Key Points
Question
Can hydrocephalus be part of the clinical spectrum of congenital Zika syndrome?
Findings
This case series describes 24 patients who developed hydrocephalus between 3 and 18 months of age and had at least 1 test positive for anti-Zika antibodies, including 14 who had symptoms and signs suggestive of hydrocephalus, 18 who had cerebellar or brainstem hypoplasia at baseline, and 2 who had no such symptoms but were found to have reduced brain volume on repeated imaging; at a second computed tomographic scan, all showed marked increases of ventricular volume and reduction of brain tissue.
Meaning
Hydrocephalus may be a complication of congenital Zika syndrome with presenting signs and symptoms that are challenging to recognize; monitoring for it, including assessing the potential harbinger of cerebellar or brainstem hypoplasia, should be part of the standard care of patients with this condition.
This case series describes clinical and imaging findings in 24 infants born with congenital Zika syndrome who developed hydrocephalus.
Abstract
Importance
Hydrocephalus is a treatable but potentially fatal complication that has not been previously described in congenital Zika syndrome (CZS).
Objective
To describe the clinical features and imaging findings in 24 patients with congenital Zika syndrome (CZS) who developed hydrocephalus.
Design, Setting, and Participants
This case series included patients with hydrocephalus who were born in October and November 2015 and followed up until mid-2017 in the 2 largest national referral centers for CZS in Brazil. The participants included consecutively enrolled children with a clinical and laboratorial diagnosis of CZS who developed clinical and/or image findings suggestive of hydrocephalus and who were confirmed to experience increased intracranial hypertension during ventriculoperitoneal shunt procedures.
Main Outcomes and Measures
To retrospectively describe clinical and image findings in these 24 patients.
Results
This multicenter cohort included 308 patients with CZS; 24 consecutive children were enrolled in this study. These children were aged between 3 to 18 months, and 13 of 24 (54%) were female. All patients presented with at least 1 positive test result for anti-Zika antibodies in cerebrospinal fluid or serum and had classic signs of CZS. At the time of hydrocephalus diagnosis, only 14 of 24 patients (58%) had symptoms and signs suggestive of hydrocephalus (mainly worsening seizures, vomiting, irritability, and/or sudden increase of head circumference percentile). Two of 24 patients (8%) had no symptoms suggestive of hydrocephalus but were found to have reduced brain volume on repeated imaging. Cerebellar or brainstem hypoplasia on baseline imaging were found in 18 of 23 patients (78%). At the second computed tomographic scan, all patients showed a marked increase of ventricular volume, compatible with communicating hydrocephalus, and reduction of brain tissue that was visibly worse than on baseline imaging for the 23 patients with repeated scans.
Conclusions and Relevance
We present evidence that hydrocephalus is a complication of CZS in at least a proportion of patients. The clinical spectrum of this condition continues to evolve, but given that presenting signs and symptoms of hydrocephalus can be challenging to recognize in CZS, we provisionally recommend that high suspicion and appropriate monitoring for hydrocephalus should be part of the standard care of patients with CZS.
Introduction
Zika virus (ZIKV) is an arthropod-borne virus of the Flaviviridae family that can cause a mild febrile illness with maculopapular rash, joint pain, and conjunctivitis.1 The first report of ZIKV infection in Brazil was in 2015,2 and a dramatic increase in cases of microcephaly was subsequently detected, especially in the northeast of the country. In April 2016, the causative relationship between microcephaly and ZIKV was established.3
The clinical spectrum of congenital Zika syndrome (CZS) is not completely understood, and confirmed cases presenting with less severe manifestations have been described.4 Presentations of CZS go beyond microcephaly to include other clinical patterns, such as retinal abnormalities with visual impairment,5 hearing loss,6 and limb abnormalities, such as arthrogryposis.7 This article describes, for the first time to our knowledge, a series of infants with CZS who developed hydrocephalus, discusses possible causes for this expansion of the clinical phenotype, and suggests that repeated neuroimaging may be necessary to detect this complication.
Methods
Patients with CZS are followed up as part of a descriptive study at 2 reference centers in Brazil: the Association for Assistance of Disabled Children of Pernambuco (in Recife) and Albert Sabin Children´s Hospital, Ceará (in Fortaleza). As part of this study, patients were identified retrospectively who developed hydrocephalus and underwent ventriculoperitoneal shunt (VPS) surgery as part of routine care.
On initial investigation, all patients underwent detailed physical and neurologic evaluations and brain imaging by nonenhanced head computed tomography (CT) or nonenhanced magnetic resonance imaging (MRI) of the head. On follow-up after 3 months of age, patients with CZS with clinical suspicion of developing hydrocephalus or unexplained worsening of other signs and symptoms, underwent repeated brain imaging. As these evaluations pointed toward an actual risk for developing hydrocephalus, we decided to systematically proceed with repeated imaging of all patients at approximately 10 months of age.
Inclusion Criteria
The diagnosis of CZS was established under the following criteria: (1) a brain imaging result suggestive of congenital infection, (2) a full negative investigation for the main causes of congenital infections producing microcephaly, and (3) detection of anti-Zika antibodies in serum and/or cerebrospinal fluid (CSF) in either the Zika IgM antibody capture enzyme-linked immunosorbent assay (Zika MAC-ELISA) and/or the plaque-reduction neutralization testing (PR-NT).8 Samples of CSF and serum from patients with CZS were tested for ZIKV infection by real-time quantitative reverse-transcription polymerase chain reaction9 and by IgM-antibody MAC-ELISA.8 A PR-NT for anti-ZIKV IgG was performed when the MAC-ELISA test result for anti-ZIKV antibodies was negative in patients with cases clinically compatible with CZS. The samples were collected at the time of suspicion of microcephaly during the early newborn period and afterward, during neurosurgery.
Additional CSF Studies
The CSF samples from patients from whom such samples were available were analyzed for total protein content and protein electrophoretic separation. Four patients had additional total cell counts analyzed.
Brain Image Analyses
Initial nonenhanced head CT scans were performed during the ZIKV outbreak in different radiology centers and with different CT equipment (with slice detectors varying from 6 to 64 images on initial CT scan), making the imaging impossible to standardize. The follow-up nonenhanced head CT scans were all performed in 4 radiology centers. The images were analyzed by 3 physicians (V.v.d.L., N.C.d.L.P., and A.N.H.) in 2 meetings. The initial and follow-up CT images from each patient were analyzed side by side at a workstation, after adjustments of window, slice thickness, and planes, to equalize the images as completely as possible. The 3 physicians answered questions at the same time, together, using a form created by the authors.
Initial CT scans were reviewed for presence and location of brain calcifications; malformations of cortical development, if present, were noted, as well as prominence of occipital bone. Increased extraaxial CSF spaces, cerebellum or brainstem hypoplasia, enlarged cisterna magna, reduced cerebral volume, or ventriculomegaly were further classified as mild, moderate, or severe.
Cerebral ventricular size, cerebral volume, extra-axial CSF spaces, and cisterna magna size were evaluated on follow-up CT scans and recorded as unchanged, reduced, or increased. The degree of prominence of the occipital bones and separation of the cranial sutures was also assessed.
Ethical Issues
All medical investigation procedures described were conducted as part of standard clinical care. Signed informed consent was obtained for publication of images presented in this article from parents of patients as part of our ongoing longitudinal studies of CZS, which were approved by the ethics boards of Association for Assistance of Disabled Children (Recife) and Albert Sabin Children´s Hospital (Fortaleza).
Statistical Analysis
Descriptive statistical analyses were performed using SPSS version 24 (IBM). A significance level of .05 (2-sided) was used for all hypothesis tests.
Results
Patient Presentation and Demographics
Our longitudinal studies of patients with CZS include a total of 308 individuals in Recife, Pernambuco, Brazil (n = 198) and Fortaleza, Ceará, Brazil (n = 110). Of these, 24 patients (7.8%) had a confirmed diagnosis of hydrocephalus with suggestive signs of increased intracranial pressure and underwent VPS neurosurgery. Dates of birth of patients with CZS and hydrocephalus ranged from October 2015 to November 2015. Twenty-one of the 24 patients (88%) were followed up in the state of Pernambuco, and the remaining 3 (13%) in the state of Ceará. Three patients (13%) were born at preterm gestation, and 21 (88%) at full term. The age of patients who fulfilled inclusion criteria for CZS with hydrocephalus were between 3 to 18 months of postnatal life. Thirteen patients of 24 (54%) were female. During VPS placement, all patients had CSF collected for further analysis. The MAC-ELISA test was performed on the CSF collected during the surgical procedure from all patients, resulting in positive findings in 23 (96%) and a negative finding in 1 (4%). Seven of 24 patients (29%) had CSF collected during the surgery analyzed for quantitative reverse-transcription polymerase chain reaction for ZIKV; all were negative. Fifteen of 24 patients’ mothers (60%) described experiencing cutaneous rashes consistent with ZIKV between the second and seventh gestational month, of whom 1 (7%) experienced this at the first trimester, 10 (67%) in the second trimester, 2 (13%) at the third trimester, and 2 (13%) without clear information on trimester. Sixteen of 24 newborns (67%) had appropriate weight for gestational age. In all patients, microcephaly and craniofacial disproportion were diagnosed at birth. Seventeen of 24 newborns (71%) had a prominent occipital bone protuberance at birth, detected by direct clinical assessment and confirmed by CT scan; 20 of 24 (80%) had redundant skin on the scalp.
Clinical Signs and Symptoms of Hydrocephalus
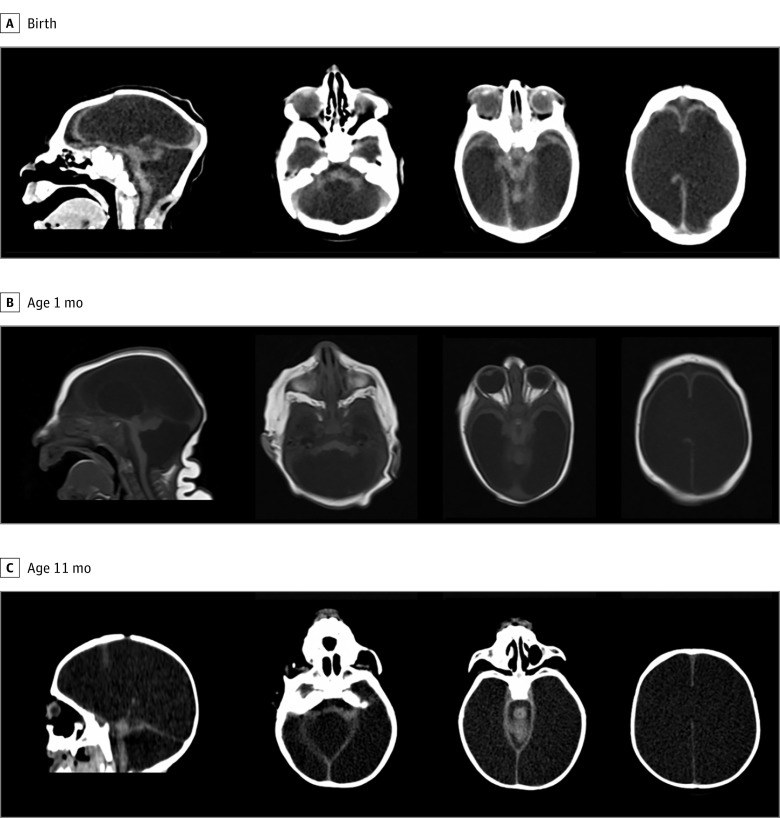
At the time of hydrocephalus diagnosis, 14 of 24 infants (58%) had symptoms and signs suggestive of hypertensive hydrocephalus, mainly vomiting, irritability, and/or sudden increases in head circumference percentile. Nonspecific neurological symptoms and signs of hydrocephalus were present in 8 of 24 patients (33%). Worsening seizures was the most frequent symptom, present in 13 patients (54%). Only 1 patient (4%) presented with vertical gaze palsy. Two of 24 patients (8%) did not present clear clinical signs for the diagnosis of hydrocephalus but presented with reduction of brain volume on repeated imaging. In Figure 1, we show the imaging examinations of 1 of these patients, with a progressive loss of the cerebral parenchyma, associated with an increase in the ventricles on examination at birth, 1 month of age, and 11 months of age, despite not presenting at the clinic with intracranial hypertension. Surgery was needed to preserve the remainder of the parenchyma.
Figure 1. Representative Brain Image of a Patient Without Clinical Evidence of Hydrocephalus but With Progressive Loss of the Cerebral Parenchyma Associated With an Increase in the Ventricles.
Considering the marked increase in ventricular size, only 5 patients (21%) presented with sudden growth of head circumference at the time of hydrocephalus diagnosis, and 2 of 24 (8%) reached an age-appropriate head circumference, while 22 of 24 (92%) remained microcephalic. All patients were noted to have CSF flowing steadily out of the dura mater during surgical entry into the subarachnoid space at the time of the VPS placement, suggesting high-pressure hydrocephalus.
Other Neurologic Disability
All 24 patients presented with severe neurological disorders with cognitive dysfunction, no interaction with the environment, and no motor skills. Dysphagia was evident in all patients. Twenty-three of 24 (96%) had epilepsy. Arthrogryposis was diagnosed in 7 of 24 patients (28%). Table 1 summarizes the clinical features of the patients, with additional details presented in eTable 1 and eTable 2 in the Supplement. All patients presented improvement of the symptoms after the VPS surgical procedure.
Table 1. Summary of Clinical Features of Patientsa.
| Characteristic | Patients, No. (%) |
|---|---|
| Total | 24 |
| Girls | 13 (54) |
| Characteristics at birth | |
| Gestational age, mean (SD) [range], wk | 38.5 (2.6) [31-41] |
| Appropriate birth weight for gestational age | 16 (67) |
| Severe microcephalyb | 20 (83) |
| Arthrogryposis | 7 (29) |
| Craniofacial dysproportion | 24 (100) |
| Characteristics at follow-up | |
| Epilepsy | 23 (96) |
| No motor milestone achieved | 24 (100) |
| Characteristics at diagnosis of hydrocephalus | |
| Age, mean (SD) [range], mo | 11.8 (3.5) [3-18] |
| Signs and symptoms suggestive of hydrocephalus | 14 (58) |
| Worsening seizures | 13 (54) |
| Vomiting | 9 (38) |
| Irritability | 7 (29) |
| Worsening dysphagia | 4 (17) |
| Drowsiness | 3 (13) |
| Dysjunction of sutures | 2 (8) |
| Hypertension | 1 (4) |
| Respiratory symptoms | 1 (4) |
| Sleep abnormality | 1 (4) |
| Vertical gaze palsy | 1 (4) |
| No symptoms suggestive of hydrocephalus | 3 (13) |
Some patients presented with more than 1 feature suggestive of hydrocephalus. Detailed features in each case can be found in eTable 1 (baseline clinical features) and eTable 2 in the Supplement (clinical features of hydrocephalus).
Severe microcephaly at birth defined as 3 SD less than the mean for gestational age and sex.
CSF Analysis Results
Sixteen of 21 patients (76%) with CSF available had their samples analyzed for total protein content and electrophoretic separation. In 14 of the 16 patients (88%), we found protein content to be normal; in 2 patients (13%), protein analysis was not possible owing to blood contamination and hemolysis in the sample. The results from electrophoretic separation were obtained from 13 samples, showing normal γ globulin, prealbumin, albumin, and α-1 and α-2 globulin fractions. Notably, in 4 patients, increased levels of β-globulin were detected. Additionally, total cell counts were performed in 4 samples and were normal. eTable 3 in the Supplement shows the main findings of the CSF analysis.
Brain Imaging
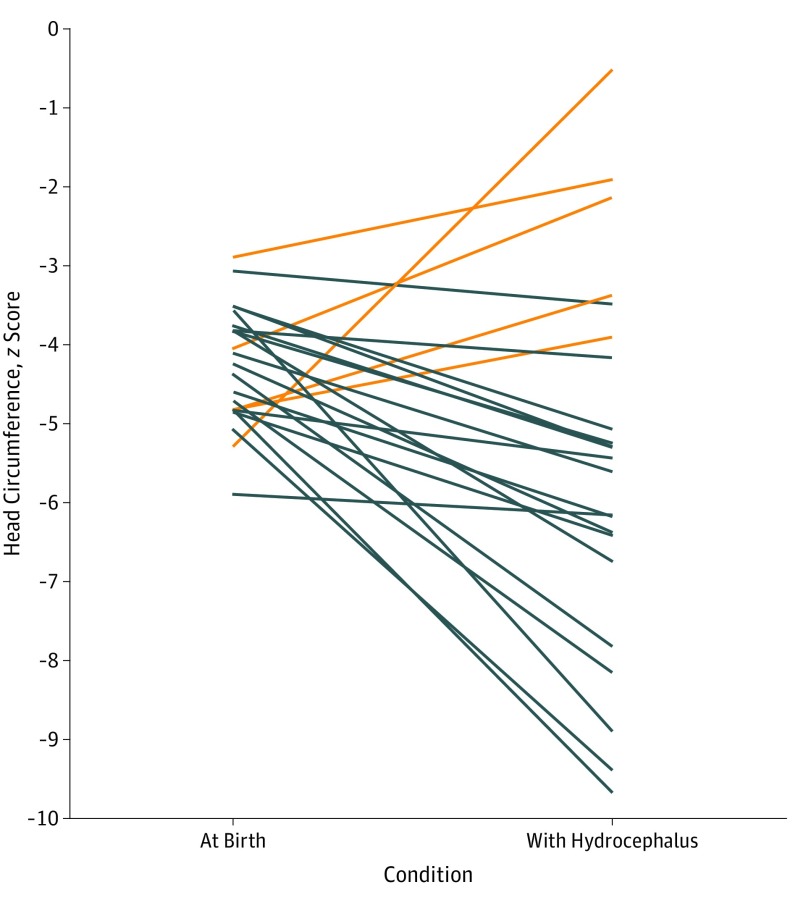
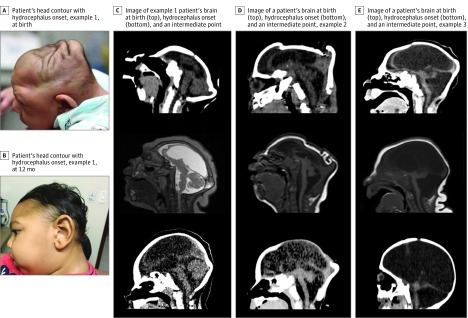
Brain images of all patients revealed malformations of cortical development and a pattern of calcifications variable in size, shape, and distribution, predominantly in the cortex and subcortical white matter (especially in the transition between the cortex and white matter). Eighteen of 23 (78%) had cerebellar or brainstem hypoplasia, and 18 patients (78%) had enlargement of the cisterna magna, including 6 with enlarged fourth ventricles. On the second CT scan, all patients showed marked increase of the ventricular volumes (including in the third and fourth ventricles in 16 of 23 patients [70%]), compatible with diffuse reduction of brain mass, together with surgically establishing communicating hydrocephalus. Representative images of patients with CZS who had hydrocephalus are shown in the eFigure in the Supplement. Most patients (n = 19) became relatively more microcephalic, despite the onset of hydrocephalus. Five patients had increases in their z scores with the onset of hydrocephalus, with 2 patients achieving normalization of the z score to −2 SD or better. The comparative head circumference z scores at birth and at diagnosis of hydrocephalus are shown in Figure 2. Several patients had change of their head contour with the onset of hydrocephalus, as shown in Figure 3.
Figure 2. Comparative Head Circumference z Scores at Birth and Diagnosis of Hydrocephalus.
Orange lines represent patients whose z scores increased with hydrocephalus onset; black lines represent patients whose z scores decreased.
Figure 3. Change of Head Contour With Onset of Hydrocephalus in Patients With Congenital Zika Syndrome.
One patient had only 1 imaging study, obtained at the time of hydrocephalus diagnosis and characterized by a malformation of cortical development with thin cerebral cortex, severe ventriculomegaly, and calcifications in the cortical and subcortical white matter and the basal ganglia. This patient also had severe hypoplasia of brainstem and cerebellum with an enlarged cisterna magna. Table 2 summarizes the brain imaging findings.
Table 2. Summary of Brain Imaging Findings in Patients Reported With Congenital Zika Syndrome and Hydrocephalus.
| Characteristic | Patients, No. (%) |
|---|---|
| Brain imaging abnormalities | 24 (100) |
| Calcification | 24 (100) |
| Ventriculomegaly | 24 (100) |
| Prominence of occipital bone | 18 (75) |
| Increased extraaxial CSF space | 22 (92) |
| Cerebellum or brainstem hypoplasia | 18 (75) |
| Enlarged cisterna magna | 18 (75) |
| At the time of hydrocephalus diagnosis | |
| Reduction brain volume in comparison with previous image | 24 (100) |
| Suture disjunction | 3 (13) |
| Increased cisterna magna on follow-up brain image | 17 (71) |
Abbreviation: CSF, cerebrospinal fluid.
Discussion
We present 24 patients with CZS who developed hydrocephalus and underwent VPS surgery. The association of severe microcephaly with hydrocephalus in some infants with CZS is surprising, and it is important for clinicians who care for children with CZS to be aware of this potential complication. Our observations stem from clinical access to the largest group of oldest surviving patients with CZS in Brazil. Consequently, we are continually gaining new insights into the evolution and the natural history of this disease, its clinical spectrum, and its pathophysiology.
It is almost a century since Dandy10 made the first experimental studies on hydrocephalus, but in most cases the underlying mechanism remains unknown. The conventional view is that CSF malabsorption owing to hindrance of the CSF circulation causes either obstructive or communicating hydrocephalus. Analyses of the intracranial hydrodynamics associated with the pulse pressure show that this is an oversimplification.10
Acute hydrocephalus is caused by an intraventricular CSF obstruction in accordance with the conventional view. In contrast, it has been suggested that chronic hydrocephalus is caused by decreased intracranial compliance.10,11 Congenital and neonatal hydrocephalus can be caused by a wide variety of developmental abnormalities or insults, including congenital infection.12 Both Taxoplasmosis gondii and cytomegalovirus have the potential to infect the developing fetus and have been identified as causes of congenital and neonatal hydrocephalus.13 Hydrocephalus in patients with congenital toxoplasmosis has traditionally been attributed to obstruction of the aqueduct of Sylvius caused by an ependymitis.14
To our knowledge, little is known about CZS and hydrocephalus. Sarno et al15 described a case of hydrops fetalis and hydranencephaly in a case of fetal demise in which they had obtained ZIKV-specific reverse-transcription polymerase chain reaction amplification products from extracts of cerebral cortex, medulla oblongata, cerebrospinal fluid, and amniotic fluid. Experimental evidence with human derived neuroprogenitor cells in vitro16,17 and in vivo, using mice18,19 and primate models,20,21 suggests that a mechanism involved in the primary loss of neural cells during brain development in CZS is the pathological induction of apoptosis17,22,23,24,25 among cortical neuroprogenitor cells.23,25,26
In patients with CZS who have been autopsied, the presence of virus particles in central nervous system cells was demonstrated by electron microscopy as well as genomic sequencing,27,28 implying that viral clearance was not achieved in the fetus after primary maternal infection. Aid et al29 showed that ZIKV can persist in CSF and lymph nodes of infected rhesus monkeys for weeks after the virus has been cleared from peripheral blood, urine, and mucosal secretions and that viral persistence in both CSF and lymph nodes correlated with the upregulation of mechanistic target of rapamycin kinase, proinflammatory, and antiapoptotic signaling pathways, as well as the downregulation of extracellular matrix signaling pathways. However, they did not detect ZIKV-specific antibodies in CSF.
The fact that we have demonstrated the presence of anti-Zika IgM antibodies in the CSF collected during VPS surgery in 96% of the patients in this study may be considered evidence that ZIKV antigen presentation occurs in the intrathecal space, perhaps owing to the development of tertiary lymphoid organs in situ.30,31 This antibody response is conceivably connected with an evolving immune sensitization to ZIKV antigens rather than to a virus-triggered inflammatory reaction because of the absence of visible necrosis in the imaging studies and the lack of other inflammatory findings as described in the literature.25,27 As shown in eTable 3 in the Supplement, the CSF analysis obtained during VPS surgery showed no evidence of ongoing inflammatory response as measured by cell count and protein concentration in the cases in which these parameters could be analyzed. In addition, autophagic behavior and modulation of apoptotic genes in host cells has been demonstrated experimentally32,33,34 as a survival strategy of the flavivirus, because preservation of the host cell alive assures virus persistence and replication.
Considering that a relatively low-grade, persistent ZIKV infection in the central nervous system of surviving infants may exist, it would allow persistence of the pathologically induced apoptosis process over time. This candidate mechanism could be implicated in the observed progressive decrease in the size of the brain mass seen on follow-up CT scans, while the production of CSF remains active. Together, these mechanisms would lead to progressive enlargement of ventricular cavities, evolving toward the severe hydrocephalus that we have so far observed. With its slow progression, this ongoing dilation may be classified as a chronic hydrocephalus-like condition11 in which the expansion of space occupied by CSF will overcome the complacency of the brain in compensating for the CSF hydrostatic pressure, thereby determining the hypertensive intracranial status.
We also suggest that the progressive increase in CSF pressure may contribute additional harm to the central nervous system and cause stretching and loss of brain mass directly as a result of both its physical compressive effect and by opposing or overcoming blood perfusion pressures. In this context, the main mechanisms that physiologically regulate the turnover of CSF (including its production at choroid plexuses, venous absorption by dural Pachionian granulations, the inflow of CSF through the periarterial pathway toward the neuropil, and the outflow of CSF and interstitial fluid throughout the paravenous vessels pathways of the lymphatic system35,36,37,38,39) would hardly be impaired because of the progressive regression of brain mass structures as a whole. Considering the imaging findings, we infer that these losses include neurons and astroglia in parallel with their attendant vascular framework. Additionally, the outflow that connects the paravenous system, with the recently described dural lymphatic system draining into deep cervical lymph nodes,37,40 would likely also be impaired by the increased hydrostatic pressure determined by the CSF constraint. To summarize, the evolution of the initial phenotype of microcephaly with ventriculomegaly to the later phenotype of severe hydrocephalus and probably high intracranial pressure is very likely multifactorial in nature.
Magnetic resonance imaging of some cases of this series showed an imaging pattern of variable degree in the severity and shape of cerebellar hypoplasia, while other patients had findings of a significant enlargement of the cisterna magna. In these cases, we may postulate a coexisting association of some degree of CSF overproduction and/or malformative points of obstructed CSF resorption. In addition, another possible mechanism of hydrocephalus in these patients is the enlargement of the fourth ventricle resulting in obstruction of the fourth ventricular outflow pathways through the lateral and median foramina, resulting in a counterclockwise rotation of the extremely hypoplastic cerebellum. In such cases, the cerebellum would be more likely involved and therefore tend to be small, with the fourth ventricle enlarged. As a result of the tetraventricular hydrocephalus, these patients may be less severely affected by microcephaly, and they would be expected to have a thin cerebral mantle.
We report here the increase in β-globulin in CSF in some patients with CZS (eTable 3 in the Supplement). The increased CSF β-globulin concentration is attributed to intrathecal synthesis owing to immune activation,40 including nervous tissues destruction and degeneration, neuroinflammation, and neuroinfections.31 In the present cases, the CSF analysis did not show increases in other defined inflammatory parameters, such as total protein concentration and cell counts. We therefore infer that increased β-globulin in some cases may be not correlated with significant exudative inflammatory reaction in the central nervous system but could result from progressive apoptosis and associated microglial activation.31
Finally, clear clinical signs and symptoms of hydrocephalus were not always present in the included patients, suggesting the need for routine central nervous system reimaging after a few months of life or in the presence of clinical worsening, even with nonspecific symptoms. Further studies are required to determine the overall risk of hydrocephalus in patients with CZS, if there is an age of maximal risk, and methods for early detection. Long-term follow-up is also needed to determine the benefits of surgical intervention. Our data indicate that the clinical picture of CZS has not yet been fully defined, and the possibility of hydrocephalus in the setting of severe microcephaly should be appreciated by clinicians caring for these children.
eTable 1. Baseline Clinical Features.
eTable 2. Clinical features at the time of hydrocephalus diagnosis.
eTable 3. Main findings in CSF analysis.
eFigure. Representative brain image near birth and at diagnosis of hydrocephalus in patients with CZS.
References
- 1.Musso D, Cao-Lormeau VM, Gubler DJ. Zika virus: following the path of dengue and chikungunya? Lancet. 2015;386(9990):243-244. doi: 10.1016/S0140-6736(15)61273-9 [DOI] [PubMed] [Google Scholar]
- 2.Zanluca C, Melo VC, Mosimann AL, Santos GI, Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015;110(4):569-572. doi: 10.1590/0074-02760150192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for casuality. N Engl J Med. 2016;374(20):1981-1987. doi: 10.1056/NEJMsr1604338 [DOI] [PubMed] [Google Scholar]
- 4.van der Linden V, Pessoa A, Dobyns W, et al. Description of 13 infants born during October 2015-January 2016 with congenital Zika virus infection without microcephaly at birth—Brazil. MMWR Morb Mortal Wkly Rep. 2016;65(47):1343-1348. doi: 10.15585/mmwr.mm6547e2 [DOI] [PubMed] [Google Scholar]
- 5.de Paula Freitas B, de Oliveira Dias JR, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil. JAMA Ophthalmol. 2016;134(5):529-535. doi: 10.1001/jamaophthalmol.2016.0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leal MC, Muniz LF, Caldas Neto SD, van der Linden V, Ramos RC. Sensorineural hearing loss in a case of congenital Zika virus. Braz J Otorhinolaryngol. 2016;pii:S1808-8694(16)30127-6. doi: 10.1016/j.bjorl.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Linden V, Rohim Filho EL, Lins OG, et al. Congenital Zika syndrome with arthrogryposis: retrospective case series study. BMJ. 2016;354:i3899. doi: 10.1136/bmj.i3899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordeiro MT, Brito CAA, Pena LJ, et al. Results of a Zikavírus (ZIKV) immunoglobulin M-specific assay are highly correlated with detection of neutralizing anti-ZIKV antibodies in neonates with congenital disease. J Infect Dis. 2016;214(12):1897-1904. doi: 10.1093/infdis/jiw477 [DOI] [PubMed] [Google Scholar]
- 9.Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14(8):1232-1239. doi: 10.3201/eid1408.080287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greitz D. Paradigm shift in hydrocephalus research in legacy of Dandy’s pioneering work: rationale for third ventriculostomy in communicating hydrocephalus. Childs Nerv Syst. 2007;23(5):487-489. doi: 10.1007/s00381-007-0303-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnamurthy S, Li J. New concepts in the pathogenesis of hydrocephalus. Transl Pediatr. 2014;3(3):185-194. doi: 10.3978/j.issn.2224-4336.2014.07.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sivagnanam M, Jha NK. Hydrocephalus: an overview In: Pant S, ed. Hydrocephalus. Zagreb, Croatia: In Tech Open; 2012:1-20. doi: 10.5772/32502 [DOI] [Google Scholar]
- 13.Simeone RM, Rasmussen SA, Mei JV, et al. A pilot study using residual newborn dried blood spots to assess the potential role of cytomegalovirus and Toxoplasma gondii in the etiology of congenital hydrocephalus. Birth Defects Res A Clin Mol Teratol. 2013;97(7):431-436. doi: 10.1002/bdra.23138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barkovich AJ, Raybaud C. Pediatric Neuroimaging. 5th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 15.Sarno M, Sacramento GA, Khouri R, et al. Zika virus infection and stillbirths: a case of hydrops fetalis, hydranencephaly and fetal demise. PLoS Negl Trop Dis. 2016;10(2):e0004517. doi: 10.1371/journal.pntd.0004517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang BL. Zika virus as a causative agent for primary microencephaly: the evidence so far. Arch Microbiol. 2016;198(7):595-601. doi: 10.1007/s00203-016-1268-7 [DOI] [PubMed] [Google Scholar]
- 17.Garcez PP, Loiola EC, Madeiro da Costa R, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352(6287):816-818. doi: 10.1126/science.aaf6116 [DOI] [PubMed] [Google Scholar]
- 18.Cugola FR, Fernandes IR, Russo FB, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534(7606):267-271. doi: 10.1038/nature18296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowall SD, Graham VA, Rayner E, et al. A susceptible mouse model for Zika virus infection. PLoS Negl Trop Dis. 2016;10(5):e0004658. doi: 10.1371/journal.pntd.0004658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RP, et al. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med. 2016;22(11):1256-1259. doi: 10.1038/nm.4193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dudley DM, Aliota MT, Mohr EL, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204. doi: 10.1038/ncomms12204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang H, Hammack C, Ogden SC, et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016;18(5):587-590. doi: 10.1016/j.stem.2016.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blomgren K, Leist M, Groc L. Pathological apoptosis in the developing brain. Apoptosis. 2007;12(5):993-1010. doi: 10.1007/s10495-007-0754-4 [DOI] [PubMed] [Google Scholar]
- 24.Frumence E, Roche M, Krejbich-Trotot P, et al. The South Pacific epidemic strain of Zika virus replicates efficiently in human epithelial A549 cells leading to IFN-β production and apoptosis induction. Virology. 2016;493:217-226. doi: 10.1016/j.virol.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 25.Jungmann P, Pires P, Araujo Júnior E. Early insights into Zika’s microcephaly physiopathology from the epicenter of the outbreak: teratogenic apoptosis in the central nervous system. Acta Obstet Gynecol Scand. 2017;96(9):1039-1044. doi: 10.1111/aogs.13184 [DOI] [PubMed] [Google Scholar]
- 26.Shao Q, Herrlinger S, Yang SL, et al. Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage. Development. 2016;143(22):4127-4136. doi: 10.1242/dev.143768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mlakar J, Korva M, Tul N, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951-958. doi: 10.1056/NEJMoa1600651 [DOI] [PubMed] [Google Scholar]
- 28.Driggers RW, Ho CY, Korhonen EM, et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med. 2016;374(22):2142-2151. doi: 10.1056/NEJMoa1601824 [DOI] [PubMed] [Google Scholar]
- 29.Aid M, Abbink P, Larocca RA, et al. Zika virus persistence in the central nervous system and lymph nodes of rhesus monkeys. Cell. 2017;169(4):610-620.e14. doi: 10.1016/j.cell.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vandvik B, Vartdal F, Norrby E. Herpes simplex virus encephalitis: intrathecal synthesis of oligoclonal virus-specific IgG, IgA and IgM antibodies. J Neurol. 1982;228(1):25-38. doi: 10.1007/BF00313407 [DOI] [PubMed] [Google Scholar]
- 31.Svatonová J. Critical evaluation of the biological role of IgM in cerebrospinal fluid in inflammatory and other diseases of the nervous system. Folia Microbiol (Praha). 2006;51(5):485-491. doi: 10.1007/BF02931596 [DOI] [PubMed] [Google Scholar]
- 32.Heaton NS, Randall G. Dengue virus and autophagy. Viruses. 2011;3(8):1332-1341. doi: 10.3390/v3081332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang Q, Luo Z, Zeng J, et al. Zika virus NS4A and NS4B proteins deregulate akt-mtor signaling in human fetal neural stem cells to inhibit neurogenesis and induce autophagy. Cell Stem Cell. 2016;19(5):663-671. doi: 10.1016/j.stem.2016.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee YR, Lei HY, Liu MT, et al. Autophagic machinery activated by dengue virus enhances virus replication. Virology. 2008;374(2):240-248. doi: 10.1016/j.virol.2008.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rekate HL. A consensus on the classification of hydrocephalus: its utility in the assessment of abnormalities of cerebrospinal fluid dynamics. Childs Nerv Syst. 2011;27(10):1535-1541. doi: 10.1007/s00381-011-1558-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iliff JJ, Wang M, Zeppenfeld DM, et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. 2013;33(46):18190-18199. doi: 10.1523/JNEUROSCI.1592-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ford ML. How brains are drained: discovery of lymphatics within the CNS. Am J Transplant. 2016;16:735-735. doi: 10.1111/ajt.13742 [DOI] [Google Scholar]
- 38.Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337-341. doi: 10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dissing-Olesen L, Hong S, Stevens B. New brain lymphatic vessels drain old concepts. EBioMedicine. 2015;2(8):776-777. doi: 10.1016/j.ebiom.2015.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raper D, Louveau A, Kipnis J. How do meningeal lymphatic vessels drain the CNS? Trends Neurosci. 2016;39(9):581-586. doi: 10.1016/j.tins.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline Clinical Features.
eTable 2. Clinical features at the time of hydrocephalus diagnosis.
eTable 3. Main findings in CSF analysis.
eFigure. Representative brain image near birth and at diagnosis of hydrocephalus in patients with CZS.