Abstract
Background
The aim of this study was to explore the role of MIAT (myocardial infarction related transcripts) in diabetic optic neuropathy and its underlying mechanism.
Material/Methods
QRT-PCR (quantitative real-time polymerase chain reaction) was performed to detect the mRNA levels of MIAT and HSPA5 (heart shock protein 5) in diabetic rat model and high-glucose cultured Müller cells. After the intracellular MIAT level was increased by lentivirus transfection, the proliferation, cell cycle, and apoptosis of Müller cells were measured using the CCK-8 (Cell Counting Kit-8) assay, flow cytometry, and TUNEL (terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling) assay, respectively. Mechanisms underlying the MIAT-related apoptosis were explored by Western blot analysis. The binding condition of microRNA-379 to MIAT and HSPA5 was confirmed by luciferase reporter gene assay.
Results
Both MIAT and HSPA5 levels were remarkably increased in high-glucose cultured Müller cells. After transfected with LV (lentivirus)-MIAT, Müller cells showed a decreased proliferation and an enhanced apoptosis with the increased expressions of pro-apoptotic proteins. However, no remarkable changes were observed in cell cycle. Further mechanistic studies found that MIAT regulated HSPA5 expression by directly binding to microRNA-379.
Conclusions
MIAT was overexpressed in the diabetic optic nerve. MIAT overexpression remarkably promoted the apoptosis of Müller cells by adsorbing microRNA-379 and thus regulating HSPA5, which was a direct target of microRNA-379.
MeSH Keywords: Apoptosis, Diabetes Complications, Optic Nerve Injuries
Background
Chronic complications of diabetes include macroangiopathy, microvascular, and neuropathy. Diabetic neuropathy can affect the central nervous system, peripheral nerves, and autonomic nerves. Ocular diabetes damages the optic nerve and then leads to diabetic optic neuropathy (DON), which has become the main cause of blindness worldwide. However, we still know very little about the pathogenic mechanism of DON [1].
Retinal ganglion cells (RGCs) and Müller cells are important components of the retina. The axons of these optic ganglion cells converge to form the optic nerves [2]. Ischemia and hypoxia caused by hypoperfusion can lead to optic nerve edema and disruption of the fiber axon flow, further resulting in insufficient supply of neurotrophic nutrition and primary damage to the optic nerves [3]. Excessive production of excitotoxins and free radicals also induce ganglion cell apoptosis and glial damage, thus leading to secondary damage to the optic nerves [4,5].
Long non-coding RNAs (lncRNAs) are transcripts with 200 nucleotides in length, which are structurally similar to those of mRNAs but do not encode proteins [4,6]. lncRNAs have been reported to be involved in various biological processes such as chromosome blotting, epigenetic regulation, cell cycle control, apoptosis, and reprogramming of pluripotent stem cells, and thus are inextricably linked to the occurrence and development of many diseases [6,7]. lncRNA-MIAT was first identified in mitotic progenitor cells and post-mitotic retinal precursor cells, and is involved in the differentiation of mouse retinal cells and myocardial infarction [8,9]. MIAT is highly conserved in mammals and is expressed in both human and mouse genomes [10]. Previously, the role of MIAT in retinal microvessels and its potential mechanisms have been elucidated [11]. The present study explored the role of MIAT in optic neuropathy and its possible mechanisms.
Material and Methods
Animals
Male Sprague-Dawley (SD) rats and db/db mice aged 4–6 weeks were obtained from the Animal Model Center of Nanjing University. The study was approved by the Shaoxing People’s Hospital Ethics Committee. All rats were randomly assigned into 2 groups and were given sufficient food and water. A diabetic rat model was established by intraperitoneal injection of streptozotocin (STZ, 60 mg/kg), and control rats received an intraperitoneal injection of citrate buffer (0.1 mol/L). Blood glucose levels of rats higher than 16.7 mM after injection for 72 h were considered as successful construction of the animal model [12]. The db/db mice were fed with high-fat diet, while WT mice were fed with normal diet. All mice were had free access to food and water. This study followed the guidelines for the protection and use of laboratory animals of ARVO (Association for Research in Vision and Ophthalmology).
Cell culture and transfection
Müller cells were isolated from diabetic rats and db/db mice. Eyeballs were removed under sterile condition and placed in DMEM for 6 h. Retinal neuroepithelial layer was careful stripped and cut into pieces less than 1 mm3. We added 0.25% trypsin and the pieces were digested at 37°C for 30 min. After filtration with copper mesh (20 um), the filtrate was transferred to centrifuge tubes for centrifugation for 3 min at 1000 rpm. The supernatant was discarded and DMEM culture medium containing 10% fetal bovine serum was added. The cells were suspended and inoculated in the culture bottle after blowing with a suction tube. After 2 days, the bottle wall was gently wiped and the cells that were loosely attached were removed. After 5–10 days, the cells fused and 0.1% trypsin was added. The cells were observed under a phase-contrast microscope. After contraction and suspension of some cells, they were sucked out and discarded. The cells were cultured in DMEM medium containing 10% fetal bovine serum. After 2–3 times, the cell morphology was basically the same, and the cells were subdivided into flasks according to 1: 2 passage. Cells were identified by immunohistochemistry after 3 to 4 passages. The rMC-1 cells (rat retinal Müller cell line) were purchased from the Shanghai Academy of Life Sciences (Shanghai, China) and cultured in RPMI-1640 (HyClone, South Logan, UT, USA) containing 10% FBS (fetal bovine serum) (Gibco, Rockville, MD, USA) at 5% CO2 and 37°C. Prior to transfection, cells were seeded in 6-well plates for 24 h until the cell confluency reached approximately 60–80%. MicroRNA-NCs, microRNA-379-5p mimics and lentiviruses (GenePharma, Shanghai, China) were diluted with serum-free and antibiotic-free medium and then mixed with Lipofectamin™ 2000 (Invitrogen, Carlsbad, CA, USA). After the mixture was allowed to stand at room temperature for 20 min, it was added to the 6-well plates, and the cells were continued to be cultured in a 37°C incubator.
High glucose treatment
The rMC-1 cells were treated with 5.5 mM and 25 mM sugar for 0, 24, 48, and 72 h, respectively. Treated cells were then harvested for subsequent experiments.
Cell cycle detection
Cells were collected after digestion and then prepared as a single-cell suspension at a concentration of 1×105 cells/mL, and then the cells were fixed in pre-cooled 75% ethanol and placed in a refrigerator at 4°C overnight. Before flow cytometry determination, cells were washed twice with PBS to remove the fixative. After adding 100 μL of RNaseA (BD Biosciences, San Jose, CA, USA), cells were protected from the light in a 37°C water bath, and 30 min later, 400 μL of PI (propidium iodide) was added to stain the cells for 30 min at 4°C in the dark.
Cell apoptosis detection
After the cells were collected and fixed as described above in cell cycle detection, the cells were transferred to flow tubes before the assay. After centrifugation at 1500 rpm for 3 min, the supernatant was removed. Corresponding buffer and antibody were added to the cell pellet, and the apoptosis was detected by flow cytometry (Partec AG, Arlesheim, Switzerland) after 15 min in the dark.
TUNEL assay
We collected and fixed 5×105 cells/mL in formaldehyde and then washed them with PBS buffer containing 2% hydrogen peroxide at room temperature. Two drops of TdT enzyme buffer (Beyotime, Shanghai, China) were then added to the cells and allowed to react at room temperature for 1 h before termination. The cells were incubated in TdT buffer for 1 h at 37°C. After washing with phosphate-buffered saline (PBS) 3 times, cells were incubated with the peroxidase-labeled anti-digoxigenin antibody in a wet box at room temperature for 30 min. TUNEL results were observed and recorded under an optical microscope (IX70, Olympus, Tokyo, Japan).
RNA extraction and qRT-PCR (quantitative real-time polymerase chain reaction)
The total RNA in the tissues was extracted by the TRIzol method (Invitrogen, Carlsbad, CA, USA). Briefly, 50–100 mg of tissue was grated and 1 mL of TRIzol and 0.2 mL of chloroform were added, followed by centrifugation at 10 000 g for 15 min. The aqueous phase was then transferred to a new tube containing 0.5 mL of isopropyl alcohol. After thorough shaking, mixing, and centrifugation, the supernatant was removed, then 75% ethanol was used to further purify the RNA extract, and an appropriate amount of DEPC (diethyl pyrocarbonate) water was added for preservation at −20°C. The reverse transcription procedure was performed according to the instructions of the Takara PrimeScript RT Master Mix kit at 37°C for 15 min and 85°C for 5 s. Reverse transcription products were stored in a 4°C refrigerator. QRT-PCR was carried out by use of the SYBR® Green Master Mix (TaKaRa, Tokyo, Japan). The primer sequences used in this experiment were: MIAT, F: GGACGTTCACAACCACACTG, R: TCCCACTTTGGCATTCTAGG; HSPA5, F: CAAGTTCTTGCCGTTCAAGG, R: AAATAAGCCTCA GCGGTTTCTT; microRNA-379-5p, F: GGGGTGGTAGACTATGGA, R: TGTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGAT ACGACCCTACG.
CCK-8 (cell counting kit-8) assay
The cells were digested and collected after 24-h–transfection, and then seeded in 96 well plates at a density of 2×103/well with 6 replicates of each group. We added 10 μL of CCK-8 solution (Dojindo, Kumamoto, Japan) to each well and incubated for another 2 h. The absorbance was measured with a microplate reader at a wavelength of 450 nm.
Western blot analysis
The cells with or without transfection were collected and washed twice with PBS, then the total protein was extracted by RIPA (radioimmunoprecipitation assay) lysate (Beyotime, Shanghai, China). The BCA (bicinchoninic acid) method was performed to determine the protein concentration. The total protein was electrophoresed on 10% polyacrylamide gels and then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). After blocking with 5% fat-free milk, the membranes were incubated with primary antibody at 4°C overnight. The membrane was incubated with the secondary antibody after washing 3 times with the buffer solution (TBST). ECL (electrochemiluminescence) chemiluminescence method (Thermo Fisher Scientific, Waltham, MA, USA) was used to indicate protein imprinting, and Image J software was used to quantify the gray value of the imprint.
Plasmid construction and luciferase activity assay
The 3′ UTR sequence of HSPA5 was downloaded and the HSPA5 wild-type sequence (HSPA5 WT 3′ UTR) and the mutant-type sequence (HSPA5 MUT 3′ UTR) were constructed accordingly. The same method was used to construct MIAT WT and MIAT MUT reporter plasmids. The cells were co-transfected with 50 pmol/L microRNA-379 mimics or negative controls and 80 ng HSPA5/MIAT wild-type or mutant plasmids. After 48-h-transfection, cells were lysed using a dual luciferase reporter gene assay system solution to detect fluorescence intensity.
Statistical analysis
SPSS 22.0 (Statistical Product and Service Solutions) statistical software (IBM, Armonk, NY, USA) was used for data analysis. Measurement data are expressed as mean±standard deviation (χ̄±s). The t test was used to analyze the difference between 2 groups. P<0.05 was considered statistically significant.
Results
MIAT was overexpressed in the diabetic animal model
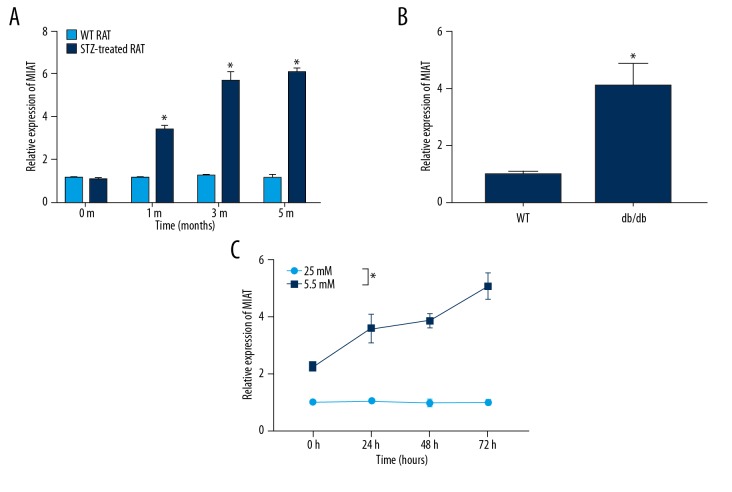
MIAT expression in Müller cells was detected at different time points in STZ-induced diabetic rats (n=6). MIAT expression in the STZ group gradually increased as the disease progressed and was remarkably higher than that of the control group (n=6), and the difference between groups was statistically significant (Figure 1A). Similarly, MIAT expression was also markedly elevated in diabetic db/db mice (n=6), and the difference between groups was statistically significant (Figure 1B). We found that MIAT in high-glucose cultured rMC-1 cells was also upregulated in a time-dependent manner compared to that of the normal-glucose cultured rMC-1 cells (n=3), and the difference between groups was statistically significant (Figure 1C). These results suggest that abnormal expression of MIAT is involved in optic nerve damage in diabetes.
Figure 1.
MIAT expression was elevated in the diabetic animal model. (A) MIAT expression was increased in STZ-induced diabetic rats. (B) MIAT expression was increased in db/db diabetic mice. (C) MIAT expression was increased in rMC-1 cells under high-glucose conditions. * p<0.05.
Overexpression of MIAT promoted apoptosis of rMC-1 cells
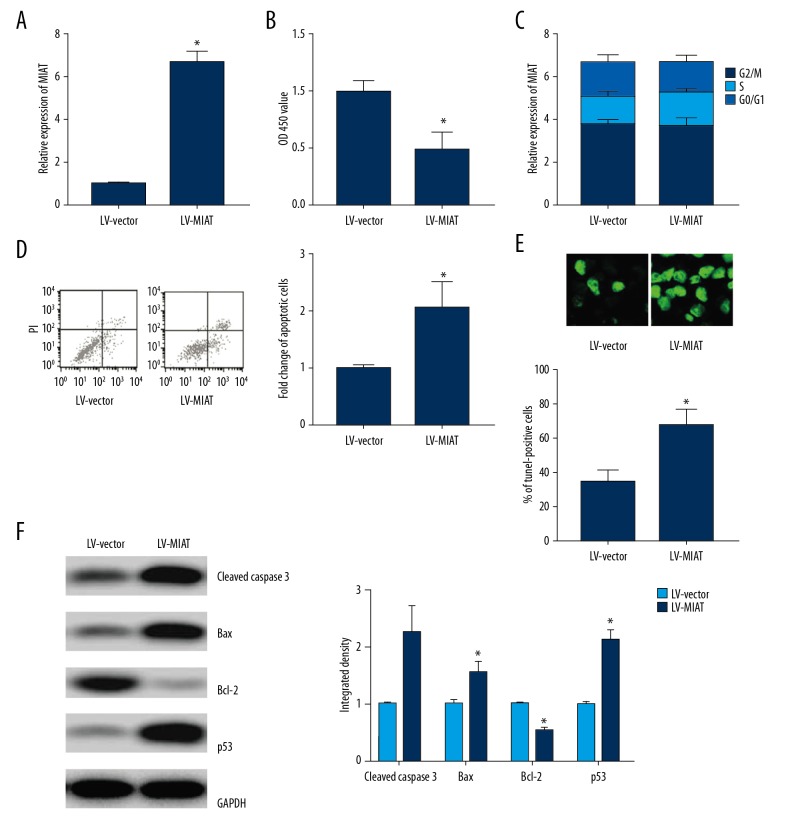
MIAT was ectopically expressed in rMC-1 cells by lentiviral transfection (n=3), and the difference between groups was statistically significant (Figure 2A). Overexpressed MIAT remarkably inhibited the proliferation of rMC-1 cells (n=3), and the difference between groups was statistically significant (Figure 2B) but had no remarkable effect on cell cycle (n=3) (Figure 2C). Flow cytometry and TUNEL assay revealed that overexpressed MIAT effectively promoted apoptosis (n=3), and the difference between groups was statistically significant (Figure 2D, 2E). Expressions of cleaved Caspase-3, Bax, and p53 were also remarkably enhanced by MIAT, while the Bcl-2 expression was attenuated, and the difference between groups was statistically significant (n=3) (Figure 2F).
Figure 2.
Overexpression of MIAT promoted apoptosis of rMC-1 cells. (A) Lentiviral transfection of rMC-1 cells increased MIAT expression. (B) Overexpression of MIAT inhibited cell proliferation. (C) Overexpression of MIAT did not affect cell cycle. (D) Overexpression of MIAT promoted apoptosis. (E) Overexpression of MIAT promoted apoptosis. (F) Effect of overexpression of MIAT on apoptosis-related protein expression was detected by Western blot. * p<0.05 vs. LV-vector.
Overexpression of MIAT enhanced HSPA5 expression
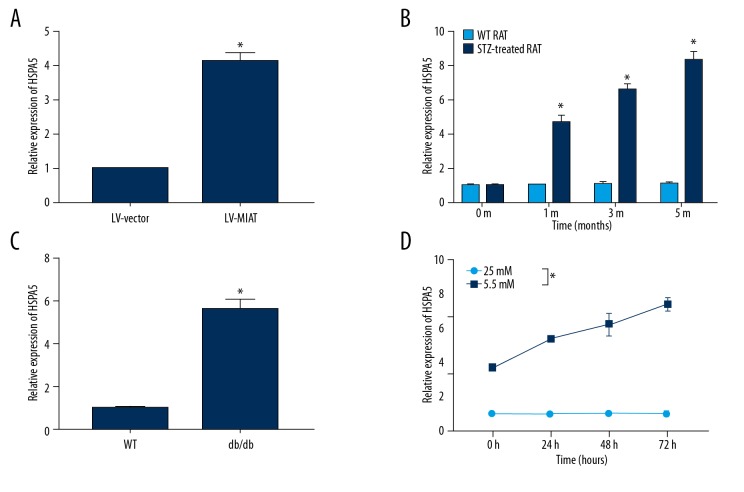
In further exploring the mechanism of MIAT, we found that HSPA5 may be regulated by MAIT based on bioinformatics prediction and functional analysis. Our data showed that overexpression of MIAT promoted HSPA5 expression (n=3), and the difference between groups was statistically significant (Figure 3A). In addition, HSPA5 was also remarkably decreased in the diabetic animal model (n=6), and the difference between groups was statistically significant (Figure 3B, 3C). Similar results were also confirmed in in vitro high-glucose cultured rMC-1 cells (n=3), and the difference between groups was statistically significant (Figure 3D).
Figure 3.
Overexpression of MIAT enhanced HSPA5 expression in diabetes. (A) Overexpression of MIAT promotes HSPA5 expression. (B) HSPA5 was overexpressed in STZ-induced diabetic rats. (C) HSPA5 was overexpressed in db/db diabetic rats. (D) HSPA5 was overexpressed in rMC-1 cells under high-glucose conditions. * p<0.05.
MIAT regulated HSPA5 expression through microRNA-379
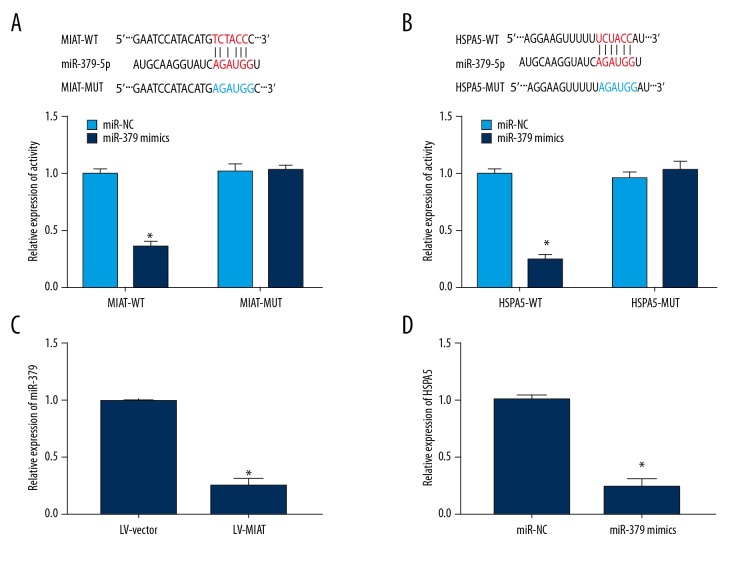
Luciferase reporter gene assay showed that microRNA-379 directly binds to MIAT and HSPA5 (Figure 4A, 4B). Overexpressed MIAT remarkably inhibited microRNA-379 expression (n=3), and the difference between both groups was statistically significant (Figure 4C). Ectopically expressed microRNA-379 markedly reduced HSPA5 expression (n=3), and the difference between groups was statistically significant (Figure 4D). The above results indicate that MIAT regulates HSPA5 expression by adsorbing microRNA-379.
Figure 4.
MIAT regulated HSPA5 expression through microRNA-379. (A) Luciferase activity assay indicated that MIAT directly bound with microRNA-379. (B) Luciferase activity assay indicated that HSPA5 directly bound to microRNA-379. (C) Overexpression of MIAT inhibited microRNA-379 expression. (D) Overexpression of microRNA-379 inhibited HSPA5 expression.
Discussion
Diabetic retinopathy used to be considered as a kind of microangiopathy. Microangiomas, loss of perivascular cells, and microvascular occlusion are the main manifestations of early lesions. Recent studies have found that changes in nerve tissue can also occur in the early stages of diabetic retinopathy, including loss of ganglion cells, decreased axons, thinner nerve fibers, increased expression of collagen fibrillary acidic protein (GFAP) in Müller cells, decreased expression of GFAP in astrocytes, degenerative changes in horizontal cells, and amacrine cells and photoreceptor cells, as well as the activation of microglia [13,14]. lncRNA-MIAT is involved in the differentiation of mouse retinal cells and myocardial infarction. In this study, we found that MIAT was overexpressed in the STZ-induced diabetic animal model and in db/db mice.
Apoptosis often depends on the expression levels of pro-apoptotic genes and anti-apoptotic genes. Many genes and proteins are involved in the regulation of optic nerve injury and apoptosis. Pro-apoptotic genes include wild-type p53, Bax, and Bcl-x, Bak, while anti-apoptotic genes include Bcl-2, Bcl-xl, and mutant p53 [15–17]. The functional proteins encoded by these genes are woven into a complex network of pathways that ultimately acts as a regulatory switch that determines cell survival [18]. The Caspase family is a class of specific aspartic cysteine proteases normally presented in the cytoplasm in an inactive form. The Caspase family is thought to be involved in all apoptotic signaling pathways. Both the expression level and the activity of caspase were increased in chronic an ocular hypertension rat model, while caspase inhibitors can effectively increase the survival rate of damaged retinal ganglion cells [19]. Our results demonstrated that overexpressed MIAT promoted expressions of cleaved caspase-3, Bax, and p53, while inhibiting the expression of Bcl-2. Results suggest that overexpressed MIAT in Müller cells under high-glucose conditions promoted cell apoptosis.
Glucose regulative protein (Grp), as an endoplasmic reticulum chaperone, is a type of stress protein produced by cells to adapt to endoplasmic reticulum stress. Grp is highly homologous to heat shock proteins (Hsps) and is considered to be a member of the heat shock protein family. The main physiological function of Grp is to assure the correct folding and assembly of proteins [20]. Grp78 (HSPA5) is currently regarded as an important member of the Grp family, which exerts an important role in tumor proliferation and drug resistance. It is reported that HSPA5 participates in the proliferation of fibrosarcoma [21]. In the process of conversion of normal tissues to adenomas or adenocarcinomas, HSPA5 in the cytoplasm also showed a corresponding overexpression trend [22]. In addition, a number of studies have demonstrated that HSPA5 expression in breast, lung, and colon cancer specimens was also significantly higher than that of paracancerous tissue [23,24]. However, inhibition of HSPA5 expression can inhibit tumor cell proliferation and promote tumor cell apoptosis [25,26]. In short, recent research on HSPA5 mainly has focused on the pathogenesis of tumors, and there is still a lack of research in the field of ophthalmology. In this study, we found that overexpression of MIAT enhanced HSPA5 and remarkably inhibited microRNA-379 expression, and ectopically expressed microRNA-379 markedly reduced HSPA5 expression, suggesting that MIAT regulates HSPA5 expression by adsorbing microRNA-379. Our results revealed that overexpressed HSPA5 might play a role in inhibiting apoptosis, which is contrary to its role in tumors. This contradiction may be due to the loss of normal cell characteristics of the tumor cells themselves. Further research on the specific mechanisms is urgently required to better understand the function of HSPA5.
Conclusions
MIAT was overexpressed in diabetic optic nerves. MIAT overexpression remarkably promoted the apoptosis of Müller cells by adsorbing microRNA-379 and thus regulating HSPA5, which is a direct target of microRNA-379.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Kramerov AA, Ljubimov AV. Stem cell therapies in the treatment of diabetic retinopathy and keratopathy. Exp Biol Med (Maywood) 2016;241:559–68. doi: 10.1177/1535370215609692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheetz MJ, King GL. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA. 2002;288:2579–88. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- 3.Roy S, Tonkiss J, Roy S. Aging increases retinal vascular lesions characteristic of early diabetic retinopathy. Biogerontology. 2010;11:447–55. doi: 10.1007/s10522-010-9263-x. [DOI] [PubMed] [Google Scholar]
- 4.Ashton N. Vascular basement membrane changes in diabetic retinopathy. Montgomery lecture, 1973. Br J Ophthalmol. 1974;58:344–66. doi: 10.1136/bjo.58.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasnoor M, Dimachkie MM, Kluding P, Barohn RJ. Diabetic neuropathy part 1: Overview and symmetric phenotypes. Neurol Clin. 2013;31:425–45. doi: 10.1016/j.ncl.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17:47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 7.Wu H, Yang L, Chen LL. The diversity of long noncoding RNAs and their generation. Trends Genet. 2017;33:540–52. doi: 10.1016/j.tig.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Ishii N, Ozaki K, Sato H, et al. Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J Hum Genet. 2006;51:1087–99. doi: 10.1007/s10038-006-0070-9. [DOI] [PubMed] [Google Scholar]
- 9.Zhu XH, Yuan YX, Rao SL, Wang P. LncRNA MIAT enhances cardiac hypertrophy partly through sponging miR-150. Eur Rev Med Pharmacol Sci. 2016;20:3653–60. [PubMed] [Google Scholar]
- 10.Metea MR, Newman EA. Signalling within the neurovascular unit in the mammalian retina. Exp Physiol. 2007;92:635–40. doi: 10.1113/expphysiol.2006.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan B, Yao J, Liu JY, et al. LncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ Res. 2015;116:1143–56. doi: 10.1161/CIRCRESAHA.116.305510. [DOI] [PubMed] [Google Scholar]
- 12.Yao L, Wu YT, Tian GX, et al. Acrolein scavenger hydralazine prevents streptozotocin-induced painful diabetic neuropathy and spinal neuroinflammation in rats. Anat Rec (Hoboken) 2017;300:1858–64. doi: 10.1002/ar.23618. [DOI] [PubMed] [Google Scholar]
- 13.Li Q, Zemel E, Miller B, Perlman I. Early retinal damage in experimental diabetes: Electroretinographical and morphological observations. Exp Eye Res. 2002;74:615–25. doi: 10.1006/exer.2002.1170. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher EL, Phipps JA, Wilkinson-Berka JL. Dysfunction of retinal neurons and glia during diabetes. Clin Exp Optom. 2005;88:132–45. doi: 10.1111/j.1444-0938.2005.tb06686.x. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y, Li X, Shi P, et al. Effects of L-carnitine on high glucose-induced oxidative stress in retinal ganglion cells. Pharmacology. 2014;94:123–30. doi: 10.1159/000363062. [DOI] [PubMed] [Google Scholar]
- 16.Fan J, Xu G, Jiang T, Qin Y. Pharmacologic induction of heme oxygenase-1 plays a protective role in diabetic retinopathy in rats. Invest Ophthalmol Vis Sci. 2012;53:6541–56. doi: 10.1167/iovs.11-9241. [DOI] [PubMed] [Google Scholar]
- 17.Lahouaoui H, Coutanson C, Cooper HM, et al. Clock genes and behavioral responses to light are altered in a mouse model of diabetic retinopathy. PLoS One. 2014;9:e101584. doi: 10.1371/journal.pone.0101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wee KB, Aguda BD. Akt versus p53 in a network of oncogenes and tumor suppressor genes regulating cell survival and death. Biophys J. 2006;91:857–65. doi: 10.1529/biophysj.105.077693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohsawa R, Kageyama R. Regulation of retinal cell fate specification by multiple transcription factors. Brain Res. 2008;1192:90–98. doi: 10.1016/j.brainres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Lee AS. The glucose-regulated proteins: Stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–10. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- 21.Dong D, Dubeau L, Bading J, et al. Spontaneous and controllable activation of suicide gene expression driven by the stress-inducible grp78 promoter resulting in eradication of sizable human tumors. Hum Gene Ther. 2004;15:553–61. doi: 10.1089/104303404323142006. [DOI] [PubMed] [Google Scholar]
- 22.Xing X, Lai M, Wang Y, et al. Overexpression of glucose-regulated protein 78 in colon cancer. Clin Chim Acta. 2006;364:308–15. doi: 10.1016/j.cca.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Fu Y, Lee AS. Glucose regulated proteins in cancer progression, drug resistance and immunotherapy. Cancer Biol Ther. 2006;5:741–44. doi: 10.4161/cbt.5.7.2970. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez PM, Tabbara SO, Jacobs LK, et al. Overexpression of the glucose-regulated stress gene GRP78 in malignant but not benign human breast lesions. Breast Cancer Res Treat. 2000;59:15–26. doi: 10.1023/a:1006332011207. [DOI] [PubMed] [Google Scholar]
- 25.Ermakova SP, Kang BS, Choi BY, et al. (−)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006;66:9260–69. doi: 10.1158/0008-5472.CAN-06-1586. [DOI] [PubMed] [Google Scholar]
- 26.Gupta P, Walter MR, Su ZZ, et al. BiP/GRP78 is an intracellular target for MDA-7/IL-24 induction of cancer-specific apoptosis. Cancer Res. 2006;66:8182–91. doi: 10.1158/0008-5472.CAN-06-0577. [DOI] [PubMed] [Google Scholar]