Abstract
Background:
The importance of alcohol and cannabis consumption for the effectiveness of treatment of chronic hepatitis C virus (HCV) infection with direct acting antivirals (DAAs) in people on opioid substitution therapy (OST) has not been investigated in detail.
Methods:
We investigated sustained virological response (SVR) rates and proportion of lost to follow-up (LTFU) between OST (n = 739) and non-OST patients (n = 7008) in the German Hepatitis C-Registry (Deutsches Hepatitis C-Register, DHC-R), which is a national multicenter prospective non-interventional real-world registry. Non-OST patients comprised patients with former/current drug use (non-OST/DU; n = 1500) and patients never consuming drugs (non-OST/NDU; n = 5508).
Findings:
SVR 12/24 rates (intention to treat [ITT]) in patients consuming no or less than 30 g/day (women) or 40 g/day (men) were significantly higher in non-OST/NDU (range 91%-92%) vs OST patients (range 83%-86%), mainly due to significantly higher LTFU rates in OST (range 11%-12%) compared with non-OST/NDU (range 2%-3%). In non-OST/NDU with high alcohol consumption of more than 30/40 g/day, SVR 12/24 rates (ITT) were lower (85%) but did not differ to OST (85%) with high alcohol consumption. No significant differences could be seen for SVR 12/24 in per-protocol (PP) analysis independent of alcohol consumption or amount of alcohol intake. Cannabis use did not significantly influence SVR 12/24 in ITT or PP or LTFU.
Conclusions:
High SVR rates could be achieved in both OST and non-OST patients irrespective of alcohol or cannabis consumption. However, LTFU is more likely in patients with current or former drug use than in patients without drug history and in patients with high alcohol consumption but occurred mainly after end of antiviral treatment (EOT), leaving a high chance for HCV elimination in these patients.
Keywords: HCV, real-world setting, PWID, OST, alcohol, cannabis, SVR12
Introduction
To reach the global World Health Organization hepatitis C virus (HCV) elimination targets of a relative reduction in new HCV infections by 80% and hepatitis related mortality by 65% until 2030 compared with 2015,1 special attention has to be drawn to the patient population most affected by hepatitis C, people who actively or had previously injected drugs (PWID). About 10 million PWID worldwide are HCV antibody positive2 and approximately 70% of new infections in industrialized countries are due to this route of infection.3 In Germany, of 4798 newly diagnosed with a positive HCV RNA test in 2017, 73% were reported to be infected by intravenous drug use.4 In modeling studies, testing, linkage to care and a high treatment uptake were identified as key factors on the road to a significant decrease in incidence and prevalence.5 In their actual treatment guidelines the Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA)6 as well as the European Association for the Study of the Liver (EASL)7 pay attention to these facts, define PWID as a key population and give special recommendations for screening and treatment. In clinical studies, opioid substitution therapy (OST) has proved to be a good basis for a treatment with direct acting antiviral (DAA),8,9 but even in PWID with recent drug use sustained virological response (SVR) rates were comparable high.10,11 Recently, we published real-world data from the Deutsches Hepatitis C-Register (DHC-R) supporting scaling up DAA therapy in PWID.12 Although SVR in intention-to-treat (ITT) analysis was lower in patients on OST than in those not receiving OST (85% and 91%, respectively), SVR rates in per-protocol (PP) analysis were the same (96% and 95%, respectively). The differences between ITT and PP analysis were driven by a higher lost to follow-up (LTFU) rate in OST. It is known that PWID on OST have a higher prevalence of alcohol13 and cannabis14,15 use, thought to be associated with polysubstance use and psychiatric comorbidity16,17 with the potential of negatively influencing the adherence to DAA therapy. As presented in this short report, we examined the influence of alcohol and cannabis consumption on DAA treatment outcome in the same DHC-R patient cohort.
Patients and Methods
The German Hepatitis C-Registry (Deutsches Hepatitis C-Register, DHC-R) is a multicenter non-interventional registry study. The present study is a further analysis of data which were recently published.12 Data were obtained by 254 centers, thereof 123 centers providing OST and the present analysis includes data through June 30, 2016. DAA treatment with sofosbuvir (SOF) + pegylated interferon + ribavirin (RBV), SOF + RBV, SOF + simeprevir (SMV) ± RBV, SOF + daclatasvir (DCV) ± RBV, SOF/ledipasvir (LDV) ± RBV, ombitasvir (OBV)/paritaprevir (PTV)/ritonavir ± RBV, OBV/PTV/r ± dasabuvir (DSV) ± RBV started between February 1, 2014 and September 30, 2015.
Opioid substitution therapy and comparator population
In Germany, OST treatment is mostly initiated and maintained in specialized private practices and psychiatric ambulances of hospitals. Most HCV treatments are initiated by gastroenterologists and infectious disease specialists who may also treat patients for opioid substitution. OST duration before start of DAA therapy and possible interruptions of OST are not documented in the DHC-R. The OST population comprised patients which are currently on opioid substitution treatment. The comparator population comprised patients which received antiviral therapy only (non-OST population). These patients were further classified by former/current drug abuse and/or HCV transmission via drug abuse (non-OST/DU) and no signs of former/current drug abuse/HCV transmission other than drugs (non-OST/NDU) as reported by the treating physician.
Assessments and endpoints
Data on alcohol and its amount (</>40 g/day in males or </>30 g/day in females) as well as cannabis consumption (yes/no) were documented at baseline. The effectiveness population (ITT) comprised patients who have completed follow-up 12 to 24 weeks after end of antiviral treatment (EOT).With respect to the PP analysis, the following patients were excluded from the ITT population: non-compliant patients and patients LTFU. Non-compliance (incomplete or irregular treatment) was evaluated by physicians’ point of view. Primary endpoint was the proportion of patients, who achieved SVR12 or SVR24 defined as HCV RNA lower limit of quantification (LLOQ, 25 IU/mL) 70-153 and 154-320 days after EOT, respectively. The proportion of LTFU comprises patients which are lost before and after end of treatment, that is, in the follow-up phase, as documented in the electronic Case Report Form.
Statistics
This analysis includes data through June 30, 2016 and considers all queries answered by 26 July 2016. Summary statistics, frequencies, and proportions were assessed dependent on the scale level of the data. Differences in specific baseline demographic and clinical characteristics between OST and non-OST patients were compared statistically using two-sided hypothesis t-test, Pearson’s χ2 test, Fisher’s exact test, or median test depending on the scale level. Differences were considered significant for P-values ⩽.05. Analyses were conducted using SPSS Windows Release 22.0.0.2 (IBM Corporation, New York, USA).
Results
In total, 7747 patients started second-generation DAA therapy before or on September 30, 2015. Of those, 739 patients received both antiviral therapy and OST (9.5%) and 7008 patients received antiviral therapy only (non-OST population). The latter comprised 1500 patients for which former/current drug abuse (non-OST/DU) and 5508 patients with no former/current drug abuse (non-OST/NDU).
A total of 528 out of 739 patients on OST and 5582 out of 7008 non-OST patients have completed therapy and at least one follow-up documentation 12 to 24 weeks after EOT. Of those, 523 OST and 5561 non-OST patients had data concerning alcohol consumption and 528 OST and 5581 non-OST patients concerning cannabis consumption (ITT population); 460 out of 739 patients on OST and 5296 out of 7008 non-OST (DU and NDU) had complete data sets including alcohol and 462 out of 739 patients on OST and 5315 out of 7008 non-OST (DU and NDU) including cannabis consumption allowing a PP analysis.
Baseline demographics of OST and non-OST (DU and NDU) patient groups stratified by alcohol and cannabis consumption are shown in Tables 1 and 2 as well as in Supplemental Tables S1 to S4. Alcohol consumption was reported in 17.9% (128/714) of OST, in 17.5% (256/1462) of non-OST/DU, and in 11.6% (631/5421) of non-OST/NDU patients. Among OST patients, 25% (32/128) consumed high amounts (>40 g alcohol/day [men]/>30 g/day [women]) of alcohol. In non-OST/DU 22.2% (57/256) and in non-OST/NDU significantly less patients (13.9%, 88/631) consumed high amounts of alcohol when compared with OST patients (P < .05). Similarly, cannabis consumption was significantly (P < .05) higher in OST patients (19.2%, 139/725) than in non-OST/DU (9.6%, 141/1474) and non-OST/NDU patients (1.2%, 66/5448). Compared with non-OST (DU and NDU) patients, OST patients differed considerably in some characteristics: Among OST patients, the prevalence of male and younger patients was higher and they were less treatment experienced than non-OST (DU and NDU) patients. In addition, all OST patients suffered from comorbidities, whereas comorbidities were documented for 70% to 80% of non-OST (DU and NDU) patients.
Table 1.
Baseline characteristics of patients with alcohol consumption.
| Patient characteristics | Non-OST/NDU (N = 631)a | Non-OST/DU (N = 256)a | OST (N = 128)a | P b | P c |
|---|---|---|---|---|---|
| Male, % (n) | 68.1 (430) | 79.3 (203) | 85.9 (110) | <.001 | n.s. |
| Age (years, mean ± SD) | 51.7 (12.0) | 47.9 (9.5) | 45.2 (8.1) | <.001 | .004 |
| Caucasian, % (n) | 96.7 (610) | 98.4 (252) | 98.4 (126) | n.s. | n.s. |
| HCV genotype, % (n) | |||||
| GT1 | 74.5 (470) | 63.7 (163) | 60.9 (78) | .002 | n.s. |
| GT1a | 33.3 (210) | 47.7 (122) | 46.1 (59) | — | — |
| GT1b | 37.9 (239) | 15.2 (39) | 12.5 (16) | — | — |
| GT1 other subtypes | 3.3 (21) | 1.2 (3) | 2.3 (3) | — | — |
| GT 2 | 6.8 (43) | 4.7 (12) | 6.3 (8) | n.s. | n.s. |
| GT 3 | 10.9 (69) | 28.5 (73) | 27.3 (359 | <.001 | n.s. |
| GT 4 | 7.8 (49) | 3.1 (8) | 5.5 (7) | n.s. | n.s. |
| GT 5 or 6 | — | — | — | — | — |
| HCV RNA (IU/mL, mean ± SD) | 3 005 425 (5 807 719) | 3 370 713 (5 633 803) | 5 566 303 (14 073 000) | .001 | .031 |
| Treatment-experienced, % (n) | 43.1 (272) | 40.6 (104) | 31.3 (40) | .014 | n.s. |
| IFN experienced, % (n/N) | 96.7 (263/272) | 95.2 (99/104) | 100.0 (40/40) | n.s. | n.s. |
| FibroScan ⩾12.5 kPa (F4), % (n/N) | 21.1 (65/308) | 26.4 (34/129) | 30.2 (16/53) | n.s. | n.s. |
| Cirrhotic patients, % (n) | 20.1 (127) | 27.0 (69) | 21.1 (27) | n.s. | n.s. |
| Platelets (×109/L, median, Q1-Q3) | 203.0 (152.0-254.0) | 186.0 (141.0-230.5) | 185.0 (132.0-227.0) | n.s. | n.s. |
| Platelets <90 × 109/L, % (n/N) | 6.6 (39/589) | 11.5 (28/244) | 11.2 (14/125) | n.s. | n.s. |
| g-GT (IU/L, median, Q1-Q3) | 78.0 (41.0-155.0) | 100.0 (55.0-201.0) | 122.5 (55.0-243.0) | .002 | n.s. |
| ALT (IU/L, median, Q1-Q3) | 70.0 (45.0-132.0) | 86.8 (56.0-125.5) | 71.5 (41.5-118.9) | n.s. | n.s. |
| Comorbidities, % (n) | 73.2 (462) | 70.7 (181) | 100.0 (128) | <.001 | <.001 |
| Cardiovascular disease, % (n) | 25.5 (161) | 16.8 (43) | 13.3 (17) | .003 | n.s. |
| Diabetes mellitus, % (n) | 8.1 (51) | 5.1 (13) | 2.3 (3) | .022 | n.s. |
| Psychiatric disorders, % (n) | 14.4 (91) | 16.0 (41) | 22.7 (29) | .024 | n.s. |
| Depression, % (n/N) | 92.3 (84/91) | 90.2 (37/41) | 89.7 (26/29) | n.s. | n.s. |
| HCV/HIV co-infection, % (n) | 17.1 (108) | 10.2 (26) | 6.3 (9) | 0.003 | n.s. |
Abbreviations: DU, former/current drug use and/or HCV transmission via drug abuse; GT, HCV genotype; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IFN, interferon alpha; non-OST, patients without OST; NDU, no former/current drug use/other mode of HCV transmission; n.s., not significant, P > .05; OST, opioid substitution therapy, g-GT, gamma glutamyl transferase; ALT, alanine transaminase.
If not otherwise indicated (specific lab data were not available for all patients).
P-value non-OST/NDU vs OST.
P-value non-OST/DU vs OST.
Table 2.
Baseline characteristics of patients with cannabis consumption.
| Patient characteristics | Non-OST/NDU (N = 66)a | Non-OST/DU (N = 141)a | OST (N = 139)a | P b | P c |
|---|---|---|---|---|---|
| Male, % (n) | 75.8 (50) | 73.0 (103) | 87.8 (122) | .041 | .002 |
| Age (years, mean ± SD) | 47.6 (10.0) | 49.0 (9.4) | 47.4 (7.8) | n.s. | n.s. |
| Caucasian, % (n) | 95.5 (63) | 97.9 (138) | 98.6 (137) | n.s. | n.s. |
| HCV genotype, % (n) | |||||
| GT1 | 71.2 (47) | 70.2 (99) | 66.2 (92) | n.s. | n.s. |
| GT1a | 51.5 (34) | 56.7 (80) | 48.2 (67) | — | — |
| GT1b | 14.4 (9) | 12.1 (17) | 14.4 (20) | — | — |
| GT1 other subtypes | 6.1 (4) | 1.4 (2) | 3.6 (5) | — | — |
| GT 2 | 4.5 (3) | 5.0 (7) | 5.0 (7) | n.s. | n.s. |
| GT 3 | 15.2 (10) | 22.7 (32) | 23.7 (33) | n.s. | n.s. |
| GT 4 | 9.1 (6) | 4.5 (60) | 3.6 (21) | n.s. | n.s. |
| GT 5 or 6 | — | — | — | — | — |
| HCV RNA (IU/mL, mean ± SD) | 3 882 842 (4 681 091) | 2 908 010 (4 181 782) | 3 224 493 (9 345 764) | n.s. | n.s. |
| Treatment-experienced, % (n) | 47.0 (31) | 42.5 (566) | 31.2 (183) | .028 | .014 |
| IFN experienced, % (n/N) | 100.0 (31/31) | 98.4 (62/63) | 97.6 (41/42) | n.s. | n.s. |
| FibroScan ⩾12.5 kPa (F4), % (n/N) | 10.3 (3/29) | 28.6 (16/56) | 24.0 (12/50) | n.s. | n.s. |
| Cirrhotic patients, % (n) | 19.7 (13/66) | 29.8 (42/141) | 23.0 (32/139) | n.s. | n.s. |
| Platelets (×109/L, median, Q1-Q3) | 191.0 (138.0-247.0) | 190.0 (137.0-252.0) | 183.0 (137.0-229.0) | n.s. | n.s. |
| Platelets <90 × 109/L, % (n/N) | 12.7 (8/63) | 7.7 (10/130) | 9.0 (12/133) | n.s. | n.s. |
| g-GT (IU/L, median, Q1-Q3) | 61.0 (39.0-133.0) | 65.5 (36.0-130.0) | 75.0 (33.0-163.0) | n.s. | n.s. |
| ALT (IU/L, median, Q1-Q3) | 59.0 (40.5-106.0) | 64.3 (46.0-105.6) | 49.7 (27.6-92.5) | n.s. | n.s. |
| Comorbidities, % (n) | 75.8 (50) | 80.1 (113) | 100.0 (139) | <.001 | <.001 |
| Cardiovascular disease, % (n) | 10.6 (7) | 12.1 (17) | 10.1 (14) | n.s. | n.s. |
| Diabetes mellitus, % (n) | 1.5 (1) | 5.0 (7) | 2.2 (3) | n.s. | n.s. |
| Psychiatric disorders, % (n) | 25.8 (17) | 26.2 (37) | 29.5 (41) | n.s. | n.s. |
| Depression, % (n/N) | 88.2 (15/17) | 86.5 (32/37) | 92.7 (28/42) | n.s. | n.s. |
| HCV/HIV co-infection, % (n) | 33.3 (22) | 12.1 (17) | 15.1 (21) | .005 | n.s. |
Abbreviations: DU, former/current drug use and/or HCV transmission via drug abuse; GT, HCV genotype; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IFN, interferon alpha; non-OST, patients without OST; NDU, no former/current drug use/other mode of HCV transmission; n.s., not significant, P > .05; OST, opioid substitution therapy, g-GT, gamma glutamyl transferase; ALT, alanine transaminase.
If not otherwise indicated (specific lab data were not available for all patients).
P-value non-OST/NDU vs OST.
P-value non-OST/DU vs OST.
Overall, the proportion of LTFU was higher in patients with current or former drug use than in patients without drug history and in patients with high alcohol consumption (Figure 1). In alcohol consuming patients, proportion of LTFU was significantly higher in OST (12/97) compared with non-OST/NDU (16/532) (Figure 1A), but occurred mainly (67%) after EOT. In cannabis consuming patients, the proportion of LTFU differed not significantly between the different patient groups (Figure 1B).
Figure 1.
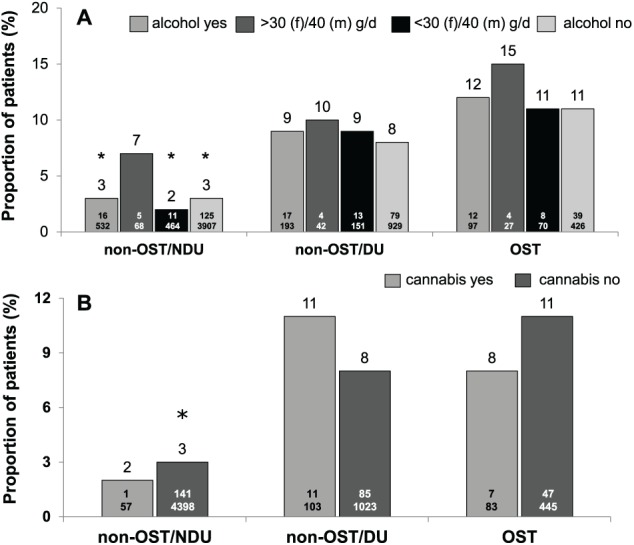
Proportion of lost to follow-up (LTFU) in non-OST and OST patient groups according to alcohol (A) and cannabis (B) consumption (ITT population). DU indicates former/current drug use and/or HCV transmission via drug abuse; non-OST, patients without OST; NDU, no former/current drug use/other mode of HCV transmission; OST, opioid substitution therapy.
*, P < .05 compared with OST.
The overall ITT SVR rate was significantly (P < .05) diminished in OST (85%) and non-OST/DU (86%) compared with non-OST/NDU patients (91%), but not in PP analysis (OST 96%, non-OST/DU 94%, non-OST/NDU 95%). When stratified by alcohol consumption (yes/no) and moderate daily intake of alcohol (⩽40 g/day [men]/⩽30 g/day [women]), non-OST/NDU patients had significantly higher SVR rates than OST patients in ITT (Figure 2A), but not in PP analysis (Figure 3A). With respect to cannabis consumption, ITT SVR rates did not differ between the three patient groups (Figure 2B). In PP analysis, SVR rates were between 93% and 96%, irrespective of cannabis consumption (Figure 3B). Relapse rates were numerically lower in OST than in non-OST/DU and non-OST/NDU patients (data not shown).
Figure 2.
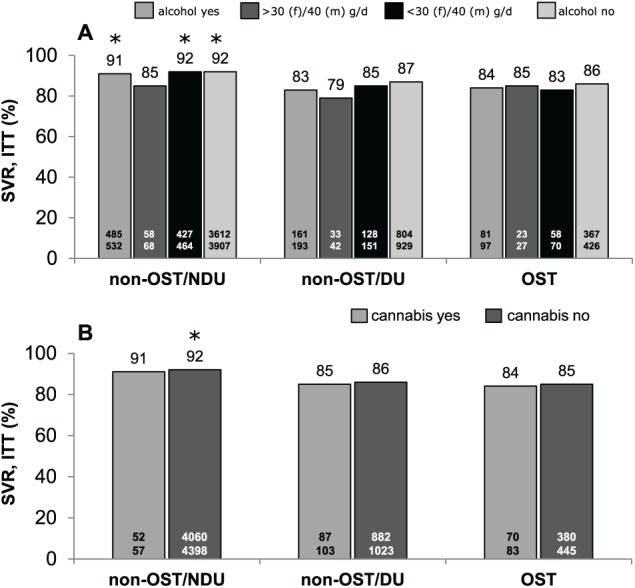
SVR 12 and/or SVR 24 rates of HCV therapy for non-OST and OST patients according to alcohol (A) and cannabis (B) consumption (ITT population). DU indicates former/current drug use and/or HCV transmission via drug abuse; ITT, intention-to-treat; non-OST, patients without OST; NDU, no former/current drug use/other mode of HCV transmission; OST, opioid substitution therapy; SVR, sustained virological response.
*, P < .05 compared with OST.
Figure 3.
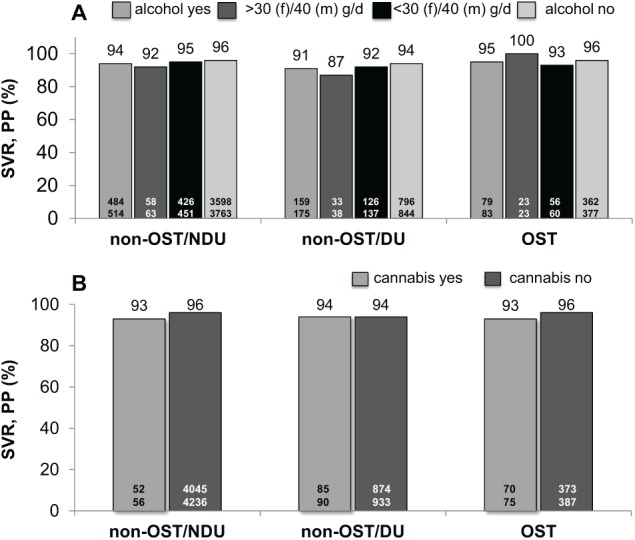
SVR 12 and/or SVR 24 rates of HCV therapy for non-OST and OST patients according to alcohol (A) and cannabis (B) consumption (PP population). DU indicates former/current drug use and/or HCV transmission via drug abuse; LTFU, lost to follow-up; non-OST, patients without OST; NDU, no former/current drug use/other mode of HCV transmission; OST, opioid substitution therapy; PP, per protocol; SVR, sustained virological response.
P > .05 compared with OST (no significant differences).
Discussion
We recently confirmed the safety and effectiveness of novel interferon-free antiviral therapy in OST patients and former drug users in a large real-world cohort,12 which is in line with two other recent reports of Mason et al18 and Norton et al19 and a large meta-analysis.20 Of note and importantly, we here report that these results were reproducible in the subset of patients with reported data on alcohol and cannabis consumption.
Due to the high treatment success of modern DAA regimens, a higher LTFU after EOT in PWID on OST in our cohort might be of less concern. Nevertheless, HCV testing, linkage to care and treatment uptake, has to be enhanced to decrease HCV incidence and prevalence in PWID and other patient groups.5 Integrated models of care in multidisciplinary teams adapted to PWID in private practices, clinics and prisons accompanied by needle syringe programs and harm reduction programs are successful21,22 but not available throughout the countries. To engage more specialists or general practitioners in DAA therapy for PWID, detailed description of this patient group under DAA therapy in a real-world setting might help to overcome prejudices and lower the barrier to initiate DAA treatment.
Cannabis use has been shown to be more prevalent in PWID on OST compared with other patient groups and the general population,14,15 as it was found in the DHC-R, respectively. Cannabis use is associated with psychiatric illnesses like mood disorders, anxiety, and psychosis.16,17 However, cannabis consumption did not influence SVR in ITT or PP analysis in our study. Beside concerns of a health risk, cannabis use should not lead to restraints with respect to antiviral treatment initiation. In our study, alcohol consumption was more prevalent in OST compared with non-OST/DU and non-OST/NDU. This is a potential concern as ethanol and hepatitis C may have additive effects in the pathogenesis of chronic liver disease.23 In PP analysis OST, non-OST/DU and non-OST/NDU performed equally with regard to SVR 12/24 independent of alcohol consumption per se or amount of alcohol intake. Still, non-OST/NDU had a significant higher chance for SVR 12/24 compared with OST in the ITT analysis which was mainly due to a significant lower LTFU rate in patients without or with a moderate alcohol intake of less than 30 g/day (women) or 40 g/day (men). The difference in LTFU lost statistical significance in patients with alcohol consumption of more than 30 g/day or 40 g/day in non-OST/NDU. These data highlight that initiating DAA therapy in alcohol consuming patients will remain an individual decision. Our data might support a strategy to lower the barrier for treatment uptake.
There are some obvious limitations of our observational study, even if prospective data have been collected. In the DHC-R, documentation of data on concomitant use of illegal drugs was scarce at the time of analysis. Unfortunately, the database lacks information on the previous duration of OST as well as data on directly observed OST. In addition, former drug use may be under-reported which may have biased the non-OST/NDU group. Rates of re-infection, still a concern especially in PWID and HCV/HIV co-infected patients, could not be reported. The role of active drug abuse could not be investigated due to the low number of patients actively consuming drugs (overall proportion <3%).
On the other hand, we have to highlight that the registry is representative for all approved DAA combinations during 2014-2015 in Germany as about 30% of all HCV therapies performed during that period were documented. Importantly, about 50% of participating centers did document both OST and non-OST patients.
This large real-world cohort of patients substantiates the effectiveness of DAAs in patients with alcohol and cannabis consumption receiving opioid substitution therapy. Our data support the finding that OST is a strong basis for the initiation of HCV therapy.24 Thus, as many patients as possible should receive antiviral treatment.
Supplemental Material
Supplemental material, DHCR_OST-Cannabis_Alcohol_supplementary_2018-10-30_xyz151015746ed84_3-converted for Alcohol and Cannabis Consumption Does Not Diminish Cure Rates in a Real-World Cohort of Chronic Hepatitis C Virus Infected Patients on Opioid Substitution Therapy—Data From the German Hepatitis C-Registry (DHC-R) by Stefan Christensen, Peter Buggisch, Stefan Mauss, Klaus HW Böker, Tobias Müller, Hartwig Klinker, Tim Zimmermann, Yvonne Serfert, Bernd Weber, Jens Reimer and Heiner Wedemeyer in Substance Abuse: Research and Treatment
Acknowledgments
The authors thank all study investigators, study nurses, and participating patients. Special thanks to Heike Pfeiffer-Vornkahl from e.factum GmbH for data analysis and statistics.
Footnotes
Funding:The German Hepatitis C-Registry (Deutsches Hepatitis C-Register, DHC-R) is a project of the German Liver Foundation (Deutsche Leberstiftung) managed by Leberstiftungs-GmbH Deutschland in cooperation with the Association of German gastroenterologists in private practice (bng, Bund Niedergelassener Gastroenterologen) with financial support from the German Center for Infection Research (DZIF) and the companies AbbVie Deutschland GmbH & Co. KG, Bristol-Myers Squibb GmbH & Co. KGaA, Gilead Sciences GmbH, Janssen-Cilag GmbH, MSD Sharp & Dohme GmbH as well as Roche Pharma AG (financial support until July 14, 2017). The authors are independent from the funding companies in data analysis, data interpretation, report writing, and publication.
Declaration of conflicting interest:SC reports personal fees from BMS, AbbVie, Janssen, ViiV, Gilead, and MSD outside the submitted work. PB reports personal fees from AbbVie, BMS, Falk, Gilead, Janssen, Merz Pharma, and MSD Pharma outside the submitted work. SM reports personal fees from AbbVie, Bristol-Meyers-Squibb, Gilead Sciences, Janssen-Cilag, MSD Sharp & Dohme/Merck, and ViiV, outside the submitted work. KHWB reports personal fees from Gilead Sciences, MSD Sharp & Dohme, Merck, AbbVie, and other from Janssen, outside the submitted work. TM reports travel grants from AbbVie, Alexion, MSD, Gilead, MERZ, Intercept, FALK; grants/research support from FALK Foundation, Intercept outside the submitted work. HK reports personal fees from AbbVie, BMS, Gilead, Hexal, Janssen, MSD, Arrowhead, and Novartis; grants/research support from AbbVie, BMS, Gilead, Janssen, MSD, Arrowhead, and Novartis outside the submitted work. TZ reports personal fees, grants, or research support from AbbVie, BMS, Gilead, MSD, Novartis, and Roche outside the submitted work. BW reports personal fees from Camurus GmbH, Indivior, Mundipharma, Gilead, BMS, AbbVie, MSD outside the submitted work. JR reports personal fees, grants/research support from Gilead, AbbVie, Janssen, and BMS outside the submitted work. YS is employee of Leberstiftungs-GmbH Deutschland. HW reports personal fees from Transgene, MSD, Roche, Gilead, Abbott, BMS, Falk, AbbVie, Novartis, GSK, Roche Diagnostics, Eiger, ITF, and MyrGmbH; grants/research support from MSD, Novartis, Gilead, Roche, Abbott, and Roche Diagnostics outside the submitted work.
Author Contributions: SC: Guarantor of article; data acquisition, data analysis, data interpretation, writing the manuscript, figures, tables; PB: patient recruitment, data acquisition; SM: patient recruitment, data acquisition; KHB: patient recruitment, data acquisition; TM: patient recruitment, data acquisition; HK: patient recruitment, data acquisition; TZ: patient recruitment, data acquisition; YS: data analysis, data interpretation, writing the manuscript, figures, tables; BW: patient recruitment, data acquisition; JR: expert advice; HW: data analysis, data interpretation, writing the manuscript, figures, tables. All authors approved the final version of the article, including the authorship list.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. World Health Organization. Global Health Sector Strategy on Viral Hepatitis. Geneva: World Health Organization; 2016. [Google Scholar]
- 2. Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Degenhardt L, Charlson F, Stanaway J, et al. Estimating the burden of disease attributable to injecting drug use as a risk factor for HIV, hepatitis C, and hepatitis B: findings from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16:1385–1398. [DOI] [PubMed] [Google Scholar]
- 4. Robert Koch-Institut. Hepatitis C im Jahr 2017. Epidemiologisches Bulletin. 2018;29 https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2018/Ausgaben/29_18.pdf?__blob=publicationFile. Accessed August 3, 2018 (Archived by WebCite® at http://www.webcitation.org/72iPz81Om) [Google Scholar]
- 5. Scott N, Doyle JS, Wilson DP, et al. Reaching hepatitis C virus elimination targets requires health system interventions to enhance the care cascade. Int J Drug Policy. 2017;47:107–116. [DOI] [PubMed] [Google Scholar]
- 6. The American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Up-dated: May 24, 2018. http://www.hcvguidelines.org. (Archived by WebCite® at http://www.webcitation.org/72iQ5rjVB)
- 7. European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69:461-511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 8. Grebely J, Dore GJ, Zeuzem S, et al. Efficacy and safety of sofosbuvir/velpatasvir in patients with chronic hepatitis C virus infection receiving opioid substitution therapy: analysis of Phase 3 ASTRAL trials. Clin Infect Dis. 2016;63:1479–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grebely J, Dore GJ, Alami NN, et al. Safety and efficacy of Glecaprevir/Pibrentasvir in patients with chronic hepatitis C Genotypes 1-6 receiving opioid substitution therapy. Paper presented at: 6th international Symposium on Hepatitis Care in Substance Users, Jersey City, NJ, September 6-8, 2017. [Google Scholar]
- 10. Dore GJ, Altice F, Litwin AH, et al. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med. 2016;165:625–634. [DOI] [PubMed] [Google Scholar]
- 11. Grebely J, Dalgard O, Conway B, et al. Efficacy and safety of sofosbuvir/velpatasvir in people with chronic hepatitis C virus infection and recent injecting drug use: the SIMPLIFY study. J Hepatol. 2017;66:513. [Google Scholar]
- 12. Christensen S, Buggisch P, Mauss S, et al. Direct acting antiviral treatment of chronic HCV-infected patients on Opioid Substitution Therapy: still a concern in clinical practice. Addiction. 2018;113:868–882. [DOI] [PubMed] [Google Scholar]
- 13. Rengade CE, Kahn JP, Schwan R. Misuse of alcohol among methadone patients. Am J Addict. 2009;18:162–166. [DOI] [PubMed] [Google Scholar]
- 14. Bawor M, Dennis BB, Varenbut M, et al. Sex differences in substance use, health, and social functioning among opioid users receiving methadone treatment: a multicenter cohort study. Biol Sex Differ. 2015;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zielinski L, Bhatt M, Eisen RB, et al. Association between cannabis use and treatment outcomes in patients receiving methadone maintenance treatment: a systematic review protocol. Syst Rev. 2016;5:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wittchen H, Frohlich C, Behrendt S, et al. Cannabis use and cannabis use disorders and their relationship to mental disorders: a 10-year prospective-longitudinal community study in adolescents. Drug Alcohol Depend. 2007;88:S60–S70. [DOI] [PubMed] [Google Scholar]
- 17. Crippa A, Zuardi AW, Martin-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24:515–523. [DOI] [PubMed] [Google Scholar]
- 18. Mason K, Dodd Z, Guyton M, et al. Understanding real-world adherence in the directly acting antiviral era: a prospective evaluation of adherence among people with a history of drug use at a community-based program in Toronto, Canada. Int J Drug Policy. 2017;47:202–208. [DOI] [PubMed] [Google Scholar]
- 19. Norton BL, Fleming J, Bachhuber MA, et al. High HCV cure rates for people who use drugs treated with direct acting antiviral therapy at an urban primary care clinic. Int J Drug Policy. 2017;47:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hajarizadeh B, Cunningham EB, Reid H, Law M, Dore GJ, Grebely J. Direct-acting antiviral treatment for hepatitis C among people who use or inject drugs: a systematic review and meta-analysis [published online ahead of print September 20, 2018]. Lancet Gastroenterol Hepatol. doi: 10.1016/S2468-1253(18)30304-2 [DOI] [PubMed] [Google Scholar]
- 21. Bruggmann P, Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin Infect Dis. 2013;57:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olafsson S, Tyrfingsson T, Runarsdottir V, et al. Treatment as prevention for Hepatitis C (TraP Hep C)—a nationwide elimination programme in Iceland using direct-acting antiviral agents. J Intern Med. 2018;283:500–507. [DOI] [PubMed] [Google Scholar]
- 23. Szabo G, Wands JR, Eken A, et al. Alcohol and hepatitis C virus—interactions in immune dysfunctions and liver damage. Alcohol Clin Exp Res. 2010;34:1675–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Butner JL, Gupta N, Fabian C, Henry S, Shi JM, Tetrault JM. Onsite treatment of HCV infection with direct acting antivirals within an opioid treatment program. J Subst Abuse Treat. 2017;75:49–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DHCR_OST-Cannabis_Alcohol_supplementary_2018-10-30_xyz151015746ed84_3-converted for Alcohol and Cannabis Consumption Does Not Diminish Cure Rates in a Real-World Cohort of Chronic Hepatitis C Virus Infected Patients on Opioid Substitution Therapy—Data From the German Hepatitis C-Registry (DHC-R) by Stefan Christensen, Peter Buggisch, Stefan Mauss, Klaus HW Böker, Tobias Müller, Hartwig Klinker, Tim Zimmermann, Yvonne Serfert, Bernd Weber, Jens Reimer and Heiner Wedemeyer in Substance Abuse: Research and Treatment


