Short abstract
Migraine is triggered by poor air quality and odors through unknown mechanisms. Activation of the trigeminovascular pathway by environmental irritants may occur via activation of transient receptor potential ankyrin 1 (TRPA1) receptors on nasal trigeminal neurons, but how that results in peripheral and central sensitization is unclear. The anatomy of the trigeminal ganglion suggests that noxious nasal stimuli are not being transduced to the meninges by axon reflex but likely through intraganglionic transmission. Consistent with this concept, we injected calcitonin gene-related peptide, adenosine triphosphate, or glutamate receptor antagonists or a gap junction channel blocker directly and exclusively into the trigeminal ganglion and blocked meningeal blood flow changes in response to acute nasal TRP agonists. Previously, we observed chronic sensitization of the trigeminovascular pathway after acrolein exposure, a known TRPA1 receptor agonist. To explore the mechanism of this sensitization, we utilized laser dissection microscopy to separately harvest nasal and meningeal trigeminal neuron populations in the absence or presence of acrolein exposure. mRNA levels of neurotransmitters important in migraine were then determined by reverse transcription polymerase chain reaction. TRPA1 message levels were significantly increased in meningeal cell populations following acrolein exposure compared to room air exposure. This was specific to TRPA1 message in meningeal cell populations as changes were not observed in either nasal trigeminal cell populations or dorsal root ganglion populations. Taken together, these data suggest an important role for intraganglionic transmission in acute activation of the trigeminovascular pathway. It also supports a role for upregulation of TRPA1 receptors in peripheral sensitization and a possible mechanism for chronification of migraine after environmental irritant exposure.
Keywords: Trigeminal, migraine, pain, TRPA1, animal model
Introduction
Air pollution and odors are known triggers of migraine, and poor air quality is correlated with an increase in emergency room visits for headache symptoms. Although evidence for an association between odor and primary headache, especially migraine exists, the exact mechanism of action of odors as migraine triggers is not known. Migraine is a complex neurologic disorder with up to 25% of patients reporting heightened sensitivity to odors, and up to 50% reporting that odors may trigger acute migraine attacks. Studies have shown that inhalation of certain odors or exposure to a variety of chemicals can cause severe headache attacks through stimulation of transient receptor potential ankyrin 1 (TRPA1) receptors.1 TRPA1 receptors are excitatory ion channels expressed in trigeminal sensory neurons which innervate the nasal and respiratory epithelium, dura, and other parts of the trigeminovascular pathway and have been linked to migraine.
Although inhaled odors and chemicals may trigger migraine symptoms, the mechanism linking activation of nasal TRPA1 receptors to peripheral and central sensitization of trigeminal pain pathways remain unknown. We propose that intraganglionic transmission within the trigeminal ganglia (TG) mediates communication between populations of neurons innervating different structures of the trigeminovascular pathway. This type of communication has been described in several ganglia and has been referred to as cross-excitation.2–7 Excitation of ganglion neurons leads to increased action potentials in neighboring neurons that are believed to be dependent on the release of diffusible chemical mediators. Cross-excitation of neuron populations may be responsible for chronic pain or sensitization.4 Within the TG, cell bodies of afferent sensory neurons from the nasal epithelium, dural blood vessels, and periorbital skin are in proximity to each other,8–10 which could enable signals from the nasal epithelium to be relayed to neurons targeting other structures. A number of chemical mediators in the TG, including glutamate,11 adenosine triphosphate (ATP),12,13 and calcitonin gene-related peptide (CGRP),14 may act as signaling molecules between the neurons. Gap junctions15,16 and satellite glia17 also appear to be important in chronic trigeminal pain. Herein, we examine the functional role of these mediators and components in the activation of the trigeminovascular pathway by environmental irritants. Specifically, we examine the effect of CGRP on the excitability of sensory neurons and describe the outcome of blocking specific neurotransmitters in the trigeminal ganglion.
We hypothesize that air pollution-induced headache is mediated by stimulation of TRPA1 receptors and subsequent activation of the trigeminovascular pathway. As evidence, we previously reported that acute nasal administration of environmental irritants increased meningeal blood flow in a TRPA1- and CGRP-dependent manner.8,18 Furthermore, inhalation pre-exposure to subacute doses of the environmental irritant and TRPA1 channel agonist acrolein sensitized blood flow and behavioral responses and induced periorbital allodynia, effects which are long-lasting.19,20 This suggests that trigeminovascular sensitization involving TRPA1 channel activation is a possible means for enhanced headache susceptibility after chemical exposure. Although we observed no changes in TRPA1 receptor mRNA from whole TG following chronic inhalation of acrolein,20 it is possible that specific relevant subpopulations of TG neurons might demonstrate changes. To further examine the role of TRPA1 and other signaling molecules in acrolein-induced sensitization, we combined retrograde labeling, laser dissection microscopy and quantitative PCR (qPCR) to identify and compare changes in message expression in selected nasal versus dural afferent neuronal populations from the TG. Our results demonstrate that TRPA1 mRNA levels are increased only in dural, but not nasal, projecting neurons in TG following acrolein exposure and suggest a possible role for these receptors in environmental irritant induced trigeminovascular sensitization.
Methods
All animal procedures were approved by the Institutional Animal Care and Use Committee at the Indiana University School of Medicine and followed the ethical guidelines of International Association for the Study of Pain.21 Experiments were performed on 225 adult male (170–250 g) Sprague–Dawley rats (Envigo, IN). Rats were housed in pairs in solid bottom cages with hardwood chip bedding with a standard 12 h light and dark cycle with free access to food and water. Animals were randomly assigned to experimental groups and weighed daily during treatment. No adverse effects of treatment were observed. All results are reported according to Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Dorsal root ganglion neuron dissociation and culture
Dorsal root ganglion (DRG) neurons from adult rats (150–180 g) were dissociated and cultured as described previously.22 In brief, DRGs were isolated from lumbar segments of spinal cords and dissociated by a combination treatment with a dispase/collagenase cocktail and mechanical disruption through a series of fire-polished glass pipettes with a decreasing inner tip diameter. The resulting suspension of single cells was plated on to poly-D-lysine-coated 15-mm glass coverslips (one thickness) for electrophysiology and imaging, or poly-D-lysine and laminin-coated wells or dishes for Western blotting. In all cases, cells were maintained in Dulbecco’s modified Eagle’s medium (Gibco, Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) and 100 units/ml penicillin and 100 mg/ml streptomycin for 16 to 24 h at 37°C under 5% CO2.
Electrophysiology
Somatic whole-cell membrane potentials were measured using patch electrodes in the whole-cell configuration with an Axopatch 200 amplifier. Data were collected and analyzed using Clampex7 software, and graphs and statistical tests were performed in SigmaPlot. Patch pipettes were constructed from N51A glass and polished on a homemade microforge at 600× magnification. All experiments were performed at room temperature (21°C–23°C). The standard external solution contained (in mM): 145 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose. The internal solution in the patch electrode contained (in mM): 130 potassium aspartate, 20 KCl, 1 EGTA, 1 MgCl2, 10 HEPES, and 10 glucose. All solutions were adjusted to a pH of 7.4 and an osmolarity of ∼305 mOsm for the internal solution and ∼297 for the external.
Two different current clamp protocols were employed, a ramp protocol, and a continuous recording protocol. For the ramp protocol, ramp currents of 400–1800 ms duration, beginning at −0.1 nA and ending at 2.1 nA were injected into the cells, and the resulting action potentials were recorded. The slope of the ramp was increased with each sweep. The gap-free protocol was used to determine the effect of CGRP application to the resting potential without current injection.
Drugs were applied locally onto the cells by gravity using a small diameter (250 µm) quartz capillary. Capsaicin stock (1 mM, Sigma) was made in ethanol, diluted to 0.1 − 1 µM with SES + 0.05 – 0.1% BSA, and applied. CGRP (Tocris) stocks (100 mM) were made in SES + 0.05 − 0.1% BSA and diluted to 100 − 300 nM. The CGRP receptor antagonist, CGRP8-37, was made at 300 nM made in SES + 0.05 − 0.1% BSA. When the antagonist was applied, it was combined with the CGRP peptide at 100 nM prior to bath application. Vehicle controls (ethanol 0.05% + 0.1% BSA) were also applied to the cells.
Retrograde labeling
Labeling of the trigeminal innervation of the middle cerebral artery (MCA) followed the procedure of O’Connor and van der Kooy.9 Briefly, male rats were anesthetized with ketamine/xylazine (80 and 10 mg/kg body weight, respectively) and a cranial window prepared. The dura was cut and reflected away to expose the right MCA and a small piece of parafilm was positioned underneath the artery. Gelfoam soaked in a 10% solution of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; Life Technologies) dissolved in ethanol was placed on top of the artery after which another piece of parafilm was placed on top of the gelfoam. The skin was sutured and the animal was placed on a heating pad during recovery. During recovery, 5 µl of hydroxystilbamidine (Fluorogold, 10% solution in DMSO; Life Technologies) was slowly administered into the right nasal cavity of the rat to label trigeminal innervation of the nasal epithelium. Animals were allowed to recover for one week prior to inhalation experiments. In addition, some of the animals received an injection of DiI into the hindpaw footpad during recovery to label neurons in the dorsal root ganglia.
Inhalation exposure
Rats were exposed to acrolein by mixing acrolein gas (Air Liquide, Plumsteadville, PA) and room air to obtain the desired concentration as described previously.20 The acrolein dosage (0.3 ppm) was chosen because it produced minimal or no detectable harmful effects in previous studies.20,23–25 In addition, it is equivalent to the limit for short-term exposure recommended by the National Institute of Occupational Safety and Health. Separate inhalation chambers (Braintree Scientific, Inc; 5.5 L total volume) and tubing were used for acrolein and control groups to avoid cross-contamination. The flow rate was maintained at 1.5 L/min and temperature and humidity were monitored in the chamber. Rats were exposed to acrolein (0.33 ± 0.03 ppm) (n = 34) 4 h per day for four days while control animals were exposed to room air with the same paradigm. Cumulative acrolein exposure for each animal was determined with monitoring badges placed in the chamber (Advanced Chemical Sensors Inc, Boca Raton, FL). On day 5, approximately 24 h after the last inhalation exposure, tissue from retrograde-labeled animals was harvested for laser dissection microscopy or laser Doppler flowmetry was conducted as described below.
Infraorbital injection
The technique for trigeminal ganglion injections followed those detailed by Neubert et al.26 Briefly, the head of an anesthetized animal was stabilized in one hand and the rostral portion of the zygomatic process of the maxillary bone palpated. A sterile 25 gauge ×20 mm needle was then inserted medial to the zygomatic process through the infraorbital foramen. Drugs dissolved in saline and 0.2% Trypan Blue solution (Sigma # T8154) were then injected (7 µl) over 1 min using a Hamilton syringe. The needle remained in the foramen for 5 min and was then slowly removed. Trypan Blue served as verification of successful drug injection into the ganglia and animals in which the dye was not visible or in which dye was observed outside the ganglion following blood flow measurements were excluded from analysis. Injected drugs included carbenoxolone (Sigma #C4790), CGRP8-37 (Tocris #1169), DL-TBOA (Tocris #1223), D-APV (Tocris #0106), and A-317491 (Santa Cruz #sc-300144). Saline and CGRP8-37 inactivated with the reducing agent TCEP-HCL (Thermoscientific #20490) were used as controls. Agents were injected under anesthesia 2 h before blood flow measurements.
Laser Doppler flowmetry
Laser Doppler flowmetry was performed as previously described between 10:00 and 15:00.18 Male rats were anesthetized with ketamine/xylazine (80 and 10 mg/kg body weight, respectively), followed by additional doses of ketamine/xylazine (40 and 5 mg/kg body weight) as needed. Body temperature was maintained at 37°C with a homeothermic blanket. For the measurement of meningeal blood flow, the skull was fixed in a stereotaxic frame and a cranial window prepared with the dura left intact.18 Dural blood flow was measured with a laser Doppler flowmeter (TSI, MN). A needle-type probe was placed over a large branch of the middle meningeal artery (MMA), distant from visible cortical blood vessels and the cranial window kept moist with synthetic interstitial solution (SIF) consisting of 135 mM NaCl, 5 mM KCl, 5 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 10 mM D-glucose (pH 7.3). Blood flow was sampled at 1 Hz with a Digidata 1320 interface using Axoscope software (Axon Instruments, CA).
Blood flow drug administration
To stimulate the nasal mucosa, 25 µl of the TRPV1 agonist, capsaicin, or vehicle solution was applied over a 30-s period at a site 2 mm into the right nostril using a Pipetman pipette.18,27 Stock solutions of the TRPV1 agonist, capsaicin (10 mM; Sigma), were dissolved in ethanol and stored at −20°C and then diluted to the desired concentration with SIF prior to use. Submaximal concentrations of capsaicin (30 nM) were used for testing the effects of the glutamate reuptake blocker where potentiation might be expected, whereas higher capsaicin doses were used when antagonists were being tested. A 30-min stabilization period preceded all blood flow measurements. Each animal in blood flow experiments was given nasal saline as a vehicle control 15 min before administering capsaicin. Saline produced less than 2% change in blood flow on average consistent with our previous published results (data not shown).8,18
Laser dissection microscopy
Brains were extracted after euthanasia and the underlying right TG removed, snap frozen on dry ice and kept at −80°C until use. In animals which received hindpaw injections of DiI, ipsilateral L4-S1 dorsal root ganglia were also harvested. Ganglia were subsequently cut using a microtome at 12 µm sections and collected on Lecia PPS membrane slides (Cat # 11505273). Laser microdissection was performed using a Leica AS LMD7000 system. Fluorescent cells were dissected using a 20× objective and collected into the cap of a 500 µL microcentrifuge vial containing 50 µL TRIzol (Life Technologies, Foster City, CA). A total of 75 DiI or Fluorogold labeled cells per sample were cut and collected respectively from each animal. The samples were gently spun and transferred to a 1.5 ml vial containing 950 µL of TRIzol and stored at −80°C.
RNA isolation and quantitative RT-PCR
RNA from homogenized TG tissue lysate (25–30 mg) was isolated and purified using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Genomic DNA was removed from isolated RNA with TURBO DNAse (Life Technologies) and yield and purity were determined on a Nanodrop ND-1000 Spectrophotometer (Thermoscientific, Franklin, MA). A260/A280 ratios were between 2.0 and 2.2 for all samples. For laser dissection samples, 1 µL of glycogen (Roche) was added and mixed well before cells were sheared with 10 passes through a 21-gauge needle to shear genomic DNA. Total mRNA was extracted according to the supplier’s manual. Single-stranded cDNA was synthesized from 1 µg mRNA using reverse transcriptase (Superscript II reverse transcriptase, Life Technologies) and Oligo(dT)12–18 primers (Life Technologies).
Quantitative PCR (qPCR) reactions were run in triplicate on an ABI PRISM 7900HT Sequence Detection System (Life Technologies). The cDNA was amplified for quantitative RT-PCR with SYBR Green PCR Master mix (Life Technologies) and gene specific primers as listed here and in Supplemental Table 1. The primers for amplification of rat TRPV1 (Trpv1, Ref NM_031982.1) message were as follows: TRPV1 forward (5′-AGG ACC CAG GCA ACT GTG-3′, TM = 58°C) and TRPV1 reverse (5′-ATC CCT CAG AAG GGG AAC C-3′, TM = 56°C). These primers span exons 15 and 16, align with nucleotides 2456–2474 and 2362–2379 and produce a 113 bp product. The primers for amplification of rat TRPA1 (Trpa1, Ref NM_207608.1) were as follows: TRPA1 forward (5′-GCC CCT GTC TCT GTA AAT AAC C-3′, TM = 55°C) and TRPA1 reverse (5′-CTT GTG TCG CTG ATG TCT TG-3′, TM = 54°C). These primers span exons 11 and 12, align with nucleotides 1276–1297 and 1402–1421 and yield a 146 bp product. The primers for β-actin (Actb, Ref NM_031144.2) were as follows: β-Actin forward (5′-CAC TTT CTA CAA TGA GCT GCG-3′, TM = 54°C) and β-actin reverse (5′-CTG GAT GGC TAC GTA CAT GG-3′, TM = 55°C). The primers span exons 4 and 5, align with nucleotides 345–365 and 473–492 and yield a 148 bp product. A mixture of cDNA template, SYBR Green Master mix and forward and reverse primers was treated with uracil N-glycosylase (Life Technologies) before undergoing the following protocol: 50°C for 2 min, 95°C for 10 min, then 45 cycles of 95°C for 15 s, 60°C for 1 min, followed by one cycle of 95°C for 15 s, 60°C for 15 s, and 95°C for 15 s. The PCR products were analyzed with ABI PRISM sequence detection software. The specificity of these amplifications was verified by melt curve analysis with detection of only a single peak. Reactions containing no reverse transcriptase or no template were run as negative controls.
Real-time qPCR data were analyzed with the ΔΔCT method as described by Livak and Schmittgen.28 Transcript levels were compared in whole TG or nasal or dural cell populations of room air (control) and acrolein exposed animals, values normalized to β-actin and calibrated to control data using the ΔΔCT method. β-actin was used as a reference gene, and its level was not altered across these experimental conditions. The quantification cycle (Cq) was defined as the number of cycles required to attain a fluorescence threshold of 0.2 units.
Data collection and statistics
For blood flow experiments, data were collected at 1 Hz. Basal blood flow was determined as the mean flow rate measured during a 4-min period prior to drug application and the effects of test compounds were calculated by comparing the peak response after drug or saline administration to the basal blood flow. Changes in blood flow were calculated relative to the basal blood flow for each animal, averaged within treatment groups, and expressed as percent changes. Comparison of blood flow changes was performed using a two-tailed Student’s t test with Welch’s correction for unequal variances. qPCR results were calculated with the ΔΔCT method as described and presented as relative expression levels.28 Data presentation and statistical analyses were performed using GraphPad Prism software (GraphPad, CA). Averaged data values are presented as means ± SEM. The significance level for all tests was set at p < 0.05.
Results
Intraganglionic administration of neurotransmitter modulators alters meningeal blood flow responses to nasal irritant
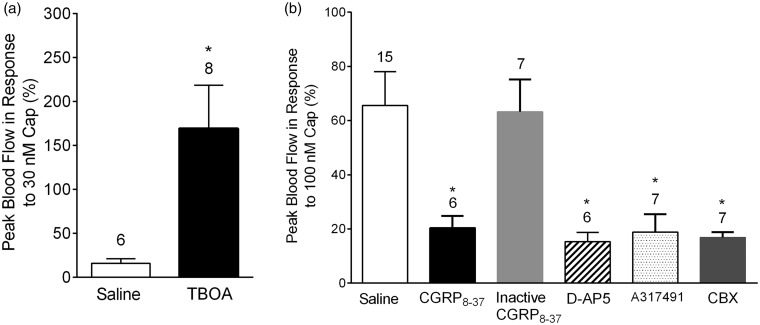
Neuronal somata within sensory ganglia can communicate with and modulate the activity of neighboring cells via the local release of chemical mediators.2,3,6,7 Evidence from in vitro and in vivo studies suggest that glutamate,11 CGRP,14 and ATP13 may act as neurotransmitters within sensory ganglia. Gap junctions and satellite glia may also have important roles in chronic trigeminal pain.15,17 To test whether these mediators contribute to irritant-induced trigeminovascular responses, we injected neurotransmitter modulators into the TG via the infraorbital foramen. The effect of local administration of modulators was assessed by measuring meningeal blood flow changes after nasal administration of the TRPV1 agonist, capsaicin (Figure 1). Injection of the glutamate reuptake inhibitor TBOA11 (1 mM) significantly potentiated peak blood flow response to 30 nM capsaicin (Figure 1(a)) compared to saline only injection (169 ± 49% (n = 6) vs. 16 ± 5% (n = 8), p = 0.0207). Furthermore, injection of the N-Methyl-D-aspartate (NMDA) receptor antagonist D-APV (10 mM) significantly reduced the peak blood flow in response to nasal administration of 100 nM capsaicin compared to saline only injection (Figure 1(b), 15 ± 3% (n = 6) vs. 65 ± 12% (n = 15), p = 0.0013). Together, these observations are consistent with a role for glutamate as an important neurotransmitter in the TG.
Figure 1.
Effects of neurotransmitter modulators on meningeal blood flow changes after trigeminal ganglion injection. (a) Ganglionic injection of TBOA, a glutamate re-uptake inhibitor, potentiates meningeal blood flow response to nasal administration of 30 nM capsaicin. (b) Effects of CGRP, NMDA, and P2X3 receptor antagonists and a gap junction inhibitor on meningeal blood flow response to nasal administration of 100 nM capsaicin. Injection of CGRP8-37, D-AP5, A317491, or the gap junction inhibitor carbenoxolone, significantly attenuated peak blood flow in response to 100 nM capsaicin compared to saline injection. In contrast, inactive CGRP8-37 had no effect on blood flow response. Values are means ± SEM. Number of animals per group is indicated. *p < 0.05 compared to blood flow change in saline-injected animals.
CGRP has a widely recognized role in migraine pain pathways and has been a recent focus for new migraine therapeutics.29 The CGRP antagonist, CGRP8-37, significantly decreased meningeal blood flow in response to capsaicin compared to saline injections (19 ± 6% (n = 7), p = 0.0036) after local administration, whereas injection of a chemically inactivated form of CGRP8-37 did not alter blood flow responses to capsaicin compared to saline injections (63 ± 12% (n = 7), p = 0.5469). ATP antagonists had similar inhibitory effects on blood flow responses compared to saline. Intraganglionic injection of the purinergic P2X3 receptor antagonist, A-317491 (60 mM), significantly reduced peak blood flow in response to 100 nm capsaicin (20 ± 4% (n = 6), p = 0.0033, compared to saline injections). Lastly, we examined the effect of gap junction blockade. Carbenoxolone16 (100 µM) injected into the TG significantly reduced peak blood flow response in response to capsaicin (17 ± 2% (n = 7), p = 0.016). Taken together, these results (Figure 1(b)) support the role of cross-talk in the TG, specifically the influence of the neurotransmitters glutamate, ATP, and CGRP and of gap junctions.
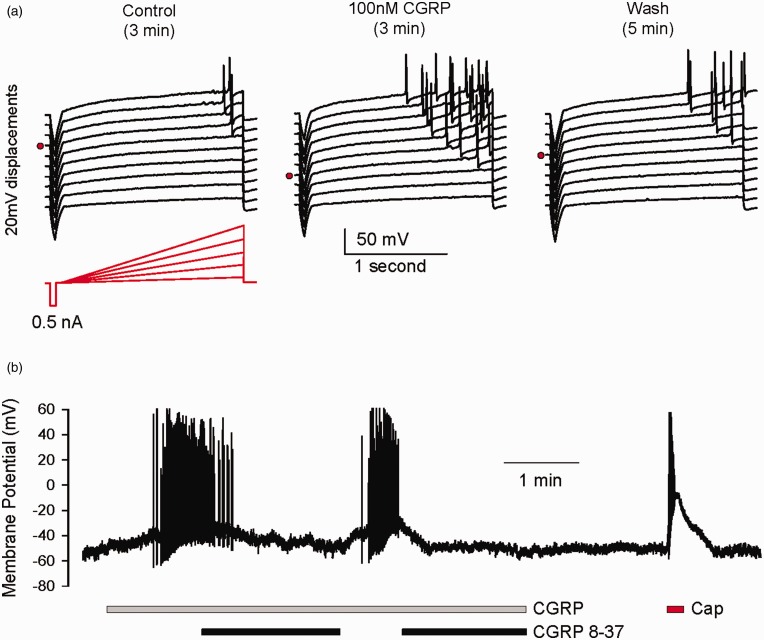
CGRP enhances the excitability of sensory neuron soma
In the case of glutamate and ATP, it is well established that sensory neuron soma express glutamate receptors and P2X receptors and that they are excitatory.11,30,31 However, it is less clear what responses, if any, CGRP, a well-documented vasodilatory peptide, would elicit from sensory neuron soma upon release. We have used whole-cell recording methods to investigate what membrane potential responses might be elicited by application of CGRP to isolated sensory neuron soma. We observed CGRP to be excitatory either through lowering the threshold for eliciting action potentials (Figure 2(a)) or eliciting spontaneous firing that was reversed by the receptor antagonist CGRP8-37 (Figure 2(b)). We currently do not know the mechanism for the increased excitability, but one group has reported a transient increase in tetrodotoxin (TTX)-resistant sodium currents in DRG neurons by CGRP.32 Nonetheless, this excitatory response is consistent with a potential sensitizing/activating role for CGRP within sensory ganglia.
Figure 2.
CGRP enhances the excitability of sensory neuron soma. (a) Voltage responses of a dissociated DRG neuron to ramp currents under control conditions, 3 min in 100 nM CGRP, and following 5 min of washout. Traces are vertically displaced by 20 mV for visual clarity. Note the ramp threshold (red dot) for each set of traces. A 0.5 nA step current preceded each ramp to monitor membrane resistance. (b) Firing of a neuron is induced by 300 nM CGRP (gray bar) which is suppressed by the CGRP receptor antagonist, CGRP8-37 (black bars). This neuron, as most observed, was capsaicin sensitive (red bar).
Laser capture dissection and qPCR of distinct nasal or meningeal projecting neuron populations in the TG
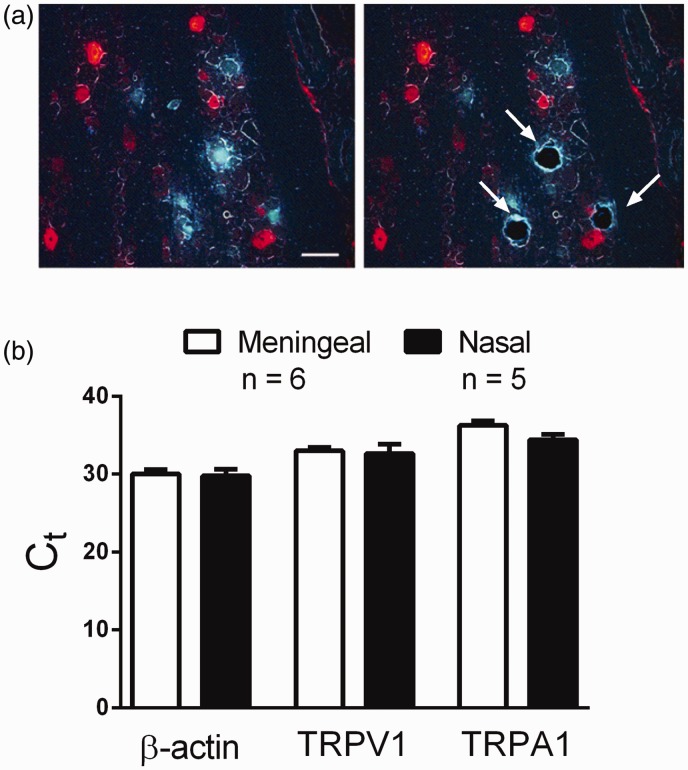
In our previous studies, we demonstrated that acrolein exposure sensitizes the trigeminovascular response to subsequent nasal administration of TRPV1 or TRPA1 agonists,19,20 but the mechanism of this response is not known. Immunocytochemistry revealed no differences in cell numbers expressing either TRPV1 receptor or CGRP in the whole TG, nor were differences detected in mRNA levels of TRPV1 or TRPA1 receptors after acrolein exposure.20 To identify mechanisms underlying the sensitization observed after acrolein exposure, we conducted additional qPCR studies of known signaling molecules in migraine pathways. Interestingly, we observed no differences in mRNA levels of CGRP, its receptor subunit components RAMP1 and CLR, the glutamate receptor GluN1, the purinergic receptor P2X3, and the gap junction hemichannel Cx43 in whole TG following acrolein or room air exposure (Supplemental Figure 1). Since the relevant nasal-meningeal pathway comprises only a small subset of trigeminal neurons, it might be that changes occurred in one or both of these subsets and could not be detected in whole TG owing to a poor signal/noise ratio. Thus, we used two retrograde tracers combined with laser capture dissection to isolate neurons specific to either nasal (FG) or meningeal sensory afferents (DiI) (Figure 3). We found the distribution of retrograde label in the TG from the nasal epithelium and dura similar to our previous findings8 with FG and DiI labeled neurons prominent in the V1 and V2 subdivisions of the ganglia. Furthermore, this labeling is consistent with other studies wherein nasal innervation of the TG33,34 or cerebral vessels9,35 was traced. Examples of TG sections before and after laser dissection to capture FG labeled cells are depicted in Figure 3(a). After laser capture of these distinct identified neurons, we compared message levels in the two populations. Under control conditions β-actin, TRPA1, or TRPV1 mRNA levels did not differ between nasal and meningeal projecting neurons (Figure 3(b)). CT values were 30.0 ± 0.6 versus 29.8 ± 0.9 for β-actin, 33.0 ± 0.4 versus 32.6 ± 0.6 for TRPV1, and 36.3 ± 0.6 versus 34.4 ± 0.7 for TRPA1 in meningeal (n = 6 rats) and nasal (n = 5 rats) cells, respectively.
Figure 3.
Laser dissection and mRNA expression levels following retrograde labeling in the trigeminal ganglion in the absence of acrolein exposure. (a) Representative image of TG section following retrograde labeling of nasal epithelium (Fluorogold; blue) and middle meningeal artery (DiI; red) before (left) and after (right) laser dissection capture. Labeled cells in the ganglion were dissected and processed separately before qPCR. (b) CT levels of β-actin, TRPA1 and TRPV1 mRNA were compared in meningeal and nasal cell populations and did not differ in the absence of acrolein. Values are means ± SEM. Number of animals per group is indicated. Scale bar 50 um.
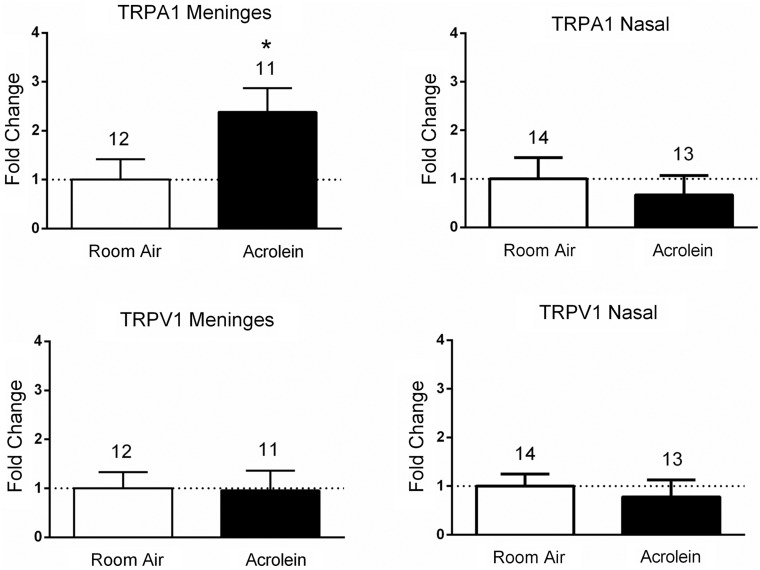
TRPA1 mRNA levels differ in nasal- and meningeal-projecting neuron populations after acrolein exposure
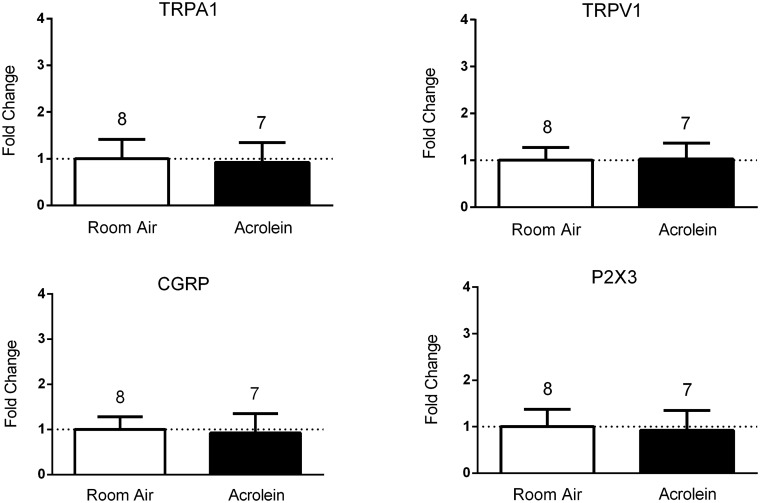
We then compared message levels in dissected cell samples from both nasal and meningeal labeled populations following inhalation pre-exposure of animals to either acrolein or room air (Figure 4). Relative expression levels of TRPV1 mRNA were not different when comparing acrolein-exposed animals to room air-exposed animals in either the meningeal population (0.95 ± 1.4 (n = 11) vs. 1.0 ± 1.15 (n = 12)) or the nasal labeled population (0.78 ± 1.26 (n = 13) vs. 1.0 ± 0.93 (n = 14)). In contrast, TRPA1 mRNA levels were significantly increased in the meningeal cell population of acrolein-exposed animals compared to room air-exposed animals (2.38 ± 1.61 (n = 11) vs. 1.0 ± 1.45 (n = 12), p < 0.05), whereas no differences were observed in the nasal cell population (0.67 ± 1.45, (n = 13) vs. 1.0 ± 1.64 (n = 14)). No change in expression of CGRP or P2X3 mRNA was observed in either nasal or meningeal labeled afferent samples following acrolein exposure (Supplemental Figure 2). The significant increase (greater than two-fold) in TRPA1 mRNA observed in the meningeal cell population may contribute to the mechanism for the sensitized response of the trigeminovascular pathway following acrolein exposure. While we cannot exclude possible contributions of mRNA from adjacent non-neuronal tissue obtained in the laser dissection, little evidence suggests that TRP channels are expressed in satellite glia surrounding the neuron soma.
Figure 4.
Fold changes in expression levels of TRPV1 and TRPA1 mRNA in meningeal and nasal afferent populations of trigeminal ganglia neurons following acrolein exposure. Acrolein exposure induced a significant increase in expression of TRPA1 mRNA in the meningeal retrograde-labeled neurons compared with room air-exposed animals. No change in expression of TRPA1 mRNA was observed in nasal epithelium labeled trigeminal neurons. Likewise, no change in TRPV1 mRNA was observed in either meningeal or nasal afferent samples following acrolein exposure compared with room air-exposed animals. Each sample’s value is normalized to β-actin values using the ΔΔCT method and averaged across groups. Values are represented as mean ± SEM. Number of animals per group are indicated. *p < 0.05 compared to mRNA expression change in room air-exposed animals.
Laser capture dissection and qPCR of dorsal root ganglia neurons
To determine if the increased expression in TRPA1 mRNA is specific to sensory neurons in TG, we also retrogradely labeled and dissected sensory neurons from L4-S1 DRG following acrolein- or room air exposure. Compared to room air-exposed animals, no differences in relative expression levels were detected in acrolein-exposed animals (Figure 5) in mRNA message levels of TRPA1 (0.92 ± 1.13 (n = 7) vs. 1.0 ± 1.18 (n = 8)), TRPV1 (1.03 ± 0.89 (n = 7) vs. 1.0 ± 0.78 (n = 8)), CGRP (0.92 ± 1.14 (n = 7) vs. 1.0 ± 0.80 (n = 8)), or P2X3 (0.92 ± 1.14 (n = 7) vs. 1.0 ± 1.06 (n = 8)). As no changes in mRNA were detected in DRG neurons, we conclude that the expression change of TRPA1 mRNA in sensory neurons projecting to the meninges in the TG is specific to that sensory pathway.
Figure 5.
Fold changes in expression of TRPA1, TRPV1, CGRP, and P2X3 in retrograde-labeled dorsal root ganglion neurons following acrolein exposure. Expression levels of mRNA do not change in sensory neurons of the dorsal root ganglion following acrolein exposure compared with room air-exposed animals. Each sample’s value is normalized to β-actin values using the ΔΔCT method and averaged across groups. Values are represented as mean ± SEM. Number of animals per group are indicated.
Discussion
Migraine is unique among primary headache syndromes due to its association with odors and poor air quality.36 Although odorants and environmental irritants are recognized as specific triggers for migraine, little is known about the etiology of this disorder. In addition, headache is cited as one of the most common symptoms of the acquired disorder, multiple chemical sensitivity, usually provoked by chemical exposure. We previously reported that TRPA1 agonists and environmental irritants such as acrolein and formaldehyde acutely activate the trigeminovascular system after nasal administration.8,18 More importantly, subacute exposure to the environmental irritant, acrolein, induces long-lasting sensitization of the migraine pain pathway.19,20 These studies were undertaken to explore the underlying mechanism by which the environmental irritant, acrolein, activates, and sensitizes the trigeminovascular system.
The trigeminovascular system is comprised of the trigeminal sensory neurons which reside in the ganglion and innervate the head, face, cerebral blood vessels, and dura. Nociceptive information arising from cerebral blood vessels during migraine is referred to the forehead. Surprisingly, the mechanism of referred pain in migraine is likely not due to a classic axon reflex as neurons innervating the MCA do not project divergent collaterals to the forehead.9,37 Instead the cells innervating the forehead “clump” around individual cells innervating the MCA. This is comparable to our finding that trigeminal cells innervating the nasal epithelium do not project divergent collaterals with cells innervating the dura but reside in close proximity within the ganglia.8 Thus, the question arises—how does nasal administration evoke changes in meningeal blood flow? We hypothesize that intraganglionic transmission mediates this activation, and we utilized a local injection of the trigeminal ganglion26 to block or modulate neurotransmission in the ganglion while minimizing damage to brain structures involved in nociception. The neurotransmitters, CGRP, glutamate, and ATP have each been implicated in cell–cell communication in sensory ganglion. While previous studies have demonstrated the expression of excitatory glutamate receptors11,30,31 and ATP receptors38 in sensory neurons, the role of CGRP has not been as well-documented. We and others have verified that sensory neurons express the CGRP coreceptors CLR and RAMP1 (data not shown).39 Our data (Figure 2) reveal that CGRP has an excitatory effect on sensory neuron soma. The mechanism responsible for the excitation is not clear but one group has reported a transient increase in TTX-resistant sodium currents in DRG neurons by CGRP.32 Our data (Figure 1) demonstrate that injection of a CGRP antagonist into the trigeminal ganglion attenuates blood flow changes providing further evidence that CGRP may be important in intraganglionic transmission. Our data are consistent with the finding of Ulrich-Lai et al.14 who reported that the TRPV1 agonist, capsaicin, evokes release of CGRP from trigeminal ganglion slices presumably from the cell soma. Similarly, our data (Figure 1) demonstrate that blocking reuptake of glutamate potentiates meningeal blood flow changes, while blocking NMDA receptors inhibits blood flow changes. This is consistent with the study of Laursen et al.11 who reported glutamate release from satellite glia cells cultured from the trigeminal ganglion and that glutamate injection into trigeminal ganglion increased electrical activity which was potentiated by TBOA and blocked by APV. ATP is also a candidate for signaling within sensory ganglion. Our data (Figure 1) indicate that purinergic P2X3 receptor antagonist attenuates trigeminovascular activation after injection in trigeminal ganglion. This concurs with the reports of Zhang et al.40 and Rozanski et al.13 which point to an important role for ATP in cell–cell transmission. Several groups also implicate satellite glia in an unusual form of cell–cell cross-talk.13,40 Evidence has substantiated the putative role of satellite glia17 and in particular gap junctions in craniofacial pain.16 We tested the role of gap junctions in acute nasal-meningeal signaling. Carbenoxolone, a gap junction blocker significantly reduced the change in meningeal blood flow seen after nasal administration of capsaicin. Likewise, blockade of gap junctions has attenuated pain signaling in other models of pain.4,16 Overall these data corroborate the theory that intraganglionic transmission is important in the trigeminovascular system.
To gain a better understanding of the mechanism of sensitization after acrolein exposure, we examined mRNA levels of candidate signaling molecules in the whole trigeminal ganglion20 and Supplemental Figure 1 in the absence and presence of acrolein exposure. Since we observed no differences in whole ganglion mRNA levels after acrolein, we isolated meningeal and nasal cell populations to quantify important trigeminal signaling molecules at a pathway-specific level. Our report demonstrates the presence of several important signaling molecules including TRPA1, TRPV1, CGRP, and ATP receptors in both meningeal and nasal cell populations (Figures 4 and 5 and Supplemental Material). Nasal trigeminal neurons have previously been shown to express TRPV1 in mice38,41 and guinea pigs,42 ATP receptors in rodents,38 and CGRP in rodents.34,43 Although TRPA1 has been described in nasal epithelium44,45 including mast cells and macrophages,46 to our knowledge, this is the first report of TRPA1 mRNA in trigeminal innervation of nasal mucosa. Trigeminal neurons innervating the cerebral blood vessels express TRPA1,35,47 TRPV1,35 CGRP,10,35 and glutamate receptor mRNA.48 We compared mRNA levels of these signaling molecules in selected cell populations in the absence or presence of acrolein exposure and observed a significant and specific increase in TRPA1 mRNA in trigeminal neurons innervating the meninges. This change was not observed in neurons innervating the nasal epithelium or in dorsal root ganglia innervating the paw. While we have not quantified changes at the TRPA1 protein level in the selected cell populations, this observation points to a selective mechanism mediating sensitivity changes in the headache circuit. It has been previously suggested that TRPA1 and TRPV1 contribute to the excitability of dura afferents35 and thus may have a role in migraine susceptibility. The upstream mediators which effect changes in TRPA1 mRNA levels are not well-understood, but TRPA1 is upregulated under inflammatory conditions49–51 or by known migraine triggers such as nitroglycerin.52 Other effectors may include endogenous lipids, the most well-known of which is anandamide, a TRPV1 and CB(1) receptor agonist53 and other lipids recently identified as TRP agonists.54 In addition to inducing trigeminal sensitization and chronic migraine phenotypes in our animal model,19,20 acrolein exposure induced changes in several endocannabinoids, including anandamide, in trigeminal tissue.55 Indeed, interactions between endocannabinoids and TRP channels are strongly implicated in pain53 and particularly migraine.56 While additional studies are necessary to understand the regulation of TRPA1 expression and putative roles of endogenous lipids, both should be considered as viable targets for pain relief in migraine.
Taken together, these data implicate intraganglionic transmission in acute signaling in the trigeminovascular pathway via signaling molecules known to be important in migraine. Herein, we also report the upregulation of TRPA1 message in this pathway after environmental irritant exposure, a putative mechanism of sensitization in chronic migraine.
Supplemental Material
Supplemental Material for Role of intraganglionic transmission in the trigeminovascular pathway by LuJuan Zhang, Phillip Edward Kunkler, Kelly L Knopp, Gerry Stephen Oxford and Joyce Harts Hurley in Molecular Pain
Acknowledgments
The authors would like to thank Dr. Richard P. Kraig for use of the laser Doppler flowmeter and Dr. Kathryn Jones for the use of the laser dissection microscope.
Author Contributions
JHH and GSO were responsible for the study design. LZ conducted laser Doppler flowmetry, retrograde labeling surgery, and RT-qPCR experiments. PEK conducted laser dissection experiments, collected data, and drafted the manuscript. KLK and GSO performed the electrophysiology experiments on dorsal root ganglion neurons. JHH performed data analysis. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from the NIEHS (ES017430) to JHH and GSO.
References
- 1.Nassini R, Materazzi S, Vriens J, Prenen J, Benemei S, De Siena G, la Marca G, Andrè E, Preti D, Avonto C, Sadofsky L, Di Marzo V, De Petrocellis L, Dussor G, Porreca F, Taglialatela-Scafati O, Appendino G, Nilius B, Geppetti P. The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain 2012; 135: 376–390. [DOI] [PubMed] [Google Scholar]
- 2.Amir R, Devor M. Chemically mediated cross-excitation in rat dorsal root ganglia. J Neurosci 1996; 16: 4733–4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devor M, Wall PD. Cross-excitation in dorsal root ganglia of nerve-injured and intact rats. J Neurophysiol 1990; 64: 1733–1746. [DOI] [PubMed] [Google Scholar]
- 4.Kim YS, Anderson M, Park K, Zheng Q, Agarwal A, Gong C, Young L, He S, LaVinka PC, Zhou F, Bergles D. Coupled activation of primary sensory neurons contributes to chronic pain. Neuron 2016; 91: 1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh EJ, Weinreich D. Chemical communication between vagal afferent somata in nodose Ganglia of the rat and the Guinea pig in vitro. J Neurophysiol 2002; 87: 2801–2807. [DOI] [PubMed] [Google Scholar]
- 6.Shinder V, Amir R, Devor M. Cross-excitation in dorsal root ganglia does not depend on close cell-to-cell apposition. Neuroreport 1998; 9: 3997–4000. [DOI] [PubMed] [Google Scholar]
- 7.Utzschneider D, Kocsis J, Devor M. Mutual excitation among dorsal root ganglion neurons in the rat. Neurosci Lett 1992; 146: 53–56. [DOI] [PubMed] [Google Scholar]
- 8.Kunkler PE, Ballard CJ, Pellman JJ, Zhang L, Oxford GS, Hurley JH. Intraganglionic signaling as a novel nasal-meningeal pathway for TRPA1-dependent trigeminovascular activation by inhaled environmental irritants. PLoS One 2014; 9: e103086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connor TP, van der Kooy D. Pattern of intracranial and extracranial projections of trigeminal ganglion cells. J Neurosci 1986; 6: 2200–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connor TP, van der Kooy D. Enrichment of a vasoactive neuropeptide (calcitonin gene related peptide) in the trigeminal sensory projection to the intracranial arteries. J Neurosci 1988; 8: 2468–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laursen JC, Cairns BE, Dong XD, Kumar U, Somvanshi RK, Arendt-Nielsen L, Gazerani P. Glutamate dysregulation in the trigeminal ganglion: a novel mechanism for peripheral sensitization of the craniofacial region. Neuroscience 2014; 256: 23–35. [DOI] [PubMed] [Google Scholar]
- 12.Goto T, Iwai H, Kuramoto E, Yamanaka A. Neuropeptides and ATP signaling in the trigeminal ganglion. Jpn Dent Sci Rev 2017; 53: 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rozanski GM, Kim H, Li Q, Wong FK, Stanley EF. Slow chemical transmission between dorsal root ganglion neuron somata. Eur J Neurosci 2012; 36: 3314–3321. [DOI] [PubMed] [Google Scholar]
- 14.Ulrich-Lai YM, Flores CM, Harding-Rose CA, Goodis HE, Hargreaves KM. Capsaicin-evoked release of immunoreactive calcitonin gene-related peptide from rat trigeminal ganglion: evidence for intraganglionic neurotransmission. Pain 2001; 91: 219–226. [DOI] [PubMed] [Google Scholar]
- 15.Sarrouilhe D, Dejean C, Mesnil M. Involvement of gap junction channels in the pathophysiology of migraine with aura. Front Physiol 2014; 5: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Cao Y, Chiang CY, Dostrovsky JO, Sessle BJ. The gap junction blocker carbenoxolone attenuates nociceptive behavior and medullary dorsal horn central sensitization induced by partial infraorbital nerve transection in rats. Pain 2014; 155: 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vit JP, Jasmin L, Bhargava A, Ohara PT. Satellite glial cells in the trigeminal ganglion as a determinant of orofacial neuropathic pain. Neuron Glia Biol 2006; 2: 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunkler PE, Ballard CJ, Oxford GS, Hurley JH. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain 2011; 152: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunkler PE, Zhang L, Johnson PL, Oxford GS, Hurley JH. Induction of chronic migraine phenotypes in a rat model after environmental irritant exposure. Pain 2018; 159: 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunkler PE, Zhang L, Pellman JJ, Oxford GS, Hurley JH. Sensitization of the trigeminovascular system following environmental irritant exposure. Cephalalgia 2015; 35: 1192–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 22.Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci 1997; 17: 3525–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassee FR, Groten JP, Feron VJ. Changes in the nasal epithelium of rats exposed by inhalation to mixtures of formaldehyde, acetaldehyde, and acrolein. Toxicol Sci 1996; 29: 208–218. [DOI] [PubMed] [Google Scholar]
- 24.Dorman DC, Struve MF, Wong BA, Marshall MW, Gross EA, Willson GA. Respiratory tract responses in male rats following subchronic acrolein inhalation. Inhal Toxicol 2008; 20: 205–216. [DOI] [PubMed] [Google Scholar]
- 25.Lyon JP, Jenkins LJ, Jr, Jones RA, Coon RA, Siegel J. Repeated and continuous exposure of laboratory animals to acrolein. Toxicol Appl Pharmacol 1970; 17: 726–732. [DOI] [PubMed] [Google Scholar]
- 26.Neubert JK, Mannes AJ, Keller J, Wexel M, Iadarola MJ, Caudle RM. Peripheral targeting of the trigeminal ganglion via the infraorbital foramen as a therapeutic strategy. Brain Res Brain Res Protoc 2005; 15: 119–126. [DOI] [PubMed] [Google Scholar]
- 27.Gottselig R, Messlinger K. Noxious chemical stimulation of rat facial mucosa increases intracranial blood flow through a trigemino-parasympathetic reflex–an experimental model for vascular dysfunctions in cluster headache. Cephalalgia 2004; 24: 206–214. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 29.Schuster NM, Rapoport AM. Calcitonin gene-related peptide-targeted therapies for migraine and cluster headache: a review. Clin Neuropharmacol 2017; 40: 169–174. [DOI] [PubMed] [Google Scholar]
- 30.Kung LH, Gong K, Adedoyin M, Ng J, Bhargava A, Ohara PT, Jasmin L. Evidence for glutamate as a neuroglial transmitter within sensory ganglia. PLoS One 2013; 8: e68312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahara Y, Noro N, Iida Y, Soma K, Nakamura Y. Glutamate receptor subunits GluR5 and KA-2 are coexpressed in rat trigeminal ganglion neurons. J Neurosci 1997; 17: 6611–6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Natura G, von Banchet GS, Schaible HG. Calcitonin gene-related peptide enhances TTX-resistant sodium currents in cultured dorsal root ganglion neurons from adult rats. Pain 2005; 116: 194–204. [DOI] [PubMed] [Google Scholar]
- 33.Anton F, Peppel P. Central projections of trigeminal primary afferents innervating the nasal mucosa: a horseradish peroxidase study in the rat. Neuroscience 1991; 41: 617–628. [DOI] [PubMed] [Google Scholar]
- 34.Hunter DD, Dey RD. Identification and neuropeptide content of trigeminal neurons innervating the rat nasal epithelium. Neuroscience 1998; 83: 591–599. [DOI] [PubMed] [Google Scholar]
- 35.Huang D, Li S, Dhaka A, Story GM, Cao YQ. Expression of the transient receptor potential channels TRPV1, TRPA1 and TRPM8 in mouse trigeminal primary afferent neurons innervating the dura. Mol Pain 2012; 8: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva-Neto RP, Rodrigues AB, Cavalcante DC, Ferreira PH, Nasi EP, Sousa KM, Peres MF, Valenca MM. May headache triggered by odors be regarded as a differentiating factor between migraine and other primary headaches? Cephalalgia 2017; 37: 20–28. [DOI] [PubMed] [Google Scholar]
- 37.McMahon MS, Norregaard TV, Beyerl BD, Borges LF, Moskowitz MA. Trigeminal afferents to cerebral arteries and forehead are not divergent axon collaterals in cat. Neurosci Lett 1985; 60: 63–68. [DOI] [PubMed] [Google Scholar]
- 38.Damann N, Rothermel M, Klupp BG, Mettenleiter TC, Hatt H, Wetzel CH. Chemosensory properties of murine nasal and cutaneous trigeminal neurons identified by viral tracing. BMC Neurosci 2006; 7: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cottrell GS, Roosterman D, Marvizon J-C, Song B, Wick E, Pikios S, Wong H, Berthelier C, Tang Y, Sternini C, Bunnett NW, Grady EF. Localization of calcitonin receptor-like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia, and the spinal cord of the rat. J Comp Neurol 2005; 490: 239–255. doi:10.1002/cne.20669 [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci U S A 2007; 104: 9864–9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dinh QT, Groneberg DA, Mingomataj E, Peiser C, Heppt W, Dinh S, Arck PC, Klapp BF, Fischer A. Expression of substance P and vanilloid receptor (VR1) in trigeminal sensory neurons projecting to the mouse nasal mucosa. Neuropeptides 2003; 37: 245–250. [DOI] [PubMed] [Google Scholar]
- 42.Taylor-Clark TE, Kollarik M, MacGlashan DW, Jr, Undem BJ. Nasal sensory nerve populations responding to histamine and capsaicin. J Allergy Clin Immunol 2005; 116: 1282–1288. [DOI] [PubMed] [Google Scholar]
- 43.Finger TE, St Jeor VL, Kinnamon JC, Silver WL. Ultrastructure of substance P- and CGRP-immunoreactive nerve fibers in the nasal epithelium of rodents. J Comp Neurol 1990; 294: 293–305. [DOI] [PubMed] [Google Scholar]
- 44.Keh SM, Facer P, Yehia A, Sandhu G, Saleh HA, Anand P. The menthol and cold sensation receptor TRPM8 in normal human nasal mucosa and rhinitis. Rhinology 2011; 49: 453–457. [DOI] [PubMed] [Google Scholar]
- 45.Nakashimo Y, Takumida M, Fukuiri T, Anniko M, Hirakawa K. Expression of transient receptor potential channel vanilloid (TRPV) 1–4, melastin (TRPM) 5 and 8, and ankyrin (TRPA1) in the normal and methimazole-treated mouse olfactory epithelium. Acta Otolaryngol 2010; 130: 1278–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toth E, Tornoczky T, Kneif J, Perkecz A, Katona K, Piski Z, Kemeny A, Gerlinger I, Szolcsanyi J, Kun J, Pinter E. Upregulation of extraneuronal TRPV1 expression in chronic rhinosinusitis with nasal polyps. Rhinology 2018; 56: 245–254. [DOI] [PubMed] [Google Scholar]
- 47.Edelmayer RM, Le LN, Yan J, Wei X, Nassini R, Materazzi S, Preti D, Appendino G, Geppetti P, Dodick DW, Vanderah TW, Porreca F, Dussor G. Activation of TRPA1 on dural afferents: a potential mechanism of headache pain. Pain 2012; 153: 1949–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Brien M, Cairns BE. Monosodium glutamate alters the response properties of rat trigeminovascular neurons through activation of peripheral NMDA receptors. Neuroscience 2016; 334: 236–244. [DOI] [PubMed] [Google Scholar]
- 49.Chung MK, Park J, Asgar J, Ro JY. Transcriptome analysis of trigeminal ganglia following masseter muscle inflammation in rats. Mol Pain 2016; 12: 1744806916668526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diogenes A, Akopian AN, Hargreaves KM. NGF up-regulates TRPA1: implications for orofacial pain. J Dent Res 2007; 86: 550–555. [DOI] [PubMed] [Google Scholar]
- 51.Nassini R, Materazzi S, Benemei S, Geppetti P. The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev Physiol Biochem Pharmacol 2014; 167: 1–43. [DOI] [PubMed] [Google Scholar]
- 52.Demartini C, Tassorelli C, Zanaboni AM, Tonsi G, Francesconi O, Nativi C, Greco R. The role of the transient receptor potential ankyrin type-1 (TRPA1) channel in migraine pain: evaluation in an animal model. J Headache Pain 2017; 18: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Storozhuk MV, Zholos AV. TRP channels as novel targets for endogenous ligands: focus on endocannabinoids and nociceptive signalling. Curr Neuropharmacol 2018; 16: 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raboune S, Stuart JM, Leishman E, Takacs SM, Rhodes B, Basnet A, Jameyfield E, McHugh D, Widlanski T, Bradshaw HB. Novel endogenous N-acyl amides activate TRPV1-4 receptors, BV-2 microglia, and are regulated in brain in an acute model of inflammation. Front Cell Neurosci 2014; 8: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leishman E, Kunkler PE, Manchanda M, Sangani K, Stuart JM, Oxford GS, Hurley JH, Bradshaw HB. Environmental toxin acrolein alters levels of endogenous lipids, including TRP agonists: a potential mechanism for headache driven by TRPA1 activation. Neurobiol Pain 2018; 3: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greco R, Demartini C, Zanaboni AM, Piomelli D, Tassorelli C. Endocannabinoid system and migraine pain: an update. Front Neurosci 2018; 12: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Role of intraganglionic transmission in the trigeminovascular pathway by LuJuan Zhang, Phillip Edward Kunkler, Kelly L Knopp, Gerry Stephen Oxford and Joyce Harts Hurley in Molecular Pain