Key Points
Question
To what extent can the beneficial effect of endovascular therapy for acute ischemic stroke on functional outcome be explained by treatment-related reduced follow-up infarct volume?
Findings
In this pooled data mediation analysis of randomized clinical trials including 1665 patients with acute ischemic stroke, follow-up infarct volume reduction was a predictor of functional outcome but only explained 12% of the benefit of endovascular therapy.
Meaning
Infarct volume assessed on posttreatment imaging is currently not a valid proxy for estimating treatment effect in phase II and III trials.
This pooled data analysis of 7 randomized clinical trials of thrombectomy for acute ischemic stroke examines whether follow-up infarct volume mediates the relationship between endovascular therapy and functional outcome in patients with acute ischemic stroke.
Abstract
Importance
The positive treatment effect of endovascular therapy (EVT) is assumed to be caused by the preservation of brain tissue. It remains unclear to what extent the treatment-related reduction in follow-up infarct volume (FIV) explains the improved functional outcome after EVT in patients with acute ischemic stroke.
Objective
To study whether FIV mediates the relationship between EVT and functional outcome in patients with acute ischemic stroke.
Design, Setting, and Participants
Patient data from 7 randomized multicenter trials were pooled. These trials were conducted between December 2010 and April 2015 and included 1764 patients randomly assigned to receive either EVT or standard care (control). Follow-up infarct volume was assessed on computed tomography or magnetic resonance imaging after stroke onset. Mediation analysis was performed to examine the potential causal chain in which FIV may mediate the relationship between EVT and functional outcome. A total of 1690 patients met the inclusion criteria. Twenty-five additional patients were excluded, resulting in a total of 1665 patients, including 821 (49.3%) in the EVT group and 844 (50.7%) in the control group. Data were analyzed from January to June 2017.
Main Outcome and Measure
The 90-day functional outcome via the modified Rankin Scale (mRS).
Results
Among 1665 patients, the median (interquartile range [IQR]) age was 68 (57-76) years, and 781 (46.9%) were female. The median (IQR) time to FIV measurement was 30 (24-237) hours. The median (IQR) FIV was 41 (14-120) mL. Patients in the EVT group had significantly smaller FIVs compared with patients in the control group (median [IQR] FIV, 33 [11-99] vs 51 [18-134] mL; P = .007) and lower mRS scores at 90 days (median [IQR] score, 3 [1-4] vs 4 [2-5]). Follow-up infarct volume was a predictor of functional outcome (adjusted common odds ratio, 0.46; 95% CI, 0.39-0.54; P < .001). Follow-up infarct volume partially mediated the relationship between treatment type with mRS score, as EVT was still significantly associated with functional outcome after adjustment for FIV (adjusted common odds ratio, 2.22; 95% CI, 1.52-3.21; P < .001). Treatment-reduced FIV explained 12% (95% CI, 1-19) of the relationship between EVT and functional outcome.
Conclusions and Relevance
In this analysis, follow-up infarct volume predicted functional outcome; however, a reduced infarct volume after treatment with EVT only explained 12% of the treatment benefit. Follow-up infarct volume as measured on computed tomography and magnetic resonance imaging is not a valid proxy for estimating treatment effect in phase II and III trials of acute ischemic stroke.
Introduction
Endovascular therapy (EVT) substantially reduces disability in patients with acute ischemic stroke with a large vessel occlusion in the anterior circulation.1,2,3,4,5,6,7 It is assumed that this positive treatment effect is caused by the salvage and preservation of brain tissue. This idea is strengthened by many studies that have shown a strong relationship between the extent of ischemic tissue injury assessed at follow-up imaging and functional outcome.8,9,10,11,12 With this in mind, follow-up infarct volume (FIV) has been suggested as an early measure of treatment efficacy because this represents a potentially more objective estimate of the pathological response to treatment than functional outcome. However, the validity of a potential surrogate outcome measure depends on the demonstration that the effects of therapy on that surrogate accurately reflects and reliably predicts the effects of therapy on the clinical end point.13 Formal testing through a mediation analysis is relevant to establishing the full potential of FIV as an early measure of treatment efficacy.
To our knowledge, only 3 studies have examined if the effect of EVT on functional outcome was mediated by FIV.5,14,15 Two of these studies5,14 reported that FIV did not mediate the relationship between treatment and functional outcome. However, no estimates were reported on the proportion of EVT effect that is explained by FIV. In the third study,15 only a small proportion of the treatment effect could be explained by FIV, but estimates were not as precise. Hence, it still remains unclear to what extent the causal relationship between EVT and functional outcome is explained by treatment-related reduction in FIV. We investigated whether FIV mediated the relationship between EVT and functional outcome by analyzing pooled patient data from 7 randomized clinical trials of thrombectomy for acute ischemic stroke.1,2,3,4,5,6,7
Methods
Data in this study are from pooled individual patient data of the Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials collaboration. This collaboration was established by trial investigators of 7 randomized clinical trials published between 2015 and 2017 that investigated the efficacy of EVT in patients with acute ischemic stroke caused by large vessel occlusions.1,2,3,4,5,6,7 A local central medical ethics committee and the research boards of all participating centers approved each trial as well as the analysis of brain scans used in this study. Written informed consent was acquired from all patients or legal representatives. Design features and inclusion criteria have previously been described.6,7,16
All patients enrolled in each trial except for the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN)1 had 24-hour follow-up brain imaging with either noncontrast computed tomography (CT) or magnetic resonance imaging (MRI). The Trial and Cost Effectiveness Evaluation of Intra-arterial Thrombectomy in Acute Ischemic Stroke trial7 protocol additionally required follow-up imaging at day 7 or at hospital discharge. Participating centers in MR CLEAN1 were required to perform follow-up imaging at 5 to 7 days. In the Extending the Time for Thrombolysis in Emergency Neurological Deficits–Intra-Arterial trial,4 the Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke trial,2 the Solitaire With the Intention For Thrombectomy as Primary Endovascular Treatment Trial,3 the Endovascular Revascularization With Solitaire Device Versus Best Medical Therapy in Anterior Circulation Stroke Within 8 Hours trial,5 and the Pragmatic Ischaemic Stroke Thrombectomy Evaluation trial,6 5-day follow-up imaging occurred at the discretion of the intervention site. This post hoc study included all patients who had available follow-up imaging data acquired at least 12 hours after symptom onset with an upper limit of 2 weeks (336 hours).
Posttreatment Outcome Measures
When multiple follow-up imaging data were available, the latest scan within the window (12 hours to 2 weeks) was selected for assessment. In case both CT and MRI were acquired, MRI was the modality of choice; within that, diffusion-weighted imaging with apparent diffusion coefficient maps was the preferred sequence. Infarcts were identified as intraaxial hypodense (CT) or hyperintense (MRI with diffusion-weighted imaging) regions within the affected hemisphere. Infarcted areas with parenchymal hemorrhage (within or adjacent to the infarct) and those extending into the contralateral hemisphere were included in the lesion. Infarcts in the ipsilateral hemisphere with characteristics of old infarct were categorized as preexistent and were not included in the FIV. In case of decompressive hemicraniectomy with no available presurgery scan, only the ischemic lesion within the theoretical boundaries of the skull was included. Thin-section data were reconstructed into images with 5-mm slice thickness. Validated software was used to segment infarcts, and volumes were calculated based on planimetry.17 Infarct volumes on MRI were calculated using planimetry and manually outlined by an experienced observer (A.M.M.B. or I.G.H.J.). All infarct boundaries were checked by an expert neuroradiologist (W.H.v.Z., L.F.M.B., or C.B.L.M.M.) and adjusted where necessary. Magnetic resonance imaging gradient echo sequence was used to identify hemorrhages. A standardized window and level setting for CT was set to limit variation between observers; window width was 30 Hounsfield units, and the center level was 35 Hounsfield units. Infarct location was defined by laterality (left or right hemisphere) and involvement of the 10 distinct anatomical regions of the Alberta Stroke Program Early CT Score (ASPECTS) template,18 assessed by one of the same expert neuroradiologists (W.H.v.Z., L.F.M.B., or C.B.L.M.M.). In case of MRI, an ASPECTS region with an infarction encompassing more than 20% of that region was classified as an infarct-positive region to minimize differences between MRI and CT. Hemorrhagic transformations were scored according to the anatomical description of the Heidelberg bleeding classification.19 In case of issues with image quality and interpretation, a consensus reading with 2 neuroradiologists was carried out. The primary outcome was the degree of disability as scored on the modified Rankin Scale (mRS) at 90 days, considered as an ordinal outcome.20 All readers were blinded to treatment assignment, trial, and clinical findings.
Mediation of the Association of EVT With Functional Outcome by FIV
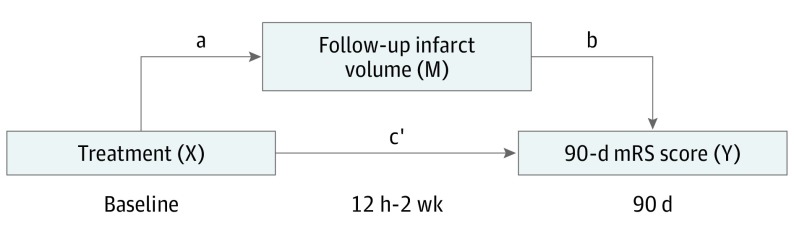
To assess FIV as a mediator of the relationship between EVT and functional outcome, mediation analysis was performed using the template described by Baron and Kenny21 and the method by Vanderweele and Vansteelandt,22 with FIV as the mediating variable. Figure 1 illustrates the hypothetical causal model where treatment type (EVT or control) determines FIV after an acute ischemic stroke and where FIV (the mediator) determines functional outcome at 90 days. To perform mediation analysis, it is necessary to test 3 pathways: step 1, the association of treatment with 90-day mRS score; step 2, the association of treatment with FIV; and step 3, the association of FIV with 90-day mRS score, controlling for treatment type. If all 3 associations are confirmed, mediation (indirect effect) can be established in a fourth step through estimation of the direct causal relationship (pathway c′) (eTable 1 in the Supplement). According to Baron and Kenny,21 the mediation of the relationship is full when c′ is 0 and partial when c′ is greater than 0. Mediation is absent when not all steps are satisfied.
Figure 1. Model of the Hypothetical Causal Pathway in Patients With Acute Ischemic Stroke.
Total effect (c) = direct effect (c′) + indirect effect (ab). mRS indicates modified Rankin Scale.
All pathways were tested using univariable and multivariable regression analysis. Follow-up infarct volume was log-transformed (log + 1) to best satisfy the linear model (distribution of residuals was normal and homoscedasticity of the data was preserved). The association of treatment with FIV (pathway a [Figure 1]) was tested using linear regression modeling and reported as adjusted and unadjusted βs with 95% CIs. All other pathways were tested using ordinal linear regression and reported as adjusted common odds ratios (acORs) and unadjusted common ORs. Multivariable modeling included infarct location, hemorrhage type, and the prespecified prognostic variables age and score on the National Institute of Health Stroke Scale (NIHSS) at baseline. The proportion of the causal relationship between EVT and functional outcome that is mediated through FIV was estimated by dividing the log ORs of the indirect effect (pathways ab) by the log OR of the total effect (pathway c) (Figure 1).22,23 Given the ordinal nature of the outcome measure, the method of Vanderweele and Vansteelandt22 was used to compute this proportion based on ORs, with 95% CIs derived from bootstrap methods. This method is specifically suitable for use with categorical outcomes and for estimation via ORs. Missing variables were included after imputation of the relevant covariate with median values of the nonmissing data. For all ORs and other parameter estimation, mixed-effects modeling with a random effect for trial was performed to account for between-trial variance.
Sensitivity Analyses
Infarcts may still evolve within the first week after onset.24,25 Accordingly, FIV assessment is dependent on the timing of image acquisition. A sensitivity analysis was conducted in patients with FIVs assessed on imaging acquired after 48 hours of symptom onset. In addition, MRI may provide more accurate estimates of FIV than CT. A second sensitivity analysis was performed with MRI data only.
Statistical Analysis
Dichotomous variables were presented as proportions. Continuous variables were tested for normality using the Shapiro-Wilk test and reported as means and standard deviations when normally distributed and as medians and interquartile ranges (IQRs) otherwise. Differences in FIVs between the EVT and control groups were tested for significance with the Wilcoxon rank sum test. The mRS score was examined for proportionality using visual and graphical methods, and no substantial departures from proportionality were found. The statistical approach in this study was based on a previous post hoc analysis by the MR CLEAN investigators.15
All statistical analyses were performed in SAS version 9.4 (SAS Institute) and R version 3.2 (The R Foundation). P values were 2-sided, and P values less than .05 indicated statistical significance in all analyses.
Results
In this analysis of the Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials collaboration,1,2,3,4,5,6,7 the median (IQR) age was 68 (57-76) years, and 781 patients (46.9%) were female. A total of 1690 of 1764 patients (95.8%) had follow-up imaging data acquired between 12 hours and 2 weeks after stroke symptom onset, with a median (IQR) period of 30 (24-137) hours. Additionally, 25 patients were excluded because of poor image quality or difficulties precluding accurate infarct determination, resulting in a total of 1665 patients for this analysis.
Baseline characteristics are shown in Table 1. A total of 821 patients (49.3%) were allocated to the EVT group and 844 (50.7%) to the control group. Overall, the median (IQR) FIV was 41 (14-120) mL, and 651 of 1650 patients (39.5%) achieved functional independence (mRS score of 0 to 2) at 3 months. Patients allocated to the EVT group had significantly smaller FIVs compared with those in the control group (median [IQR] FIV, 33 [11-99] vs 51 [18-134] mL; P = .007) (Table 2). Successful reperfusion (Thrombolysis in Cerebral Ischemia [TICI] scores 2B to 3) was achieved in 523 of 690 patients (75.8%) in the EVT group with evaluable angiographic imaging. In the EVT group, the median FIV was 28 mL in patients with substantial reperfusion vs 86 mL in those without substantial reperfusion (TICI score 0-2a) (P < .001). In 26 patients, a consensus reading of the imaging outcome measures between neuroradiologists was needed.
Table 1. Baseline Characteristicsa.
| Characteristic | No./Total No. (%) | P Value | |
|---|---|---|---|
| EVT (n = 821) | Control (n = 844) | ||
| Age, median (IQR), y | 68 (57-76) | 68 (58-76) | .79 |
| Female | 388/821 (47.3) | 393/844 (46.6) | .81 |
| Left hemisphere infarct | 381/809 (47.1) | 409/840 (48.7) | .52 |
| Time from onset to follow-up imaging acquisition, median (IQR), h | 29 (24-138) | 31 (24-141) | .61 |
| Imaging modality | .36 | ||
| CT | 675/821 (82.2) | 708/844 (83.9) | |
| MRI | 146/821 (17.8) | 136/844 (16.1) | |
| NIHSS score at baseline, median (IQR) | 17 (14-20) | 17 (13-21) | .93 |
| Alteplase (tPA) delivered | 724/821 (88.2) | 772/844 (91.5) | .03 |
| Diabetes | 119/819 (14.5) | 151/842 (17.9) | .06 |
| Hypertension | 439/819 (53.6) | 497/843 (59.0) | .03 |
| Tobacco use | 280/742 (37.7) | 286/777 (36.8) | .71 |
| Time from onset to alteplase (tPA), median (IQR), min | 115 (85-155) | 119 (85-161) | .08 |
| Time from onset to randomization, median (IQR), min | 181 (141-240) | 184 (140-248) | .80 |
| Time from onset to reperfusion, median (IQR), min | 291 (230-355) | NA | NA |
| ASPECTS at baseline, median (IQR) | 8 (7-9) | 8 (7-9) | .20 |
| Occlusion location | .91 | ||
| Not available | 47/821 (5.7) | 46/844 (5.5) | |
| ICA | 201/821 (24.5) | 221/844 (26.2) | |
| M1 | 507/821 (61.8) | 517/844 (61.3) | |
| M2 | 65/821 (7.9) | 59/844 (7.0) | |
| Other | 1/821 (0.1) | 1/844 (0.1) | |
| Collateral score | .75 | ||
| 0 | 6/606 (1.0) | 7/627 (1.1) | |
| 1 | 90/606 (14.9) | 107/627 (17.1) | |
| 2 | 265/606 (43.7) | 265/627 (42.3) | |
| 3 | 245/606 (40.4) | 248/627 (39.6) | |
Abbreviations: ASPECTS, Alberta Stroke Program Early CT Score; CT, computed tomography; EVT, endovascular therapy; ICA, internal carotid artery; IQR, interquartile range; M1, first segment of the middle cerebral artery; M2, second segment of the middle cerebral artery; MRI, magnetic resonance imaging; NA, not applicable; NIHSS, National Institutes of Health and Stroke Scale; tPA, tissue plasminogen activator.
Missing variables were excluded from analysis.
Table 2. Outcomes per Treatment Allocation Group.
| Characteristic | No. (%) | P Value | |
|---|---|---|---|
| EVT (n = 821) | Control (n = 844) | ||
| Follow-up infarct volume, median (IQR), mL | 33 (11-99) | 51 (18-134) | .007 |
| mRS score at 90 d, median (IQR) | 3 (1-4) | 4 (2-5) | <.001 |
| Hemorrhagea | |||
| Hemorrhagic infarct | |||
| Type 1 | 116 (14.1) | 113 (13.4) | .67 |
| Type 2 | 101 (12.3) | 94 (11.1) | .49 |
| Parenchymal hematoma | |||
| Type 1 | 70 (8.5) | 55 (6.5) | .14 |
| Type 2 | 68 (8.3) | 50 (5.9) | .07 |
| Remote parenchymal hematoma | 13 (1.6) | 8 (0.9) | .28 |
| Intraventricular hemorrhage | 20 (2.4) | 23 (2.7) | .76 |
| Subarachnoid hemorrhage | 23 (2.8) | 14 (1.7) | .14 |
| Subdural hemorrhage | 0 | 3 (0.4) | .25 |
| Reperfusion, No./total No. (%) | |||
| TICI score 2B-3b | 518/684 (75.7) | NA | NA |
| TICI score 0-2A | 166/684 (24.3) | NA | NA |
Abbreviations: EVT, endovascular therapy; IQR, interquartile range; mRS, modified Ranking Scale; NA, not applicable; TICI, Thrombolysis in Cerebral Ischemia.
Hemorrhages scored according to the anatomical description of the Heidelberg bleeding classification.19
A TICI score of 2B to 3 indicates successful reperfusion.
Mediation Analysis
Endovascular therapy was independently associated with a better functional outcome (step 1: acOR, 2.28; 95% CI, 1.55-3.36; P < .001) and with smaller log-transformed FIV (step 2: β, −0.13; 95% CI, −0.19 to −0.07; P < .001) (Table 3). In adjusted analysis, log-transformed FIV was a predictor of functional outcome (step 3: acOR, 0.46; 95% CI, 0.39-0.54; P < .001); EVT was similarly a predictor associated with good functional outcome (step 4: OR, 2.22; 95% CI, 1.52-3.21; P < .001) (Table 3) (eTable 2 in the Supplement). Other independent predictors associated with functional outcome included age (acOR, 0.62 per 10 years; 95% CI, 0.57-0.67; P < .001) (ie, the odds of increasing 1 point in mRS score for every 10 years were 62%), baseline NIHSS score (acOR, 0.82 per 5 points; 95% CI, 0.74-0.90; P = .001), hemorrhagic infarct type 2 (acOR, 0.73; 95% CI, 0.54-0.99; P = .04), intraventricular hemorrhage (acOR, 0.29; 95% CI, 0.13-0.64; P = .002), and involvement of the ASPECTS internal capsula (acOR, 0.45; 95% CI, 0.35-0.58 P < .001) and M5 (acOR, 0.77; 95% CI, 0.60-0.99; P = .04) regions.
Table 3. Proportion of Association of Treatment With Ordinal 90-Day Modified Rankin Scale Score Mediated By Follow-up Infarct Volumea.
| Pathwayb | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| Measure | Value (95% CI) | P Value | Measure | Value (95% CI) | P Value | |
| a | β | −0.28 (−0.41 to −0.14) | <.001 | β | −0.13 (−0.19 to −0.07) | <.001 |
| b | cOR | 0.45 (0.42-0.48) | <.001 | acOR | 0.46 (0.39-0.54) | <.001 |
| c | cOR | 2.17 (1.38-3.41) | <.001 | acOR | 2.28 (1.55-3.36) | <.001 |
| c′ | cOR | 1.87 (1.25-2.81) | .002 | acOR | 2.08 (1.44-3.00) | <.001 |
Abbreviations: acOR, adjusted common odds ratio; cOR, common odds ratio.
Follow-up infarct volume was log-transformed (log + 1) to best satisfy the linear model.
Pathway a represents the regression coefficient of the association of treatment (control or endovascular therapy) with follow-up infarct volume; b, the association of follow-up infarct volume with 90-day modified Rankin Scale score; c, the association of treatment with 90-day modified Rankin Scale score; and c′, the association of treatment with 90-day modified Rankin Scale score, controlling for follow-up infarct volume. Multivariable regression analysis included follow-up infarct volume, location, hemorrhage type, age, and National Institutes of Health and Stroke Scale score.
In the mediation analysis, steps 1 to 3 were satisfied. Step 4 indicated partial mediation of FIV on the association of treatment with 90-day mRS score; after adjustment for the mediator FIV, EVT still had a substantial association with functional outcome. For the adjusted model, the mediator FIV explained 12% (95% CI, 1-19) of the association of EVT with functional outcome. This proportion was 18% (95% CI, 3-34) in the unadjusted model.
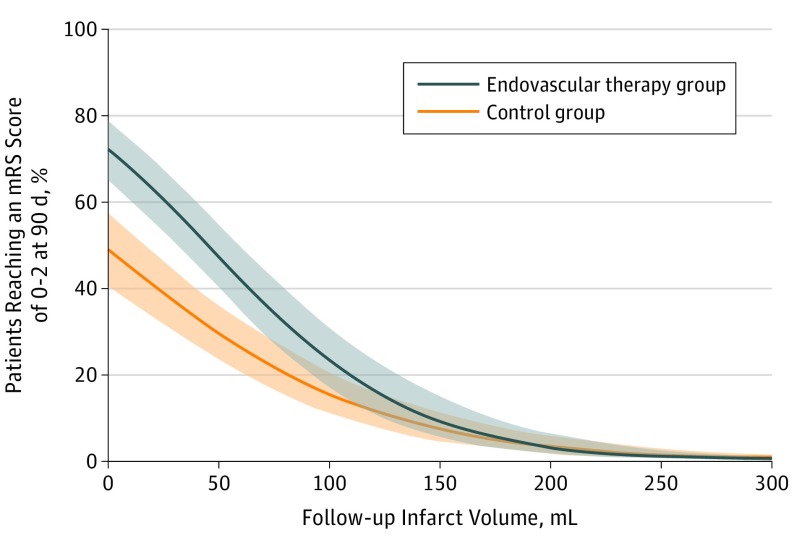
Figure 2 depicts the relationship of FIV with estimated probability of functional independence, stratified by treatment type and adjusted for baseline characteristics. The difference in estimated probability between treatments was mainly present in patients with smaller FIVs, where the absolute benefit of EVT appears highest. eFigure 1 and eFigure 2 in the Supplement show this relation for patients in the EVT group who achieved successful reperfusion (TICI score 2B to 3) and who did not achieve any reperfusion (TICI score 0), respectively. These illustrate an average increased likelihood of good outcome when reperfusion therapy was successful, but these associations lack statistical precision because of the dominant proportion of patients with successful reperfusion.
Figure 2. Association of Follow-up Infarct Volume With Estimated Probability of Functional Independence by Treatment and Adjusted for Baseline Characteristics.
The colored regions indicate 95% CIs. mRS indicates modified Rankin Scale.
Sensitivity Analyses
Results of the sensitivity analyses are shown in eTable 3 and eTable 4 in the Supplement. The first sensitivity analysis, which only included follow-up imaging past 48 hours of symptom onset (n = 688), showed no substantial differences from the main analysis (eTable 3 in the Supplement). The proportions of the relationship between EVT and functional outcome explained by FIV were 7% (95% CI, −5 to 22) and 23% (95% CI, −2 to 55) for the adjusted and unadjusted analysis, respectively. The second sensitivity analysis with MRI only (n = 279) showed proportions of 0% (95% CI, −25% to 24%) in adjusted analyses and 10% (95% CI, −38% to 56%) in unadjusted analyses (eTable 4 in the Supplement). However, treatment was not significantly associated with FIV (pathway a [Figure 1]). The lack of statistically significant findings as well as the wide confidence intervals signify that mediation in the MRI group could not be estimated, probably owing to limited sample size.
Discussion
Our analysis showed that FIV at subacute points only partially mediates the relationship between EVT and functional outcome at 3 months, despite being a predictor associated with functional outcome. Only 12% of the variance in functional outcome as captured by mRS score could be attributed to a difference in FIV, suggesting that there are additional unrecognized mechanisms that underpin the benefits of EVT or that there are limitations to the measurement of ischemic injury.
Several studies have previously addressed the association of FIV with functional outcome after an acute occlusion of the proximal anterior circulation.9,10,11,12 In concordance with our results, all have demonstrated that FIV is a predictor associated with functional outcome. Those studies also found that patients treated with EVT had significantly smaller FIVs compared with controls. Therefore, it is surprising that reduced infarct volume did not more fully explain the beneficial effect of EVT on functional outcome. As such, our study holds important information on the mismatch between radiological and clinical outcome measures after treatment. This mismatch might be a result of imprecise mRS or FIV measurements and varying infarct sizes because of differences in follow-up acquisition time. However, the facts that patients were randomized and that our sensitivity analysis in patients with late follow-up imaging did not alter the results argue that these factors cannot clarify the small proportion of the mediation explained by FIV. Because acute stroke therapies are designed to save brain tissue, our study indicates that we cannot simply assume that the amount of saved ischemic tissue as estimated from neuroimaging is key in such therapies.
Following our results, FIV may be used for estimating patient outcome, but more insight must be gathered in the mismatch between FIV and mRS score after EVT before FIV is found suitable as a valid proxy for treatment effect. There may be unrecognized mechanisms that affect this pathway, such as the eloquence of certain brain areas. A 2015 study26 demonstrated large differences between brain regions in functional outcome when affected by a stroke, even when corrected for infarct volume. We used laterality and involvement of follow-up ASPECTS regions as a measure of infarct location in our analysis and found that involvement of the internal capsule and M5 regions were associated with functional outcome. These areas are linked to the motor cortex and corticospinal tract, which underlines the importance of brain eloquence. This association is additionally likely because the mRS score is heavily weighted toward motor functions, particularly walking. In addition, mRS score may be an insensitive scale for capturing other important neurological deficits following an infarction, such as aphasia, neglect, and fine motor coordination.
In this study, we found that patients in the EVT group had significantly better functional outcome than patients in the control group even after controlling for FIV. This finding remained consistent in our sensitivity analyses with comparable effect sizes. The difference between treatment groups was mainly driven by patients with smaller infarcts in whom the effect of treatment was more pronounced. This effect was even stronger in patients who had successful reperfusion.
Several hypotheses could explain this phenomenon. First, studies have reported significant infarct growth between 24 hours and 1 week in follow-up imaging24 (whether or not driven by edema), but little is known about the course of infarction after the follow-up imaging period of 1 week. It could be that patients randomized to the control group continue to have hypoperfusion and, consequently, true infarct growth even a week after ictus. Second, the binary definition of infarcted vs noninfarcted may be an oversimplification. There may be variation in the severity of injury within tissue defined as infarcted, and this may potentially be less marked in patients receiving EVT. Moreover, tissue outside the defined infarct may have undetected injury (eg, selective neuronal loss), and this may be less severe in patients receiving EVT.27 Future studies are encouraged to use more sophisticated imaging approaches to increase insight in the pathophysiological process. Follow-up imaging near the 90-day mRS evaluation time or magnetization transfer ratio imaging to assess axonal damage might help to address this issue. Third, it is possible that the apparent benefits of EVT outside of FIV restriction are not from treatment alone but may also result from differences in aftercare. However, evidence is scarce to support these hypotheses.28
Mediation analysis is increasingly applied in biomedical sciences as it provides information to help in answering questions on why an intervention succeeded (or failed). In this study, we estimated the proportion of the association of EVT with functional outcome mediated by FIV via calculation of the difference between the regression coefficients, which is specifically intended for categorical outcomes and for estimation of effects via the OR.22 However, as evidence on ordinal regression models in mediation analysis is still limited, different statistical approaches may in likewise manner be suitable to estimate the proportion of the mediation,29 such as calculating the product of regression coefficients, as suggested by Liu et al,30 rather than the difference between them.
Of note is that FIV was log-transformed to best satisfy a linear relation with mRS. As such, the ORs are on a multiplicative instead of an additive scale; each time FIV doubles, a corresponding change in outcome is realized instead of effect per absolute mL change in FIV. Consequently, the interpretation of the results on the association of FIV with outcome is not very intuitive, but this study was designed to assess the proportion of the association of EVT with functional outcome mediated by FIV more than to investigate the association of FIV with outcome itself.
Limitations
Our study has limitations. First, FIV measurements might be less accurate on CT, and treatment may have other pathophysiological effect that cannot be seen on CT. However, in a sensitivity analysis using MRI data only, the size of the association of EVT with functional outcome explained by FIV did not differ significantly from the main analysis in both the adjusted and unadjusted analyses. Nevertheless, mediation could not be established because of an absence of an association of treatment with FIV. This may be explained by the small number of patients in this subanalysis with subsequent wide CIs, prohibiting an accurate estimation of the proportion of mediation. In addition, MRI is also not immune to FIV measurement error. However, it is noteworthy that a previous study investigating the association of FIV with functional outcome showed similar strengths of the association of both MRI and CT with mRS score as well as early and late imaging.12 Second, the fact that the last scan of each patient was selected for FIV assessment could have led to a bias, as patients with complications and deterioration would have had more late imaging.
Third, as old infarcts were excluded from the FIV, patients with a history of stroke might have ended up with a relatively poor clinical outcome, thereby weakening the overall association of FIV with functional outcome. However, as all observers were blinded to information outside of relevant imaging material, the impact of old infarcts on the mediation analysis can be neglected. Fourth, despite the 90-day mRS score being a well-validated and accepted scale, it remains a subjective patient-derived measure, as patients could not be blinded to treatment type. Being aware of this potential bias, all trials made attempts to blind the observer to treatment arm when evaluating mRS score and made use of standardized questionnaires to reduce variability. Although we could not completely reduce the impact of the study not being blinded, every effort was made to minimize its effect.
Fifth, to obtain trustworthy results from mediation analysis, unmeasured confounding must not exist between parameters in the hypothetical causal model (Figure 1). This is a strong assumption, especially when we consider that the independent contributions of the many interconnected biological processes to the final clinical outcome are not fully understood and are likely to vary among individuals. However, we can expect that this unmeasured confounding effect is minimal, as patients across all trials were randomized and all observers were blinded to information outside of relevant imaging material. Sixth, the proportions of the association of EVT with functional outcome explained by FIV could never reach the theoretical value of 0%. This is because our model would no longer suffice in such a situation, as the mediator FIV would not remain significant. The same applies for the hypothetical value of 100%, as all variables (even when that biological pathway is completely nonexistent) exert some form of influence, albeit being noise. As ordinal regression models in mediation analysis are only scarcely applied, evidence on these models is limited, leaving room for unidentified limitations. Further work needs to be done to fully understand the pathway of acute stroke treatment to infarction and of infarction to clinical outcome.
Conclusions
In this analysis of EVT for patients with acute ischemic stroke, FIV predicted functional outcome; however, a reduced infarct volume after successful treatment with EVT only explained 12% of the benefit of treatment. As it is currently measured, FIV is not yet a valid proxy for estimating treatment effect in phase II or III trials of acute ischemic stroke.
eTable 1. Four-step approach for testing mediation.
eTable 2. Associations of predictors with 90-day modified Rankin Scale score in multivariable modeling.
eTable 3. Mediating effect of FIV on the association between treatment and ordinal 90-day mRS in subgroup of patients with imaging obtained after 48 hours after onset only.
eTable 4. Mediating effect of FIV on the association between treatment and ordinal 90-day mRS in subgroup of patients with MRI modality only.
eFigure 1. Relation between adjusted FIV and estimated probability of functional independence between EVT, control, and reperfusion patients.
eFigure 2. Relation between adjusted FIV and estimated probability of functional independence between EVT, control, and nonreperfusion patients.
References
- 1.Berkhemer OA, Fransen PS, Beumer D, et al. ; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 3.Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators . Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. doi: 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 4.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. ; EXTEND-IA Investigators . Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009-1018. doi: 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 5.Jovin TG, Chamorro A, Cobo E, et al. ; REVASCAT Trial Investigators . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296-2306. doi: 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 6.Muir KW, Ford GA, Messow C-M, et al. ; PISTE Investigators . Endovascular therapy for acute ischaemic stroke: the Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) randomised, controlled trial. J Neurol Neurosurg Psychiatry. 2017;88(1):38-44. doi: 10.1136/jnnp-2016-314117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bracard S, Ducrocq X, Mas JL, et al. ; THRACE investigators . Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15(11):1138-1147. doi: 10.1016/S1474-4422(16)30177-6 [DOI] [PubMed] [Google Scholar]
- 8.Yoo AJ, Chaudhry ZA, Nogueira RG, et al. . Infarct volume is a pivotal biomarker after intra-arterial stroke therapy. Stroke. 2012;43(5):1323-1330. doi: 10.1161/STROKEAHA.111.639401 [DOI] [PubMed] [Google Scholar]
- 9.Zaidi SF, Aghaebrahim A, Urra X, et al. . Final infarct volume is a stronger predictor of outcome than recanalization in patients with proximal middle cerebral artery occlusion treated with endovascular therapy. Stroke. 2012;43(12):3238-3244. doi: 10.1161/STROKEAHA.112.671594 [DOI] [PubMed] [Google Scholar]
- 10.Albers GW, Goyal M, Jahan R, et al. . Relationships between imaging assessments and outcomes in Solitaire With the Intention For Thrombectomy as Primary Endovascular Treatment for acute ischemic stroke. Stroke. 2015;46(10):2786-2794. doi: 10.1161/STROKEAHA.115.010710 [DOI] [PubMed] [Google Scholar]
- 11.Al-Ajlan FS, Goyal M, Demchuk AM, et al. ; ESCAPE Trial Investigators . Intra-arterial therapy and post-treatment infarct volumes: insights from the ESCAPE randomized controlled trial. Stroke. 2016;47(3):777-781. doi: 10.1161/STROKEAHA.115.012424 [DOI] [PubMed] [Google Scholar]
- 12.Boers AMM, Jansen IGH, Beenen LFM, et al. . Association of follow-up infarct volume with functional outcome in acute ischemic stroke: a pooled analysis of seven randomized trials [published online April 7, 2018]. J Neurointerv Surg. doi: 10.1136/neurintsurg-2017-013724 [DOI] [PubMed] [Google Scholar]
- 13.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8(4):431-440. doi: 10.1002/sim.4780080407 [DOI] [PubMed] [Google Scholar]
- 14.Al-Ajlan FS, Al Sultan AS, Minhas P, et al. ; REVASCAT Investigators . Posttreatment infarct volumes when compared with 24-hour and 90-day clinical outcomes: insights from the REVASCAT randomized controlled trial. AJNR Am J Neuroradiol. 2018;39(1):107-110. doi: 10.3174/ajnr.A5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Compagne KCJ, Boers AMM, Marquering HA, et al. ; MR CLEAN Investigators . Follow-up infarct volume as a mediator of endovascular treatment effect on functional outcome in ischaemic stroke [published online July 9, 2018]. Eur Radiol. 2018. doi: 10.1007/s00330-018-5578-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 17.Boers AM, Marquering HA, Jochem JJ, et al. ; MR CLEAN investigators . Automated cerebral infarct volume measurement in follow-up noncontrast CT scans of patients with acute ischemic stroke. AJNR Am J Neuroradiol. 2013;34(8):1522-1527. doi: 10.3174/ajnr.A3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pexman JH, Barber PA, Hill MD, et al. . Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol. 2001;22(8):1534-1542. [PMC free article] [PubMed] [Google Scholar]
- 19.von Kummer R, Broderick JP, Campbell BCV, et al. . The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981-2986. doi: 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 20.Saver JL. Novel end point analytic techniques and interpreting shifts across the entire range of outcome scales in acute stroke trials. Stroke. 2007;38(11):3055-3062. doi: 10.1161/STROKEAHA.107.488536 [DOI] [PubMed] [Google Scholar]
- 21.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173-1182. doi: 10.1037/0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- 22.Vanderweele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. 2010;172(12):1339-1348. doi: 10.1093/aje/kwq332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedman LS, Graubard BI, Schatzkin A. Statistical validation of intermediate endpoints for chronic diseases. Stat Med. 1992;11(2):167-178. doi: 10.1002/sim.4780110204 [DOI] [PubMed] [Google Scholar]
- 24.Bucker A, Boers AM, Bot JCJ, et al. ; MR CLEAN Trial Investigators (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands) . Associations of ischemic lesion volume with functional outcome in patients with acute ischemic stroke: 24-hour versus 1-week imaging. Stroke. 2017;48(5):1233-1240. doi: 10.1161/STROKEAHA.116.015156 [DOI] [PubMed] [Google Scholar]
- 25.Krongold M, Almekhlafi MA, Demchuk AM, Coutts SB, Frayne R, Eilaghi A. Final infarct volume estimation on 1-week follow-up MR imaging is feasible and is dependent on recanalization status. Neuroimage Clin. 2014;7:1-6. doi: 10.1016/j.nicl.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ernst M, Forkert ND, Brehmer L, et al. . Prediction of infarction and reperfusion in stroke by flow- and volume-weighted collateral signal in MR angiography. AJNR Am J Neuroradiol. 2015;36(2):275-282. doi: 10.3174/ajnr.A4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baron J-C, Yamauchi H, Fujioka M, Endres M. Selective neuronal loss in ischemic stroke and cerebrovascular disease. J Cereb Blood Flow Metab. 2014;34(1):2-18. doi: 10.1038/jcbfm.2013.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pendlebury ST, Lee MA, Blamire AM, Styles P, Matthews PM. Correlating magnetic resonance imaging markers of axonal injury and demyelination in motor impairment secondary to stroke and multiple sclerosis. Magn Reson Imaging. 2000;18(4):369-378. doi: 10.1016/S0730-725X(00)00115-6 [DOI] [PubMed] [Google Scholar]
- 29.VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health. 2016;37(1):17-32. doi: 10.1146/annurev-publhealth-032315-021402 [DOI] [PubMed] [Google Scholar]
- 30.Liu H, Zhang Y, Luo F. Mediation analysis for ordinal outcome variables In: Millsap R, Bolt D, van der Ark L, Wang WC, eds. Quantitative Psychology Research. Cham, Switzerland: Springer; 2015:429-450. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Four-step approach for testing mediation.
eTable 2. Associations of predictors with 90-day modified Rankin Scale score in multivariable modeling.
eTable 3. Mediating effect of FIV on the association between treatment and ordinal 90-day mRS in subgroup of patients with imaging obtained after 48 hours after onset only.
eTable 4. Mediating effect of FIV on the association between treatment and ordinal 90-day mRS in subgroup of patients with MRI modality only.
eFigure 1. Relation between adjusted FIV and estimated probability of functional independence between EVT, control, and reperfusion patients.
eFigure 2. Relation between adjusted FIV and estimated probability of functional independence between EVT, control, and nonreperfusion patients.