Abstract
Objective.
To evaluate the phytochemical content and antioxidant potential of the acetone, aqueous, and methanol extracts of the fruit, leaf, and root of Cucumis africanus L.f.
Methods.
Total phenolic, flavonoid, and proanthocyanidin contents were evaluated using spectrophotometric methods. The free radical scavenging activity of the acetone, methanol, and aqueous extracts of the fruit, leaf, and root were evaluated against DPPH+, ABTS+, and phosphomolybdenum. Alkaloid and saponin contents were also determined.
Results.
The acetone extract of the fruit revealed the highest polyphenol content when compared with the other solvent extracts analyzed. Total phenol content of the parts tested ranged from 3.66 ± 0.17 to 44.98 ± 3.41, flavonoid content ranged from 4.63 ± 3.33 to 401.33 ± 7.89, and proanthocyanidin content ranged from 8.84 ± 2.65 to 504 ± 36.6. Significant amount of alkaloids present was observed in the fruits, leaf, and root (10.68 ± 0.68, 14.12 ± 1.67, and 12.15 ± 4.74), respectively, while saponin content was 33.33 ± 11.55, 26.67 ± 11.55, and 20.00 ± 0.00 for the fruit, leaf, and root, respectively. Solvent extracts showed significant antioxidant activity, with acetone showing highest antioxidant ability in correlation with the polyphenol contents. Based on the IC50 values, acetone extract of the root revealed the best DPPH radical scavenging ability, the leaf aqueous extract had the highest IC50 value for ABTS, and the methanol extract of the leaf was best for phosphomolybdenum assays.
Conclusion.
This study suggests that fruit, leaf, and root of Cucumis africanus could be a potential source of natural antioxidant and justifies its use in ethnomedicine.
Keywords: antioxidant, Cucumis africanus, phytochemical, wild vegetables, free radicals, polyphenol
Plants have long been used as a source of food and medicine. They not only serve as vegetables of high nutritive value, but their different parts (leaf, fruit, and root) are used for health remedial purposes. The beneficial effects of plant products can be attributed to the biological activities of their phytochemical and antioxidant constituents such as phenolic compounds, proanthocyanidin, vitamins, carotenoids, flavonoids, and saponin.1,2 Recently, there has been an increasing interest in natural antioxidants, with the aim of utilizing them to reduce the toxic effects of free radicals in the human body.3 The free radical scavenging properties of medicinal plants considered as natural antioxidants are utilized in several medical applications because of the assurance of effectiveness and safety.4
Wild vegetables are potential sources of these phytochemicals and antioxidants that play a vital role in health promotion, especially in the rural populations, which have better access to these plants. These phytochemicals are responsible for many biological effects, including antioxidant, anti-inflammatory, antimicrobial, and anticancer activities,5 and therefore, they may find applications in formulation of various pharmaceutical drugs.
Antioxidants are chemical substances that the inhibit oxidation process by preventing the formation of free radicals that cause damage to healthy cells,6 thus treating and managing chronic diseases such as cardiovascular diseases, diabetes, obesity, and some forms of cancers.7
Cucumis africanus L.f. is a wild vegetable belonging to the family Cucurbitaceae. It is indigenous to Africa and is known by several common names including wild cucumber, bitter apple, small wild apple or thorn cucumber (English), and Ithanganza (Xhosa). It is found in places such as the woodlands of Angola and Zimbabwe to Namibia, Botswana, and South Africa. In South Africa, the fruit and shoot are consumed as vegetables,8 and the whole plant is also used for the management of obesity and wound healing.9 The fruit, leaf, or root is also used as an emetic, purgative, or enema in the treatment of various ailments. However, being a vegetable growing in the wild, in spite of the long history of its use, no attempt has been made to evaluate the parameters that could support its use as a medicinal plant. To address this dearth of information, the present study is designed to explore the phytochemical profile and antioxidant activity of the shoot, fruit, and root of Cucumis africanus L.f.
Materials and Method
Chemicals and Reagents
All reagents and chemicals including gallic acid, rutin, quercetin, aluminum chloride (AlCl3), ferric chloride (FeCl3·7H2O), Folin-Ciocalteu, oxalic acid, trichloroacetic acid (TCA), sulfuric acid (H2SO4), hydrochloric acid (HCl), Na2CO3, ammonium molybdate, potassium ferricyanide (K3Fe(CN)6), catechin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and 2,2′-azinobis (3-ethylobenzothiazoline-6-sulphonic acid diammonium salt) (ABTS) are products of Sigma-Aldrich, South Africa. Others such as anhydrous sodium carbonate, sodium nitrite (NaNO2), ethanol, methanol, n-butanol, sodium acetate, butylated hydroxyoluene (BHT), diethyl ether, glacial acetic acid, sulfanilic acid, potassium persulfate, and sodium nitroprusside were also purchased from Sigma-Aldrich. All the reagents used were of analytical grade.
Collection of Plant Materials
The whole plant and fruits were collected from its natural habitat in Murunwa village, Vhembe Biosphere Reserve, Limpopo Province, South Africa. The site is located on latitude 22° 56′ 0″ S, 30° 28′ 0″ E and about 600 m above sea level. It was authenticated by Prof Cupido, a taxonomist in the University of Fort Hare, and a voucher specimen (Abif-Med, 2018/1) was deposited at the Giffen herbarium for future reference.
Preparation of Extracts
The freshly collected plant materials were cleaned to remove adhering dust, oven dried at 40°C, pulverized, and stored in airtight containers. Hundred grams of the oven-dried plant parts was extracted separately in 1000 mL of each of the solvents: methanol, acetone, and water. The samples were then filtered under vacuum with Whatman No. 1 filter paper and evaporated using a rotary vacuum evaporator. The aqueous extracts were dried under reduced pressure using a freeze-drier. The crude extract recovery of each solvent was expressed in percentage and stored in the refrigerator at 4°C until needed for use.
Phytochemical Screening
Phytochemical analysis was carried out to determine the alkaloid, saponin, flavonoid, proanthocyanidin, and total phenol contents of the leaf, root, and fruit of Cucumis africanus.
Total Phenolic Content
Total phenolic content was evaluated by method described by Wintola and Afolayan.10 An aliquot of 0.5 mL of each part of the plant extract and rutin standards were prepared (1 mg/mL) and added to 2.5 mL of Folin-Ciocalteu reagent, which was previously diluted with distilled water (1:10 v/v) and 2 mL of sodium carbonate (Na2CO3). The tubes containing the mixtures were properly vortexed and allowed to stand for 30 minutes at 40°C for color development. Absorbance was measured at 765 nm using a spectrophotometer (AJI-C03 UV-VIS). Results were expressed as mg/g gallic acid equivalent (mg/g GAE) using the equation based on the standard curve: y = 10.875x + 0.1025, R 2 = 0.9966. Where y is the absorbance, x is the concentration, and R 2 is the determined coefficient.
Proanthocyanidin Content
The method described by Ohikhena et al11 was used to evaluate the proanthocyanidin content of the sample. A total of 0.5 mL of the different solvent extracts in varying concentrations (0.02 to 1 mg/mL) of catechin (standard) was mixed with 3 mL of 4% (w/v) vanillin methanol and 1.5 mL of hydrochloric acid and the solution was vortexed properly. The solution was incubated for 15 minutes at 27°C and absorbance was measured at 500 nm. The experiment was done in triplicates. Proanthocyanidin content was extrapolated using the calibration graph equation: y = 0.9342x + 0.116, R 2 = 0.9866, and expressed as mg catechin equivalent (CE/g).
Flavonoid Content
The aluminum chloride colorimetric assay as described by Ohikhena et al11 was used to determine the flavonoid content. Briefly, 0.5 mL aliquots of the solvent fractions in varying concentrations (0.2-1 mg/mL) of queretin standard and the solvent of dissolution (control) were placed in different test tubes. Thereafter, 2 mL of distilled water was added to each test tube and then 0.15 mL of 5% sodium nitrite was also added to the mixture. The mixture was left to stand for 6 minutes. Then, 0.15 mL of AlCl3 (10%) was added to the solution and left to stand for another 5 minutes and then 1 mL of 1 M sodium hydroxide was added made up to 5 mL with distilled water. The absorbance was measured at 420 nm and the experiment was done in triplicates. The flavonoid content was calculated using the calibration curve equation, y = 1.5645x + 0.1135, R 2 = 0.9848, and expressed as mg of quercetin equivalent (QE/g).
Alkaloid Content
The alkaloid content was determined according to the method of Onyilagha and Islam.12 A total of 0.5 g of the powdered plant sample was weighed and soaked in 200 mL of 10% acetic acid in ethanol. The mixture was covered and left for 4 hours, after which it was filtered and the filtrate was concentrated on a water bath to one fourth of the initial volume. Concentrated ammonium hydroxide was added in drops to the extract until precipitation was completed. After this, the whole solution was allowed to settle and the collected precipitates were washed with dilute ammonium hydroxide and then filtered again. The residue collected was dried and weighed. The alkaloid content was determined using the following formula: % Alkaloid = Final weight of sample/Initial weight of extract × 100.
Saponin Content
Total saponin content was determined by the method described by Obadoni and Ochuko.13 A total of 0.5 g of the powdered sample was mixed in 200 mL of 20% ethanol. This was placed on a shaker for 30 minutes. Thereafter, the plant sample was heated in a water bath at 55°C for 4 hours. The mixture was filtered and the residue was extracted again with another 200 mL of 20% ethanol. The combined extracts were reduced to 40 mL over the water bath at 90°C. The concentrated solution obtained was then transferred into a 250 mL separating funnel and extracted twice using 20 mL diethyl ether. The ether layer was discarded, while the aqueous layer was retained and 60 mL n-butanol was added. The n-butanol extracts were washed twice with 10 mL of 5% sodium chloride. The remaining solution was heated in a water bath and then oven dried at 40°C to a constant weight. The percentage saponin content was calculated as follows: % Saponin = Final weight of sample/Initial weight of sample × 100.
Antioxidant Assays
Antioxidant potentials of the plant extracts were evaluated using DPPH radical scavenging, ABTS, nitric oxide scavenging, and total antioxidant capacity assays.
2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Assay
The scavenging ability of the plant extract on DPPH was determined using the method described by Wintola and Afolayan10 with modifications in the concentrations. One milliliter of DPPH prepared in methanol (0.135 mM) was mixed with 1 mL of varying concentrations ranging from 0.2 to 1 mg/mL of plant extracts and standards. The mixture was vortexed thoroughly and left in the dark at room temperature for 30 minutes. The absorbance was measured at 517 nm using a spectrophotometer. The scavenging ability of the plant extract on DPPH was calculated as follows: DPPH scavenging activity (%) = [(Abs control − Abs sample)/(Abs control)] × 100, where Abs control is the absorbance of DPPH + methanol; Abs sample is the absorbance of DPPH radical + sample (extract/standard).
ABTS Radical Scavenging Assay
The scavenging activity of the different plant extracts against ABTS radical was determined by the method described by Unuofin et al.14 The stock solution was prepared by reacting 7 mM ABTS solution and 2.45 mM potassium persulfate solution (1:1) and allowing the mixture to stand for 12 hours in the dark at room temperature to produce a bluish green ABTS radical. The solution was further diluted by mixing 1 mL of the ABTS+ solution with methanol until an absorbance of 0.700 ± 0.01 at 734 nm was attained. One milliliter of plant extract was allowed to react with 1 mL of the ABTS solution and the absorbance was measured at 734 nm after 7 minutes. The ABTS scavenging capacity of the extracts were compared with that of BHT and rutin and percentage inhibition was calculated as follows: ABTS radical scavenging activity (%) = [(Abs control − Abs sample)/(Abs control)] × 100, where Abs control is the absorbance of ABTS radical + methanol; Abs sample is the absorbance of ABTS radical + sample extract/standard.
Total Antioxidant Capacity (Phosphomolybdenum) Assay
The total antioxidant capacity was determined by the phosphomolybdenum method as described by Ohikhena et al.11 Briefly, 0.3 mL of the different solvent extracts and standard (0.025-0.4 mg/mL) were put in test tubes and dissolved in 3 mL of reagent solution (0.6 M sulfuric acid, 4 mM ammonium molybdate, and 28 mM sodium phosphate). The test tubes were covered and incubated at 95°C in a water bath for 95 minutes. The mixture was allowed to cool to room temperature and the absorbance was measured at 695 nm. Ascorbic acid and BHT were used as standards. The percentage inhibition was calculated as follows: [(Absorbance of sample − Absorbance of control)/(absorbance of sample)] × 100.
Statistical Analysis
Statistical analysis was carried out by one-way analysis of variance test using a statistical package program (Minitab, release version 17). The results were expressed as mean ± standard deviation of 3 replicates, and significant levels were tested at P < .05.
Results
Phytochemical Content
The total phenolic, proanthocyanidin, and flavonoid contents of the fruit, leaf, and root of Cucumis africanus were analyzed and are summarized in Table 1. Results from this study indicated a variation in polyphenol compounds in the different extracts and the different plant parts. The acetone extract of the fruit gave the highest quantity of total phenol (44.98 ± 3.41 mg GAE/g), proanthocyanidin (504 ± 36.6 mg CE/g), and flavonoids (401.33 ± 7.89 mg QE/g). Acetone proved to be the most effective solvent for extracting phenolic compounds when compared with the aqueous and methanol extracts, with water showing very poor extractability of these compounds.
Table 1.
Total Phenol, Flavonoid, and Proanthocyanidin Contents of the Fruit, Leaf, and Root of Cucumis africanus*.
| Plant Extracts | Total Phenol (mg/g GAE) | Flavonoids (mg/g QE) | Proanthocyanidin (mg/g CE) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fruit | Leaf | Root | Fruit | Leaf | Root | Fruit | Leaf | Root | |
| Acetone | A44.98 ± 3.41a | B25.23 ± 2.51b | C20.24 ± 0.64a | A401.33 ± 7.89a | B342.03 ± 5.49a | C289.50 ± 14.80a | A504 ± 36.6a | B275.57 ± 8.81a | C154.93 ± 1.46a |
| Water | B7.32 ± 0.42c | A9.633 ± 0.27c | C3.66 ± 0.17b | ND | A4.63 ± 3.33c | ND | ND | ND | ND |
| Methanol | B17.46 ± 3.21b | A42.31 ± 3.40a | B15.36 ± 4.71a | B20.63 ± 6.88b | A228.96 ± 11.11b | B11.67 ± 2.09b | ND | A86.18 ± 7.49b | B8.84 ± 2.65b |
Abbreviations: GAE, gallic acid equivalent; QE, quercetin equivalent; CE, catechin equivalent; ND, not detected.
*The results are expressed as mean ± standard deviation (n = 3). Different lowercase superscripts are significantly different (P < .05) in the same plant part but different solvents. Different uppercase superscripts indicate significant differences (P < .05) between different plant parts in the same solvents. Means with different letters are significantly different (a > b > c).
Other phytochemical constituents analyzed in the fruit, leaf, and root of Cucumis africanus were alkaloids and saponin. Quantitative determination of the alkaloid contents in the plant parts indicated that the alkaloid contents were relatively low (P < .05) when compared with the saponin content and showed no significant difference between the plant parts. Alkaloid content of the fruits, leaf, and root was 10.68 ± 0.68, 14.12 ± 1.67, and 12.15 ± 4.74, respectively. On the other hand, saponin content of the plant parts was high and showed no significant difference between the tested plant parts. Saponin content in decreasing order was fruit (33.33 ± 11.55) > leaf (26.67 ± 11.55) > root (20.00 ± 0.00).
Antioxidant Activity
DPPH Radical Scavenging Assay
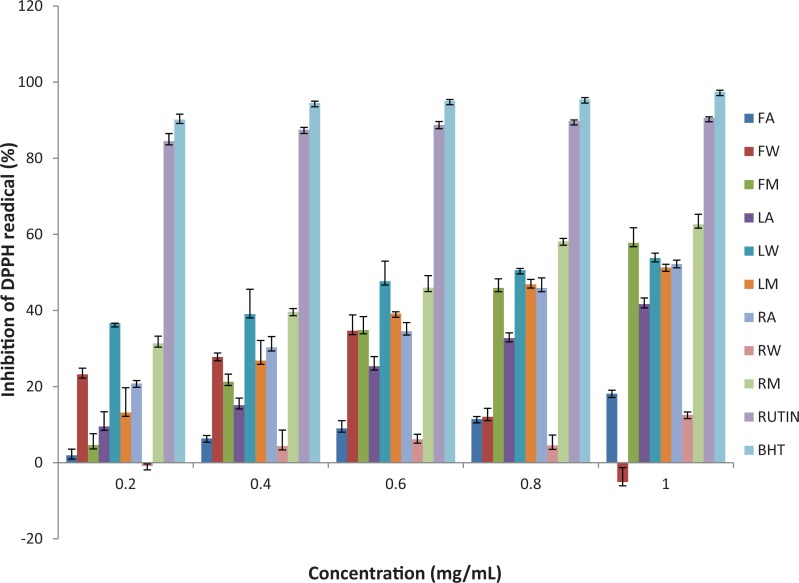
The DPPH radical scavenging activities of the extracts and standards (BHT and rutin) are presented in Figure 1. Both plant extracts and standards significantly reduced the DPPH radical with increasing concentrations. At the highest concentration tested (1 mg/mL), the highest DPPH scavenging activity was observed in BHT and the lowest in fruit aqueous extract. The standards and extracts in decreasing order of activity is BHT > rutin > root methanol extract > fruit methanol extract > leaf aqueous extract > root acetone extract > leaf methanol extract > leaf acetone extract > fruit acetone extract > root aqueous extract > fruit aqueous extract. Significant antioxidant activity was observed by methanol and acetone extracts even though methanol revealed better hydrogen donating ability. The IC50 values (Table 2) of the tested extracts/standards were in the following increasing order: rutin < BHT < root methanol extract < leaf aqueous extract < fruit methanol extract < root acetone extract < leaf methanol extract < leaf acetone < fruit acetone extract < root aqueous extract > fruit aqueous extract. Values ranged from 0.000009 to >1 mg/mL.
Figure 1.
DPPH radical scavenging activity of the different solvent fractions of the fruit, leaf, and root of Cucumis africanus.
FA, LA, and RA, acetone extract of fruit, leaf, and root; FW, LW, and RW, aqueous extracts of fruit, leaf, and root; FM, LM, and RM, methanol extracts of the fruit, leaf, and roots, respectively.
Table 2.
IC50 Values of Various Solvent Extracts of Fruit, Leaf, and Root of Cucumis africanus and Standard Drugs (BHT and Rutin).
| DPPH | ABTS | TAC | ||||
|---|---|---|---|---|---|---|
| Extracts/Standard | IC50 (mg/mL) | R 2 | IC50 (mg/mL) | R 2 | IC50 (mg/mL) | R 2 |
| Acetone | ||||||
| Fruit | >1 | 0.965 | 0.188 | 0.942 | >0.4 | 0.971 |
| Leaf | >1 | 0.994 | 0.054 | 0.990 | 0.287 | 0.978 |
| Root | 0.927 | 0.979 | > 0.08 | 0.980 | > 0.4 | 0.994 |
| Aqueous | ||||||
| Fruit | >1 | 0.541 | 0.05 | 0.965 | > 0.4 | 0.981 |
| Leaf | 0.798 | 0.957 | 0.072 | 0.963 | > 0.4 | 0.927 |
| Root | >1 | 0.509 | > 0.08 | 0.981 | > 0.4 | 0.864 |
| Methanol | ||||||
| Fruit | 0.826 | 0.993 | >0.08 | 0.932 | 0.36 | 0.973 |
| Leaf | 0.955 | 0.977 | 0.069 | 0.998 | 0.187 | 0.989 |
| Root | 0.66 | 0.985 | 0.077 | 0.992 | 0.376 | 0.964 |
| Rutin | 0.000009 | 0.9298 | 0.016 | 0.996 | — | — |
| BHT | 0.000016 | 0.996 | 0.012 | 0.964 | 0.00018 | 0.983 |
| Ascorbic acid | — | — | — | — | 0.0002 | 0.972 |
ABTS Radical Scavenging Activity
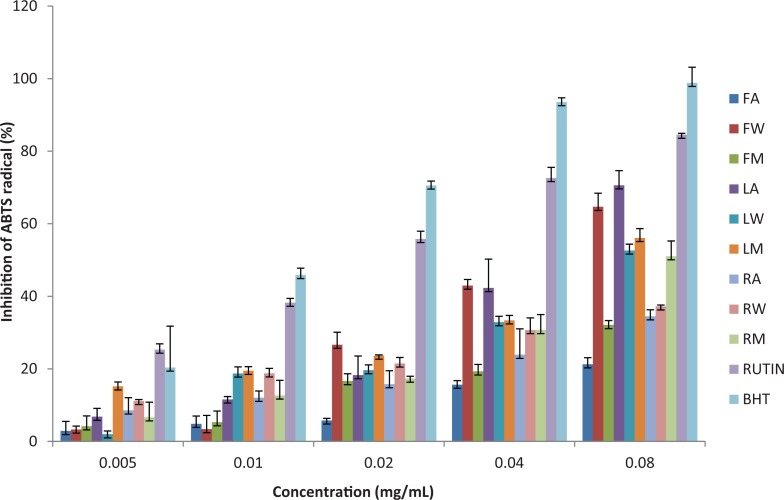
The ABTS scavenging activity of the extracts and standards increased with increasing concentrations (Figure 2). The standards/extracts possess significant ABTS scavenging activities. At the highest concentration tested (0.08 mg/mL), the highest percentage mean inhibition was obtained by BHT and the lowest by fruit acetone extract. The ABTS scavenging activities of the standards/extracts in decreasing order is as follows: BHT > rutin > leaf acetone extract > fruit aqueous extract > leaf acetone extract > leaf aqueous extract > root methanol extract > root acetone extract > root aqueous extract > fruit methanol extract > fruit acetone extract. The IC50 values of the standards/extracts in decreasing order of scavenging activity is as follows: BHT < rutin < fruit aqueous extract < leaf acetone extract < leaf methanol extract < leaf aqueous extract < root methanol extract < root acetone extract < fruit acetone extract < root aqueous extract < fruit methanol extract. Values ranged from 0.012 to >1.
Figure 2.
ABTS radical scavenging activity of the different solvent fractions of the fruit, leaf, and root of Cucumis africanus.
FA, LA, and RA, acetone extract of fruit, leaf, and root; FW, LW, and RW, aqueous extracts of fruit, leaf, and root; FM, LM, and RM, methanol extracts of the fruit, leaf, and roots, respectively.
Total Antioxidant Capacity (Phosphomolybdenum) Assay
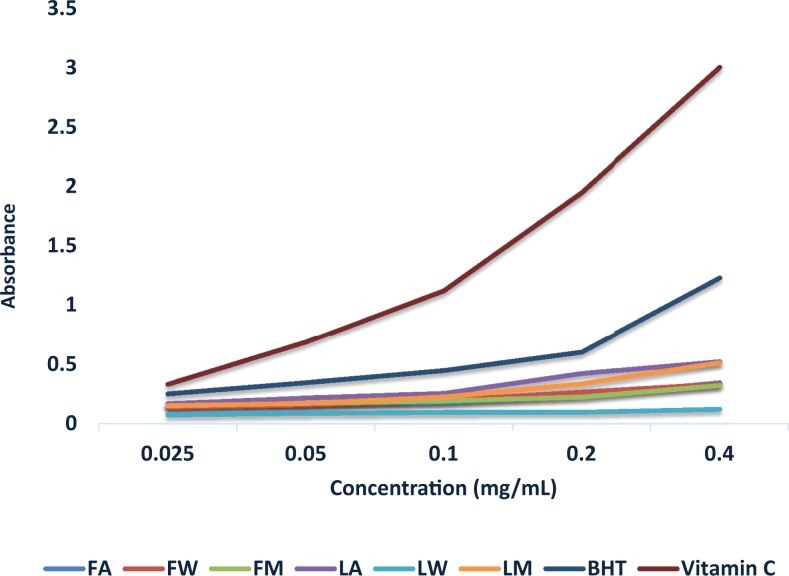
The TAC of the extracts/standards was determined based on the reduction of molybdenum(VI) to molybdenum(V) and the formation of a green phosphate/molybdenum(V) complex. The extracts showed concentration-dependent total antioxidant capacity (Figure 3). At the lowest concentration, the plant extracts showed very low antioxidant activity. Based on the IC50, the standards (vitamin C and BHT) showed higher antioxidant activity compared with the solvent extracts at values far less than the lowest concentration used for this assay.
Figure 3.
Total antioxidant capacity of the different solvent fractions of the fruit, leaf, and root of Cucumis africanus.
FA, LA, and RA, acetone extract of fruit, leaf, and root; FW, LW, and RW, water extracts of fruit, leaf, and root; FM, LM, and RM, methanol extracts of fruit, leaf, and roots, respectively.
Discussion
Plants contain natural antioxidants that possess beneficial effects in health maintenance, management of diseases, and alleviating the harmful effects of toxic agents.15 Phenolic compounds are a class of antioxidant agents that can inhibit and scavenge free radicals due to their redox properties.16 Different solvents possess different extraction abilities, which are dependent on the polarity of the extraction medium and the type of solvents used.17 The present study revealed that the distribution of polyphenols differed significantly between plant parts for all the solvents used. This correlates with the observation made by Iloki-Assanga et al.18 The percentage yield of the solvent extracts in decreasing order is as follows: leaf methanol extract > root methanol extract > fruit acetone > leaf aqueous extract > fruit methanol extract > leaf acetone extract > root aqueous extract > aqueous fruit extract > acetone fruit extract > acetone root extract.
From this study, it was observed that fruit, leaf, and root of Cucumis africanus have significant amounts of polyphenol compounds most effectively extractable by acetone, fairly by methanol, and very poorly by water (Table 1). This correlates with the observation made by Unuofin et al14 that acetone was the most effective solvent for extracting polyphenol compounds in Kedrostis africana of the curcurbitaceae family. Although our findings indicate that Cucumis africanus possesses antioxidant properties, further research on the active ingredients from the leaves and fruit are been conducted confirm its consumption safety in order to further its therapeutic applications.
From research, it is confirmed that the pharmacological property of flavonoids integrates with their activities.19 The high flavonoid content observed in the acetone extract of all the tested parts of the plant can support its ethno-medicinal use in the management of obesity and other related diseases. Proanthocyanidin are also phytochemicals of interest in medicine and nutrition. It possesses a convincing antioxidant capacity and has been shown to protect body from tissue damage, cancer, and to improve blood circulation by strengthening the capillaries, arteries, and veins.20
Therefore, the high content observed in this study could point to the potential of the plant for prevention or management of diseases and may contribute synergistically to the significant antioxidant potency of the plant and support its ethno-medicinal use for the treatment of oxidative stress–related diseases. Alkaloids and saponins are relevant for their analgesic and antispasmodic effects. Saponins possess a wide range of pharmacological activities, including expectorant, anti-inflammatory, vasoprotective, gastroprotective, and antimicrobial properties.20 They are also important in human diets to reduce the risk of coronary heart disease.21 Therefore, the high saponin content observed in all the tested parts of the plant may give support to the consumption and traditional usage of this plant for the treatment of diseases.
The scavenging activity of the plant extract against ABTS+ and DPPH+ was comparable to the standard drugs used in this study. It was observed that all the solvent extracts showed DPPH scavenging activities only at very high concentrations (0.2-1 mg/mL). At the highest concentrations of the extracts, methanol extract of the fruit exhibited the highest scavenging activity. The activity of all the solvent extracts was relatively lower than that of the standards. ABTS radical is a blue chromophore produced by the reaction of ABTS and potassium persulfate after incubation in the dark. The reactions of extract with this radical cation decolorized the blue chromophore with increasing concentrations. The ABTS activity of all the solvent extracts was relatively lower and the IC50 higher compared to the standards. At the highest concentration of the extracts, acetone extracts of the leaf gave the highest ABTS scavenging activities. Appreciable values of scavenging activities obtained in this study indicate that they are a potent source of antioxidants since the reducing power of a compound is an indication of its antioxidant activity.
The scavenging activity of ABTS and DPPH radicals by the extract was observed to be different at the highest concentration. This correlates with the opinion that plants with DPPH scavenging ability may not inhibit ABTS radical, which is due to their different solubility and methods of preparations.22
In conclusion, this study revealed the effect of different solvents on the extraction of phytochemicals from and antioxidant activities of Cucumis africanus, which is in agreement with high phenolic contents observed in the plant and this correlates with similar observation by Mansouri et al23 on Cucumis melo. The high phytochemical constituents and antioxidant activities of the fruit, leaf, and root of Cucumis africanus is indicative of their ability to maintain health and treat various diseases and therefore supports its use in ethno-medicine. Cucumis africanus may have great importance in combating oxidative stress. Therefore, expanding its application in health maintenance is highly recommended.
Acknowledgments
The authors acknowledge the financial support of Govan Mbeki Research and Development Centre, University of Fort Hare, Alice 5700, Eastern Cape, South Africa (Grant Number C127).
Footnotes
Author Contributions: TOA, GAO, and AJA conceived and designed the study. TOA performed the experiments and wrote the draft manuscript. GAO and AJA coordinated and revised the manuscript. TOA, GAO and AJA read and approved the final manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Govan Mbeki Research and Development Centre, University of Fort Hare, Eastern Cape, South Africa (Grant Number C127).
Ethical Approval: Ethical approval was granted by the University of Fort Hare Animal and Plant Use Research Ethics Committee, South Africa, with Protocol Number AFO111SABI01
References
- 1. Dinda B, Debnath S, Harigaya Y. Naturally occurring secoiridoids and bioactivity of naturally occurring iridoids and secoiridoids. A review, part 2. Chem Pharma Bull (Tokyo). 2007;55:689–728. [DOI] [PubMed] [Google Scholar]
- 2. Francis G, Kerem Z, Makkar HP, Becker K. The biological action of saponins in animal systems: a review. Br J Nutr. 2002;88:587–605. [DOI] [PubMed] [Google Scholar]
- 3. Zaouali Y, Bouzaine T, Boussaid M. Essential oils composition in two Rosmarinus officinalis L. varieties and incidence for antimicrobial and antioxidant activities. Food Chem Toxicol. 2010;48:3144–3152. [DOI] [PubMed] [Google Scholar]
- 4. Al-Snafi AE. Medicinal plants with antioxidant and free radical scavenging effects (part 2): plant based review. IOSR J Pharm. 2016;6:62–82. [Google Scholar]
- 5. Mertz C, Gancel AL, Gunata Z, et al. Phenolic compounds, carotenoids and antioxidant activity of three tropical fruits. J Food Compos Anal. 2009;22:381–387. [Google Scholar]
- 6. Benbrook CM. Elevating Antioxidant Levels in Food Through Organic Farming and Food Processing. Washington, DC: Organic Center; 2005. [Google Scholar]
- 7. Lillioja S, Neal AL, Tapsell L, Jacobs DR., Jr Whole grains, type 2 diabetes, coronary heart disease, and hypertension: links to the aleurone preferred over indigestible fiber. Biofactors. 2013;39:242–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maanda MQ, Bhat RB. Wild vegetable use by Vhavenda in the Venda region of Limpopo Province, South Africa. Phyton. 2010;1:79:189–194. [Google Scholar]
- 9. Afolayan AJ, Mbaebie BO. Ethnobotanical study of medicinal plants used as anti-obesity remedies in Nkonkobe Municipality of South Africa. Pharmacogn J. 2010;2:368–373. [Google Scholar]
- 10. Wintola OA, Afolayan AJ. Phytochemical constituents and antioxidant activities of the whole leaf extract of Aloe ferox Mill. Pharmacogn Mag. 2011;7:325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ohikhena FU, Wintola OA, Afolayan AJ. Quantitative phytochemical constituents and antioxidant activities of the Mistletoe, Phragmanthera capitata (Sprengel) Balle extracted with different solvents. Pharmacogn Res. 2018;10:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Onyilagha JC, Islam S. Flavonoids and other polyphenols of the cultivated species of the genus Phaseolus. Int J Agric Biol. 2009;11:231–234. [Google Scholar]
- 13. Obadoni BO, Ochuko PO. Phytochemical studies and comparative efficacy of the crude extracts of some haemostatic plants in Edo and Delta States of Nigeria. Global J Pure Appl Sci. 2002;8:203–208. [Google Scholar]
- 14. Unuofin JO, Otunola GA, Afolayan AJ. Phytochemical screening and in vitro evaluation of antioxidant and antimicrobial activities of Kedrostis africana (L.) Cogn. Asian Pac J Trop Biomed. 2017;7:901–908. [Google Scholar]
- 15. Mishra K, Ojha H, Chaudhury NK. Estimation of antiradical properties of antioxidants using DPPH assay: a critical review and results. Food Chem. 2012;130:1036–1043. [Google Scholar]
- 16. Jimoh FO, Adedapo AA, Afolayan AJ. Comparison of the nutritive value, antioxidant and antibacterial activities of Sonchus asper and Sonchus oleraceus. Rec Nat Prod. 2011;2:29–42. [Google Scholar]
- 17. Alothman M, Bhat R, Karim AA. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009;115:785–788. [Google Scholar]
- 18. Iloki-Assanga SB, Lewis-Luján LM, Lara-Espinoza CL, et al. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. Food Chem. 2015;115:785–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shi J, Yu J, Pohorly J, Young JC, Bryan M, Wu Y. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. J Food Agric Environ. 2003;1:42–47. [Google Scholar]
- 20. Di Majo D, La Guardia M, Giammanco S, La Neve L, Giammanco M. The antioxidant capacity of red wine in relationship with its polyphenolic constituents. Food Chem. 2008;111:45–49. [Google Scholar]
- 21. Oakenfull D. Saponins in food—a review. Food Chem. 1981;7:19–40. [Google Scholar]
- 22. Igbinosa OO, Igbinosa IH, Chigor VN, et al. Polyphenolic contents and antioxidant potential of stem bark extracts from Jatropha curcas (Linn). Int J Mol Sci. 2011;12:2958–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mansouri A, Embarek G, Kokkalou E, Kefalas P. Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera). Food Chem. 2005;89:411–420. [Google Scholar]