Abstract
Background:
With the introduction of newer anti-cancer agents, the adverse effects have become more rampant which call for concern in the treatment of patients with cancer. Hence, the assessment and management of dermatological adverse effects of anti-cancer therapy have become a significant part of the care of patients with cancer and require proper and close collaboration between the dermatologists and the oncologists.
Aims:
To assess the frequency and pattern of mucocutaneous adverse reactions to cancer chemotherapy and chemoradiation and grade them according to their severity and to identify hematological and biochemical changes related to cancer chemotherapy-induced mucocutaneous adverse reactions.
Materials and Methods:
This was a descriptive study done among 226 patients in an Indian tertiary care hospital, who presented with mucocutaneous adverse reactions to either chemotherapy alone or combination of chemotherapy and radiation to dermatology, medical oncology and radiotherapy outpatient departments. Detailed history and examination were undertaken. Visual analog score (VAS) was employed to quantify pain and pruritus. Correlation of various biochemical and hematological parameters with chemotherapy-induced adverse reactions was attempted and grading of adverse reactions was done based on the severity scale of Common Terminology Criteria for Adverse Events (CTCAE).
Results:
The common cutaneous adverse reactions observed in our study were nail changes (194 patients; 85.84%), followed by skin changes (191; 84.51%), hair changes (159, 70.35%), mucosal changes (34, 15.04%), and other miscellaneous manifestations. Grade 1 manifestations comprised of 49.91% of total manifestations followed by Grade 2 (45.45%) and Grade 3 (5.64%). In addition to bleomycin, other chemotherapeutic agents also had been shown to produce flagellate dermatitis in our study.
Conclusion:
Nail changes, skin changes, hair changes and mucosal changes occurred frequently as a significant side effect of chemotherapy, which a physician should be aware of, while selecting a chemotherapeutic drug.
KEY WORDS: Adverse cutaneous reactions, cancer, chemoradiation, chemotherapy
Introduction
In recent years, a tremendous breakthrough has been made towards the knowledge of specialty of cancer and its management. However, with its growing prevalence, cancer management progresses to be a challenge for the current era of clinical medicine. Different treatment options such as radiotherapy, chemotherapy, combination therapy, immunotherapy, and hormonal therapy are being used to treat cancer patients in the current practice.[1] Increasingly skeptical remedial regimens and modernized instruments have drastically revolutionized the functionality and mitigated, but not completely eliminated the adverse reactions they cause to the body. Thus, appraisal and management of mucocutaneous adverse reactions have become a vital part in the care of patients with cancer.[1] Early recognition and treatment of mucocutaneous adverse drug reactions facilitate early control and cure of the manifestations, decreasing morbidity and allowing further continuation and maintenance of cancer therapy. Hence, this study was conducted to assess the various mucocutaneous adverse reactions of cancer chemotherapy and chemoradiation and proper cataloging and grading of these adverse reactions for proper communication and better administration of supportive measures.
Materials and Methods
This was a descriptive study conducted at an Indian tertiary care hospital in south India on 226 patients who presented with mucocutaneous adverse reactions due to either chemotherapy or combination of chemotherapy and radiation in dermatology, medical oncology and radiotherapy outpatient departments during the period from July 2014 to June 2016. The institute's ethics committee clearance was obtained before undertaking the study. All patients on cancer chemotherapy (with or without radiotherapy) presenting with mucocutaneous adverse reactions after initiation of chemotherapy were screened and recruited after obtaining a written informed consent and those patients with graft versus host disease or any prior primary dermatoses were excluded from the study. All the patients were subjected to a detailed history and clinical examination comprising general, systemic and dermatological examinations. Mucocutaneous adverse reactions were described and recorded along with other clinical data. The parameters recorded were demographic details (age, gender, occupation, and smoker/alcoholic), diagnosis and duration of cancer, and treatment details such as drugs/regimen, dosage of drug, formulation, bolus/infusion, route of administration, frequency of administration, cycles of drug therapy, and duration of treatment. Pain and pruritus were scored using visual analog scale (VAS) of 0–10. To differentiate from infectious and other diseases, tests such as nail clipping for KOH, nail fungal culture, and nail plate histopathology were done as and when needed. Complete hemogram, renal function tests, and liver function tests were recorded for all cases. Skin biopsy was done as and when required as a part of regular management of cases of adverse reactions.
Statistical analysis was performed using IBM's Statistical Package for the Social Sciences (SPSS) version 22 software (Amonk, NY: IBM). Comparison of various parameters between the two groups of chemotherapy and chemoradiation was done using Chi-square test. The Common Terminology Criteria for Adverse Events (CTCAE) grading, pruritus score, and pain score in the two groups were compared using Mann–Whitney U-test.
Results
In our study of 226 patients with malignancy and skin rash, 126 (55.75%) were on chemotherapy alone and 100 (44.25%) were on a combination of radiotherapy and chemotherapy. The mean age of the study population was 44.76±16.04 years (range 3--85 years; median 46 years) with females outnumbering males with a sex ratio of 1.33:1. Carcinoma breast (64; 28.31%) was the most common malignancy recorded in our study followed by leukemia (42; 18.58), gastro-intestinal tract carcinoma (28; 12.39), carcinoma cervix (23; 10.18) and others. The mean duration of malignancy was 14.48±14.73 months (range 1--84 months; median 10 months). The mean duration of chemotherapeutic treatment was 6.60±10.25 months (range 1--84 months; median 4 months). Duration of malignancy was significantly high in patients on combination therapy than those on chemotherapy alone (P=0.013; Mann-Whitney U-test). There was no statistically significant difference in the duration of treatment between those on chemotherapy alone and those on combination therapy (P=0.272; Mann-Whitney U-test). Most of the patients were on adjuvant chemotherapy (122; 53.98%) followed by palliative therapy (38; 16.81%), induction therapy (31; 13.72%), maintenance therapy (25; 11.06%), and neoadjuvant therapy (10; 4.42%).
Mucocutaneous adverse reactions
The most common mucocutaneous adverse drug reaction noted were nail changes (194; 85.84%), followed by skin changes (191; 84.51%), hair changes (159; 70.35%), mucosal changes (34; 15.04%), and other miscellaneous manifestations.
Cutaneous changes of cancer chemotherapy
Of the 226 patients included in our study, 191 (84.51%) patients had at least one cutaneous adverse drug reaction. The most common cutaneous adverse drug reaction observed was skin pigmentation (105; 46.46%), followed by pruritus (101; 44.69%), acral erythema/palmar-plantar erythrodysesthesia (PPE) (62; 27.43%), xerosis (46; 20.35%), and others as provided in Table 1.
Table 1.
Skin related changes of cancer chemotherapy
| Skin change | Patients only on chemotherapy (126) n (%) | Patients on combination therapy (100) n (%) | Total (226) n (%) |
|---|---|---|---|
| Skin hyperpigmentation | 55 (43.65) | 50 (50) | 105 (46.46) |
| Pruritus | 55 (43.65) | 46 (46) | 101 (44.69) |
| Palmar-plantar erythrodysesthesia (PPE) | 35 (27.78) | 27 (27) | 62 (28.76) |
| Xerosis | 18 (14.28) | 28 (28) | 46 (20.35) |
| Acneiform eruptions | 6 (4.76) | 11 (11) | 17 (7.52) |
| Flagellate dermatitis | 7 (5.55) | 8 (8) | 15 (6.64) |
| Photosensitivity | 6 (4.76) | 6 (6) | 12 (5.31) |
| Serpentine supravenous hyperpigmentation | 2 (1.59) | 8 (8) | 10 (4.42) |
| Intertrigo | 4 (3.17) | 5 (5) | 9 (3.98) |
| Inflammation of preexisting keratosis | 3 (2.38) | 5 (5) | 8 (3.54) |
| Papulopustular rash | 3 (2.38) | 5 (5) | 8 (3.54) |
| Maculopapular rash | 7 (5.55) | 0 | 7 (3.10) |
| Urticaria | 3 (2.38) | 3 (3) | 6 (2.65) |
| Skin induration | 3 (2.38) | 3 (3) | 6 (2.65) |
| Skin atrophy | 2 (1.59) | 3 (3) | 5 (2.21) |
| Skin infection | 1 (0.79) | 2 (2) | 3 (1.33) |
| Bullous dermatitis | 2 (1.59) | 1 (1) | 3 (1.33) |
| Erythema multiforme/SJS/TEN | 1 (0.79) | 1 (1) | 2 (0.88) |
| Skin ulceration | 0 | 2 (2) | 2 (0.88) |
| Wound complication | 0 | 2 (2) | 2 (0.88) |
| Autoimmune reactions | 1 (0.79) | 0 | 1 (0.44) |
| Fat atrophy | 1 (0.79) | 0 | 1 (0.44) |
| Skin hypopigmentation | 1 (0.79) | 0 | 1 (0.44) |
| Others | 3 (2.38) | 2 (2) | 5 (2.21) |
Note: SJS - Stevens Johnson syndrome, TEN - Toxic epidermal necrolysis
Pigmentation was the most common cutaneous adverse reaction seen in 105 (46.46%) patients. Localized skin pigmentation (acral areas mostly) was seen in 47 (44.76%) patients whereas generalized pigmentation was seen in 58 (55.24%) patients. Pruritus was significantly more common in patients on combination regimens (88; 87.13%) than in those on single drug (13; 12.87%) (P<0.0001; Chi-square test). The pruritus was assessed subjectively using VAS. The mean VAS score was 39.40±17.40. VAS score was significantly high in those who received chemotherapy alone (43.45±17.53) when compared to those who received combination therapy (34.56±16.12) (P=0.0097; Mann–Whitney U-test). Twenty-eight (60.87%) patients with xerosis had associated pruritus which was statistically significant (P=0.0004; Chi-square test). Acral erythema/PPE was seen in 62 (28.76%) patients [Figure 1a and b]. It was significantly more common in patients on combination regimens (45; 72.58%) than those on single drug (17; 27.42%) (P<0.0001; Chi-square test). PPE was associated with pain which was assessed subjectively using VAS. The mean VAS score noted was 27.98±14.36. Xerosis was seen in 46 (20.35%) patients. Xerosis was significantly higher in those with anemia (35; 76.09%) with mean hemoglobin 9.75±2.01 (g/dl) (P=0.0004; Chi-square test). Twenty-eight (60.87%) patients with xerosis had associated pruritus which was statistically significant (P=0.0004; Chi-square test).
Figure 1.
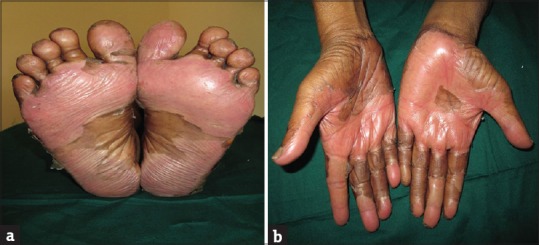
(a and b) Gemcitabine/carboplatin combination therapy induced palmoplantar erythrodysesthesia (PPE) in a patient of carcinoma ovary (Grade 3)
Nail, hair, and mucosal changes
Of the 226 patients, 194 (85.84%) patients had at least one nail related change, 159 (70.35%) patients had at least one hair related change, and 50 (18.3%) patients had at least one oral mucosal change. Nail discoloration was common in patients on combination therapy (70%), when compared to those on chemotherapy alone (64.28%), but it was not statistically significant (P=0.936; Chi-square test). Of the 151 patients who had nail discoloration, melanonychia was the most common finding seen in 132 (86.84%) patients, followed by 8 (5.26%) patients each of leukonychia and half-and-half nails (5.26%) and 3 (1.97%) patients of Muehrcke's nail [Figure 2a and b]. Alopecia was the most common hair-related change seen in 143 (63.27%) patients, of which 53 (37.06%) patients had localized alopecia whereas 38 (26.57%) patients had anagen effluvium and 52 (36.36%) patients had telogen effluvium. Oral mucositis was the most common mucosal change seen in 32 (14.16%) patients. Among 32 patients with oral mucositis, 28 patients had anemia with mean hemoglobin of 8.93±2.34 (g/dl). Oral mucosal infection was seen in 11 (4.87%) patients. Six patients had oral candidiasis, three patients developed herpetic stomatitis, and two patients had impetigo of lips. Four patients developed gingivitis and five patients developed herpes labialis on combination therapy. The detailed record of the above mucocutaneous features is given in Table 2.
Figure 2.
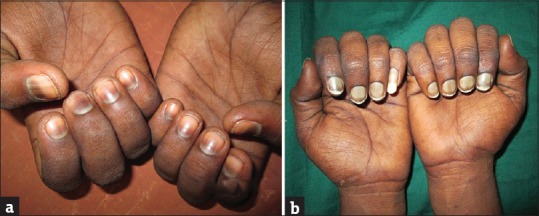
(a) Transverse melanonychia in a patient of Hodgkin's lymphoma on CHOP regimen. (b) Leukonychia in a patient of B- ALL on 6-Mercaptopurine / adriamycin/ cyclophosphamide/ methotrexate/ L-asparaginase
Table 2.
Nail, hair and mucosa related changes
| Change | Patients only on chemotherapy (126) n (%) | Patients on combination therapy (100) n (%) | Total (226) n (%) |
|---|---|---|---|
| Nail related changes | |||
| Nail discoloration | 81 (64.28) | 70 (70) | 151 (66.81) |
| Nail ridging | 31 (24.60) | 34 (34) | 65 (28.76) |
| Onycholysis | 17 (13.49) | 23 (23) | 40 (17.70) |
| Beau’s line | 15 (11.90) | 22 (22) | 37 (16.37) |
| Paronychia | 16 (12.70) | 15 (15) | 31 (13.72) |
| Brittle nail | 11 (8.73) | 15 (15) | 26 (10.18) |
| Nail infection | 3 (2.38) | 5 (5) | 8 (3.54) |
| Hair related changes | |||
| Alopecia | 84 (37.16) | 59 (26.11) | 143 (63.27) |
| Hirsutism | 5 (2.21) | 5 (2.21) | 10 (4.42) |
| Hypertrichosis | 4 (1.77) | 4 (1.77) | 8 (3.54) |
| Trichomegaly | 4 (1.77) | 2 (0.88) | 6 (2.65) |
| Hair coiling | 1 (0.44) | 2 (0.88) | (1.32) |
| Mucosa related changes | |||
| Oral mucositis | 20 (15.87) | 12 (12) | 32 (14.16) |
| Mucosal infection | 1 (0.79) | 10 (10) | 11 (4.87) |
| Herpes labialis | 1 (0.79) | 1 (1) | 5 (2.21) |
| Gingivitis | 4 (3.17) | 0 | 4 (1.77) |
Miscellaneous changes
Hyperhidrosis of palms and soles was seen in 5 (2.21%) patients. Neutrophilic eccrine hidradenitis involving scalp, face, and extremities was exclusively seen as adverse drug reaction caused by cytarabine in 4 (1.77%) patients. One patient developed eccrine syringe squamous metaplasia involving scalp and arms on long-term capecitabine therapy. Type 1 hypersensitivity was seen in six patients as urticaria. Three patients developed vasculitis-like lesions and two patients developed type 4 hypersensitivity as erythema multiforme-like lesions. Local reaction developed in 10 patients as erythema, pain, and inflammation at injection site associated with localized edema. Three patients on vincristine-based regimen developed drug extravasation as erythema, edema, blistering, and necrosis over the injection site. Periorbital edema was observed in eight patients. Two patients developed flushing on L-asparaginase-based therapy.
Grading of mucocutaneous adverse reactions
Grading of mucocutaneous adverse reactions was based on Common Terminology Criteria for Adverse Events (CTCAE) v4.0 and consisted of mild as Grade 1, moderate as Grade 2, and severe as Grade 3. There was no manifestation in Grade 4 and Grade 5. Grade 1 manifestations comprised of 49.91% of total manifestations, followed by Grade 2 (45.45%) and Grade 3 (5.64%).
Discussion
Mucocutaneous manifestations of cancer chemotherapy and chemoradiation are very common and subtle and may result in debilitating and even life-threatening complications in patients undergoing such treatment which not only affect their quality of life but also increase the morbidity and mortality. These manifestations are protean and can present as asymptomatic findings to disabling symptoms. In the past, alopecia was the most common dermatologic adverse reaction that developed with chemotherapeutic agents. With the advent of newer targeted therapies, there has been a greater incidence and severity of dermatologic adverse reactions such as hand–foot syndrome, paronychia, papulopustular eruptions, drug extravasation, neutrophilic eccrine hidradenitis, erythema multiforme, and bullous dermatosis.[2]
Pavey et al.,[3] in their descriptive study of 53 patients on chemotherapy, observed that the most common mucocutaneous adverse reactions were nail changes (33; 62.2%), followed by hair changes (20; 37.7%), skin changes (19; 33.9%), and mucosal changes (2; 3.7%). However, in our study, we observed nail and skin changes to be more common when compared to hair and mucosal changes and with higher prevalence when compared to their study.
Nail changes can involve the nail plate, nail bed, periungual area, or all of these. Nail abnormalities commonly seen with chemotherapeutic agents are nail hyperpigmentation which includes melanonychia, leukonychia, half-and-half nail, Muehrcke's nails, Terry's nails, Beau's lines, onychomadesis, onychoschizia, onychorrhexis, paronychia, onycholysis, and pyogenic granuloma-like lesions.[4] Chromonychia induced by chemotherapeutic agents has different forms, the most common being melanonychia.[5] Although several chemotherapeutic agents have been implicated to cause these nail changes, the most common drugs associated are cyclophosphamide, doxorubicin, and hydroxyurea.[6] In our study, among 151 patients who had nail discoloration, melanonychia was seen in 132 (86.84%) patients who were on FAC regimen, paclitaxel/docetaxel/cisplatin/carboplatin combination therapy, FEC regimen, cisplatin, and docetaxel monotherapy.
Hong et al.,[7] in their prospective study of 84 patients with advanced non-small cell lung cancer treated with docetaxel/cisplatin combination therapy, recorded nail changes in 26% of patients, including 11% with Grade 3 toxicity. In our study, nail changes were recorded in 85.84% of patients treated with multiple chemotherapeutic drugs, of which 75.55% had Grade 1 toxicity, 20.28% had Grade 2 toxicity, and 4.17% had Grade 3 toxicity.
Onycholysis was seen in 40 (17.70%) patients in our study who were on oxaliplatin/capecitabine combination therapy, paclitaxel/docetaxel/cisplatin/carboplatin combination therapy, capecitabine, gefitinib monotherapy, and others. Previous studies have reported onycholysis to be associated with etoposide, capecitabine, doxorubicin, and taxanes.[4] Changes such as Beau's lines and brittle nails were significantly associated with anemia and hypoalbuminemia in our study.
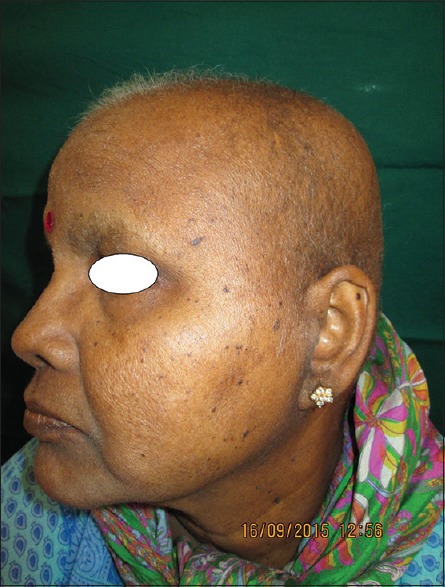
Ghunawat et al.[8] in their study of five patients reported melasma-like pigmentation in patients who were on imatinib. Our study also recorded four cases who had melasma-like pigmentation who were on imatinib and imatinib/hydroxyurea combination [Figure 3].
Figure 3.
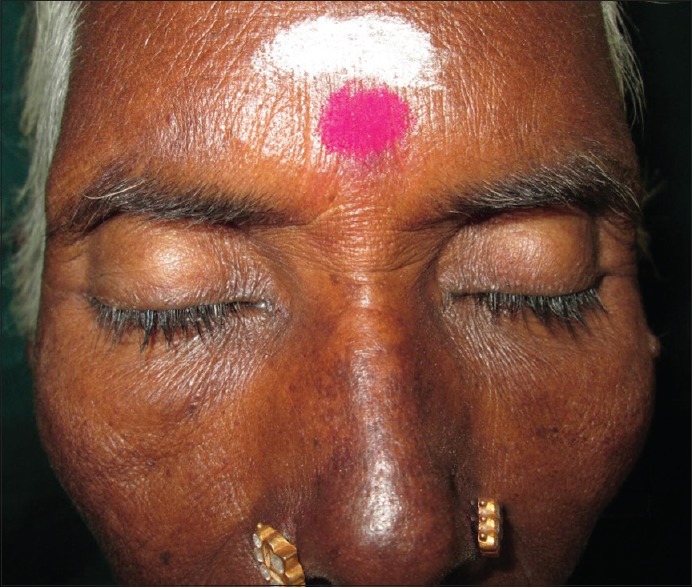
Trichomegaly of eyelashes with melasma of nose and malar areas in a patient of chronic myeloid leukemia on imatinib
Hueso et al.,[9] in their descriptive study of 2186 patients, recorded 44 (2.01%) cases of acral erythema. Our study reported 28.76% of patients with acral erythema who were on gemcitabine/carboplatin combination, FAC regimen, FEC regimen, imatinib/hydroxyurea combination, cytarabine, and docetaxel monotherapy, which was comparatively very high than reported in previous studies.
Fabbrocini et al.[10] in their study of 100 patients observed 34 patients who were treated with epidermal growth factor receptor (EGFR) inhibitors: cetuximab, erlotinib, lapatinib, gefitinib, and panitumumab; 19 (55.88%) patients developed papulopustular reactions, 14 (41.17%) patients showed dry skin and 10 (29.41%) nail alterations, and only 6 (17.64%) patients suffered from hair changes including anagen effluvium. In our study, we observed papulopustular reaction in eight patients who were on gefitinib, FAC regimen, crizotinib, and chlorambucil/bendamustine combination.
Hackbarth et al.[11] in their prospective study of 91 female cancer patients on chemotherapy reported an overall incidence of skin, nail, and hair side effects as 86.8% (n=79). In their study, 17 (18.7%) patients developed PPE, and 21 (23.1%) patients developed nail changes such as subungual hematoma, onycholysis, and leukonychia or nail loss, while 69 (75.8%) patients developed hair loss. In our study, PPE was observed in 62 (28.76%) patients who were on oxaliplatin/capecitabine combination therapy, capecitabine, and paclitaxel/docetaxel/cisplatin/carboplatin combination. PPE was significantly associated with pain which was assessed with VAS score. In our study, 9.68% of patients who developed PPE had Grade 3 toxicity, 66.13% of patients had Grade 2 toxicity, whereas Grade 1 toxicity was found in 19.35% of patients.
In a series of 15 patients treated with multiple chemotherapy including bleomycin, 10 (66%) developed flagellate dermatitis.[12] In our study, 15 (6.64%) patients developed flagellate pattern of skin rash who were on bleomycin/etoposide/cisplatin combination [Figure 4a and b]. Flagellate dermatitis has not been described for many chemotherapeutic drugs other than bleomycin for a long time. However, in our study, we observed flagellate dermatitis associated with docetaxel/paclitaxel, FEC regimen/docetaxel combination, cytarabine, capecitabine, and chlorambucil/bendamustine combination.
Figure 4.
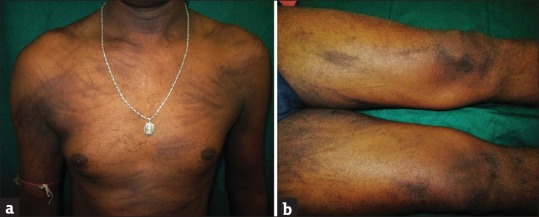
(a and b) Flagellate dermatitis over trunk and thighs in a patient of germ cell tumor on bleomycin/ etoposide/ cisplatin combination therapy
Susser et al.[12] in their study reported anagen effluvium to be the most common hair-related change. Our study recorded anagen effluvium in 26.57% of patients [Figure 5] whereas telogen effluvium was recorded in 36.36% of patients. Drugs commonly causing alopecia were FAC regimen, paclitaxel/docetaxel/cisplatin/carboplatin combination, FEC regimen, cyclophosphamide/vincristine/daunorubicin/L-asparaginase combination, doxorubicin/bleomycin/vinblastine/dacarbazine combination, and taxanes. Hypertrichosis is commonly caused by EGFR inhibitors.[13] Our study reported hypertrichosis in eight patients who were on imatinib, gefitinib, and temozolomide.
Figure 5.
Anagen effluvium in a patient of carcinoma breast on FAC regimen
Lalla et al.[14] in their study reported overall incidence of mucositis to be 20%–40% in patients who were on chemotherapy. Our study reported mucositis in 14.16% of patients, of which Grade 1 toxicity was found in 20.59%, Grade 2 in 56.25%, and Grade 3 in 20.59% [Figure 6].
Figure 6.
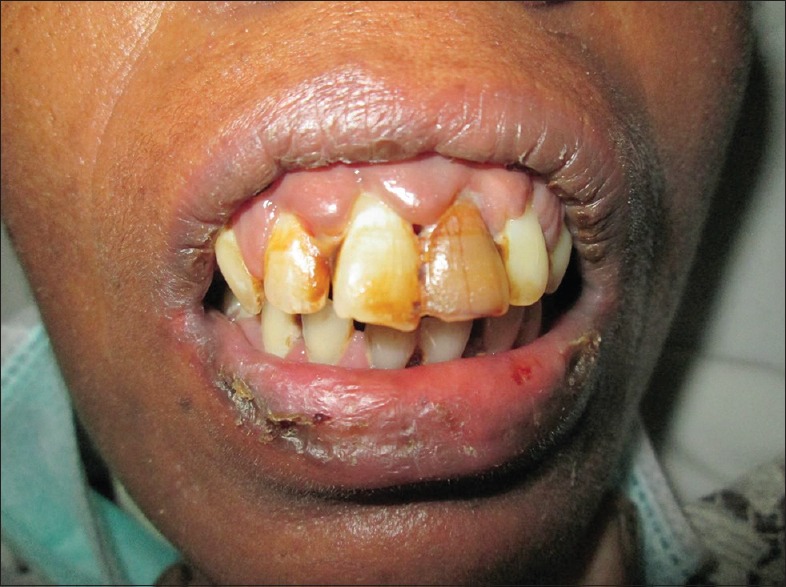
Gingivitis and oral mucositis in a patient of chronic lymphocytic leukemia on chlorambucil/bendamustine combination therapy
Limitation
As this study was cross-sectional in nature, the causal association of the drug, its dosage, and its side effect cannot be conclusively drawn. The total sample size was small and an absolute number of cases in many subgroups were not adequate to elucidate their characteristics.
Conclusion
In our study, nail changes, skin changes, hair changes, and mucosal changes occurred frequently as significant side effects in patients who were under chemotherapy and chemoradiation. Physicians should be aware of the potential impact of dermatological toxicity, especially when selecting appropriate chemotherapy agents. Furthermore, this important topic of toxicity profile should be integrated in medical informed consent of our patients, and medical education should be given to alleviate the morbidity related to chemotherapy and radiation therapy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: Current advances and future directions. Int J Med Sci. 2012;9:193–9. doi: 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wyatt AJ, Leonard GD, Sachs DL. Cutaneous reactions to chemotherapy and their management. Am J Clin Dermatol. 2006;7:45–63. doi: 10.2165/00128071-200607010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Pavey RA, Kambil SM, Bhat RM. Dermatological adverse reactions to cancer chemotherapy. Indian J Dermatol Venereol Leprol. 2015;81:434. doi: 10.4103/0378-6323.159950. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Sibaud V, Mateus C, Verschoore M, Charles C, Lanoy E, et al. Nail toxicities induced by systemic anticancer treatments. Lancet Oncol. 2015;16:e181–9. doi: 10.1016/S1470-2045(14)71133-7. [DOI] [PubMed] [Google Scholar]
- 5.Lopes M, Jordão C, Grynszpan R, Sodré C, Ramos-E-Silva M. Chromonychia secondary to chemotherapy. Case Rep Dermatol. 2013;5:163–7. doi: 10.1159/000351874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W, Yu YS, Liu YH, Sheen JM, Hsiao CC. Nail changes associated with chemotherapy in children. J Eur Acad Dermatol Venereol. 2007;21:186–90. doi: 10.1111/j.1468-3083.2006.01887.x. [DOI] [PubMed] [Google Scholar]
- 7.Hong J, Park SH, Choi SJ, Lee SH, Lee KC, Lee JI, et al. Nail toxicity after treatment with docetaxel: A prospective analysis in patients with advanced non-small cell lung cancer. Jpn J Clin Oncol. 2007;37:424–8. doi: 10.1093/jjco/hym042. [DOI] [PubMed] [Google Scholar]
- 8.Ghunawat S, Sarkar R, Garg VK. Imatinib induced melasma-like pigmentation: Report of five cases and review of literature. Indian J Dermatol Venereol Leprol. 2016;82:409–12. doi: 10.4103/0378-6323.182387. [DOI] [PubMed] [Google Scholar]
- 9.Hueso L, Sanmartín O, Nagore E, Botella-Estrada R, Requena C, Llombart B, et al. Chemotherapy-induced acral erythema: A clinical and histopathologic study of 44 cases. Actas Dermosifiliogr. 2008;99:281–90. [PubMed] [Google Scholar]
- 10.Fabbrocini G, Cameli N, Romano MC, Mariano M, Panariello L, Bianca D, et al. Chemotherapy and skin reactions. J Exp Clin Cancer Res. 2012;31:50. doi: 10.1186/1756-9966-31-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hackbarth M, Haas N, Fotopoulou C, Lichtenegger W, Sehouli J. Chemotherapy-induced dermatological toxicity: Frequencies and impact on quality of life in women's cancers. Results of a prospective study. Support Care Cancer. 2008;16:267–73. doi: 10.1007/s00520-007-0318-8. [DOI] [PubMed] [Google Scholar]
- 12.Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol. 1999;40:367–98. doi: 10.1016/s0190-9622(99)70488-3. [DOI] [PubMed] [Google Scholar]
- 13.Agero AL, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657–70. doi: 10.1016/j.jaad.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Lalla RV, Saunders DP, Peterson DE. Chemotherapy or radiation-induced oral mucositis. Dent Clin North Am. 2014;58:341–9. doi: 10.1016/j.cden.2013.12.005. [DOI] [PubMed] [Google Scholar]


