cells7 and oligodendrocytes8 and causes demyelination.6 The toxin has been shown to recognise cells expressing myelin and lymphocyte protein (MAL18) including human T-cells.19 Against this background, we investigated whether antibodies to epsilon toxin are more frequently found in MS patients. We used Western blotting because this methodology was used to screen sera in the study from the United States.12
Our data suggest that seroreactivity towards epsilon toxin, measured using Western blotting or Pepscan, was more frequent in CDMS, CIS or ON patients than in controls. Using Western blotting, the overall incidence of immunoreactivity in CDMS patients in the United Kingdom (24%) was higher than the incidence reported in the United States (10%). Seroreactivity to epsilon toxin also occurred in the control group, also at a higher incidence (10%) than previously reported (1%). These findings are consistent with the increased sensitivity of the assay we have used.12 MS patients often show elevated levels of antibodies in sera, though it is not clear what these antibodies are directed against.20 Our finding that antibody reactivity occurred with some control sera, albeit at a lower frequency, indicates that the responses we detected in CDMS patients were not simply due to the elevated level of antibodies as a consequence of MS disease.20
Reactivity in the control group also suggests that exposure to epsilon toxin does not necessarily result in the development of MS. In an attempt to understand whether exposure to epsilon toxin is associated with the subsequent development of disease, we looked at patients with CIS. Around 30%–70% individuals with CIS develop MS.21,22 We found that 71% (5 out of 7) CIS individuals with antibody to epsilon toxin went on to develop CDMS, but this finding would need to be confirmed by screening a larger group.
The intensity of the responses we saw in CDMS patients and in controls were broadly similar by Western blotting. However, with the exception of control BUH0226, the responses that we saw were weak. Different serotypes of epsilon toxin have not been described, but we cannot discount the possibility of this. Another possible explanation for the weak responses is that antibodies are directed against a different structural form of epsilon toxin. During insertion into host-cell membranes, the protein would undergo structural changes, thereby altering epitopes.2 Antibodies directed against a different structural form might be unable to neutralise the toxin. Small differences in the epitope recognised by antibodies can profoundly influence their abilities to neutralise other toxins.23
There was little overlap between results obtained using Western blotting and peptide scanning. This is surprising since both methods should primarily detect linear epitopes. However, Western blots can be sensitive to the denaturation state of the electrophoresed antigen.24 In addition, since the Western blotting and Pepscan studies were carried out in different laboratories, it is possible that differences in sample handling affected the results. During this study, we also carried out some preliminary work to peptide map sera from eight patients with CIS/ON. In this pilot study, we found that five sera showed reactivity with peptides. Further studies should investigate a larger cohort of CIS/ON sera.
Several previous studies have found that dysbiosis of C. perfringens in the gut is not associated with MS,25–29 though one study did find that C. perfringens levels were elevated in patients diagnosed with neuromyelitis optica.25 However, these studies characterised the population at the species level. It is known that C. perfringens strains, which normally colonise the human gut, are not able to produce epsilon toxin. One hypothesis is that replacement with strains producing the toxin triggers MS,12 and this would not be apparent from changes in the gut microbiome. During this project, we searched human gut metagenome data sets at the NCBI human microbiome project (70 healthy volunteers) for the presence of the epsilon toxin gene. We identified the gene encoding alpha toxin, which is common to all C. perfringens strains, but we did not identify matches with the epsilon toxin gene.
A key question is whether immunoreactivity towards epsilon toxin is indicative of toxin exposure and whether the toxin plays a role in the development of MS. Some elements of our study support this suggestion, especially when viewed in combination with the previous findings.12 Of CDMS samples, tested by Western Blotting or Pepscan, 26 (43%) were positive under one or the other methods, whereas only 10 (16%) of the control sera were positive. This indicates that MS patients are more than twice as likely to possess antibodies to epsilon toxin. However, our finding that responses were generally weak, rather than showing a spectrum of responses, is unusual. The evidence for a role of epsilon toxin in the aetiology of MS warrants further investigation.
Supplemental Material
Supplemental material, MSJ767327_supplementary_data for Evidence of Clostridium perfringens epsilon toxin associated with multiple sclerosis by Sariqa Wagley, Monika Bokori-Brown, Helen Morcrette, Andrea Malaspina, Caroline D’Arcy, Sharmilee Gnanapavan, Nicholas Lewis, Michel R Popoff, Dominika Raciborska, Richard Nicholas, Ben Turner and Richard W Titball in Multiple Sclerosis Journal
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: N.L. is a shareholder in MS Sciences Ltd.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the MS Sciences Ltd and by the NIHR Exeter CRF.
ORCID iD: Richard W Titball  https://orcid.org/0000-0002-0162-2077
https://orcid.org/0000-0002-0162-2077
Contributor Information
Sariqa Wagley, College of Life and Environmental Sciences, University of Exeter, Exeter, UK.
Monika Bokori-Brown, College of Life and Environmental Sciences, University of Exeter, Exeter, UK.
Helen Morcrette, College of Life and Environmental Sciences, University of Exeter, Exeter, UK.
Andrea Malaspina, Blizard Institute, Queen Mary University of London, London, UK.
Caroline D’Arcy, West London Neuroscience Centre, Charing Cross Hospital, London, UK.
Sharmilee Gnanapavan, Blizard Institute, Queen Mary University of London, London, UK.
Nicholas Lewis, MS Sciences Limited, London, UK.
Michel R Popoff, Bactéries Anaérobies et Toxines, Institut Pasteur, Paris, France.
Dominika Raciborska, Blizard Institute, Queen Mary University of London, London, UK.
Richard Nicholas, Division of Brain Sciences, Department of Medicine, Imperial College London, London, UK.
Ben Turner, Clinical Research Centre, Barts Health NHS Trust, London, UK.
Richard W Titball, College of Life and Environmental Sciences, University of Exeter, Exeter, UK.
References
- 1. McDonel JL. Toxins of Clostridium perfringens types A, B, C, D and E. In: Dorner F, Drews J. (eds) Pharmacology of bacterial toxins. Oxford: Pergamon Press, 1986, pp. 477–517. [Google Scholar]
- 2. Bokori-Brown M, Savva CG, Fernandes Da, Costa SP, et al. Molecular basis of toxicity of Clostridium perfringens epsilon toxin. FEBS J 2011; 278: 4589–4601. [DOI] [PubMed] [Google Scholar]
- 3. Finnie JW. Pathogenesis of brain damage produced in sheep by Clostridium perfringens type D epsilon toxin: A review. Aust Vet J 2003; 81: 219–221. [DOI] [PubMed] [Google Scholar]
- 4. Nagahama M, Sakurai J. High-affinity binding of Clostridium perfringens epsilon-toxin to rat brain. Infect Immun 1992; 60: 1237–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dorca-Arevalo J, Soler-Jover A, Gibert M, et al. Binding of epsilon-toxin from Clostridium perfringens in the nervous system. Vet Microbiol 2008; 131: 14–25. [DOI] [PubMed] [Google Scholar]
- 6. Wioland L, Dupont JL, Doussau F, et al. Epsilon toxin from Clostridium perfringens acts on oligodendrocytes without forming pores, and causes demyelination. Cell Microbiol 2015; 17: 369–388. [DOI] [PubMed] [Google Scholar]
- 7. Soler-Jover A, Dorca J, Popoff MR, et al. Distribution of Clostridium perfringens epsilon toxin in the brains of acutely intoxicated mice and its effect upon glial cells. Toxicon 2007; 50: 530–540. [DOI] [PubMed] [Google Scholar]
- 8. Lonchamp E, Dupont JL, Wioland L, et al. Clostridium perfringens epsilon toxin targets granule cells in the mouse cerebellum and stimulates glutamate release. PLoS ONE 2010; 5: e13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Popoff MR. Epsilon toxin: A fascinating pore-forming toxin. FEBS J 2011; 278: 4602–4615. [DOI] [PubMed] [Google Scholar]
- 10. Gleeson-White MH, Bullen JJ. Clostridium welchii epsilon toxin in the intestinal contents of man. Lancet 1955; 268: 384–385. [DOI] [PubMed] [Google Scholar]
- 11. Kohn J, Warrack GH. Recovery of Clostridium welchii type D from man. Lancet 1955; 268: 385. [DOI] [PubMed] [Google Scholar]
- 12. Rumah KR, Linden J, Fischetti VA, et al. Isolation of Clostridium perfringens type B in an individual at first clinical presentation of multiple sclerosis provides clues for environmental triggers of the disease. PLoS ONE 2013; 8: e76359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cases M, Llobet A, Terni B, et al. Acute effect of pore-forming Clostridium perfringens ε-toxin on compound action potentials of optic nerve of mouse. eNeuro 2017; 4: ENEURO.0051-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cole AR, Gibert M, Popoff M, et al. Clostridium perfringens epsilon-toxin shows structural similarity to the pore-forming toxin aerolysin. Nat Struct Mol Biol 2004; 11: 797–798. [DOI] [PubMed] [Google Scholar]
- 16. Bokori-Brown M, Hall CA, Vance C, et al. Clostridium perfringens epsilon toxin mutant Y30A-Y196A as a recombinant vaccine candidate against enterotoxemia. Vaccine 2014; 32: 2682–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Posthumus WP, Lenstra JA, Schaaper WM, et al. Analysis and simulation of a neutralizing epitope of transmissible gastroenteritis virus. J Virol 1990; 64: 3304–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rumah KR, Ma Y, Linden JR, et al. The myelin and lymphocyte protein MAL is required for binding and activity of Clostridium perfringens ε-toxin. PLoS Pathog 2015; 11: e1004896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frank M. MAL, a proteolipid in glycosphingolipid enriched domains: Functional implications in myelin and beyond. Prog Neurobiol 2000; 60: 531–544. [DOI] [PubMed] [Google Scholar]
- 20. Weber MS, Hemmer B, Cepok S. The role of antibodies in multiple sclerosis. Biochim Biophys Acta 2011; 1812: 239–245. [DOI] [PubMed] [Google Scholar]
- 21. Miller D, Barkhof F, Montalban X, et al. Clinically isolated syndromes suggestive of multiple sclerosis, part I: Natural history, pathogenesis, diagnosis, and prognosis. Lancet Neurol 2005; 4: 281–288. [DOI] [PubMed] [Google Scholar]
- 22. Brownlee WJ, Miller DH. Clinically isolated syndromes and the relationship to multiple sclerosis. J Clin Neurosci 2014; 21: 2065–2071. [DOI] [PubMed] [Google Scholar]
- 23. Tempest PR, White P, Williamson ED, et al. Efficient generation of a reshaped human mAb specific for the alpha toxin of Clostridium perfringens. Protein Eng 1994; 7: 1501–1507. [DOI] [PubMed] [Google Scholar]
- 24. Yermakova A, Vance DJ, Mantis NJ. Sub-domains of ricin’s B subunit as targets of toxin neutralizing and non-neutralizing monoclonal antibodies. PLoS ONE 2012; 7: e44317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cree BA, Spencer CM, Varrin-Doyer M, et al. Gut microbiome analysis in neuromyelitis optica reveals overabundance of Clostridium perfringens. Ann Neurol 2016; 80: 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berer K, Gerdes LA, Cekanaviciute E, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A 2017; 114: 10719–10724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cekanaviciute E, Yoo BB, Runia TF, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A 2017; 114: 10713–10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jangi S, Gandhi R, Cox LM, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun 2016; 7: 12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tremlett H, Fadrosh DW, Faruqi AA, et al. Gut microbiota composition and relapse risk in pediatric MS: A pilot study. J Neurol Sci 2016; 363: 153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSJ767327_supplementary_data for Evidence of Clostridium perfringens epsilon toxin associated with multiple sclerosis by Sariqa Wagley, Monika Bokori-Brown, Helen Morcrette, Andrea Malaspina, Caroline D’Arcy, Sharmilee Gnanapavan, Nicholas Lewis, Michel R Popoff, Dominika Raciborska, Richard Nicholas, Ben Turner and Richard W Titball in Multiple Sclerosis Journal