Key Points
Question
For metastatic colorectal cancer, what is the incremental cost-effectiveness of adding capecitabine and bevacizumab maintenance treatment after standard induction chemotherapy?
Findings
This economic evaluation study finds that compared with observation, capecitabine and bevacizumab maintenance therapy adds average per-patient benefits of 0.14 quality-adjusted life-years (QALYs), with incremental costs of $105 217 and an incremental cost-effectiveness ratio of $725 601 per QALY. To reduce the cost to $59 039 per unadjusted life-year (median household income) total drug costs must be reduced from $6173 to $452 per 3-week chemotherapy cycle.
Meaning
High US drug prices represent the best target for improving the cost-effectiveness of capecitabine and bevacizumab maintenance therapy for metastatic colorectal cancer, which is not currently cost-effective.
This Markov model study assesses the cost-effectiveness of capecitabine and bevacizumab maintenance therapy after induction chemotherapy for treatment of metastatic colorectal cancer.
Abstract
Importance
Unregulated drug prices increase cancer therapy costs. After induction chemotherapy, patients with metastatic colon cancer can receive maintenance capecitabine and bevacizumab therapy based on improved progression-free survival, but whether this treatment’s cost justifies its benefits has not been evaluated in the United States.
Objective
This study sought to determine the influence of capecitabine and bevacizumab drug prices on cost-effectiveness from a Medicare payer’s perspective.
Design, Setting, and Participants
The incremental cost-effectiveness of capecitabine and bevacizumab maintenance therapy was determined with a Markov model using a quality-of-life penalty based on outcomes data from the CAIRO phase 3 randomized clinical trial (RCT), which included 558 adults in the Netherlands with unresectable metastatic colorectal cancer who had stable disease or better following induction chemotherapy. The outcomes were modeled using Markov chains to account for patients who had treatment complications or cancer progression. Transition probabilities between patient states were determined, and each state’s costs were determined using US Medicare data on payments for capecitabine and bevacizumab treatment. Deterministic and probabilistic sensitivity analyses identified factors affecting cost-effectiveness.
Main Outcomes and Measures
Life-years gained were adjusted using CAIRO3 RCT quality-of-life data to determine quality-adjusted life-years (QALYs). The primary end point was the incremental cost-effectiveness ratio, representing incremental costs per QALY gained using a capecitabine and bevacizumab maintenance regimen compared with observation alone.
Results
Markov model estimated survival and complication outcomes closely matched those reported in the CAIRO3 RCT, which included 558 adults (n = 197 women, n = 361 men; median age, 64 and 63 years for patients in the observation and maintenance therapy groups, respectively) in the Netherlands with unresectable metastatic colorectal cancer who had stable disease or better following induction chemotherapy. Incremental costs for a 3-week maintenance chemotherapy cycle were $6601 per patient. After 29 model iterations corresponding to 60 months of follow-up, mean per-patient costs were $105 239 for maintenance therapy and $21.10 for observation. Mean QALYs accrued were 1.34 for maintenance therapy and 1.20 for observation. The incremental cost-effectiveness ratio favored maintenance treatment, at an incremental cost of $725 601 per QALY. The unadjusted ratio was $438 394 per life-year. Sensitivity analyses revealed that cost-effectiveness varied with changes in drug costs. To achieve an incremental cost-effectiveness ratio of less than $59 039 (median US household income) per unadjusted life-year would require capecitabine and bevacizumab drug costs to be reduced from $6173 (current cost) to $452 per 3-week chemotherapy cycle.
Conclusions and Relevance
Antineoplastic therapy is expensive for payers and society. The price of capecitabine and bevacizumab maintenance therapy would need to be reduced by 93% to make it cost-effective, a finding useful for policy decision making and payment negotiations.
Introduction
Colorectal cancer ranks third in the number of diagnoses and deaths in the United States, with 135 430 new diagnoses and 50 260 deaths estimated for 2017.1 The price of antineoplastic drugs represents a large and growing source of high health care costs,2 and American drug prices, already the highest in the world, will increase by 50% between 2010 and 2020.2,3 Most new cancer drugs now cost more than $100 000 per year, with certain regimens exceeding $1 000 000 per year.2 Despite higher drug costs, benefits have lagged, and average cost per life-year gained have increased from $54 000 per life-year in 1995 to $207 000 per life-year in 2013.3
The CAIRO phase 3 (CAIRO3) randomized clinical trial (RCT) provided evidence supporting the use of capecitabine and bevacizumab maintenance treatment for unresectable metastatic colorectal cancer (mCRC).4 This trial randomized patients with stable mCRC or better after initial capecitabine, oxaliplatin, and bevacizumab treatment to observation alone or a capecitabine and bevacizumab maintenance regimen. On disease progression, patients resumed full capecitabine, oxaliplatin, and bevacizumab treatment until the primary end point of a second progression event (measured from randomization). Patients in the capecitabine and bevacizumab maintenance therapy group had significantly longer time to a first progression event (8.5 vs 4.1 months, P < .001) and second progression event (11.7 vs 8.5 months, P < .001) compared with the observation group, with no difference in overall survival (21.6 vs 18.1 months, P = .06). Higher rates of grade 3 to 4 complications occurred in the maintenance therapy group than in the observation group (60% vs 34%), with similar quality of life (QoL) until disease progression.4 Given the longer progression-free survival and trend toward longer overall survival without QoL detriments, the CAIRO3 RCT established capecitabine and bevacizumab maintenance therapy as an option for mCRC.5
While capecitabine and bevacizumab maintenance therapy doubled the time to a first progression event compared with observation, the CAIRO3 RCT did not consider the additional costs associated with maintenance therapy. Recently, Franken et al6 performed a cost-effectiveness analysis, finding that maintenance therapy conferred 0.21 quality-adjusted life-years (QALYs) at an incremental cost-effectiveness ratio (ICER) of €175,452 per QALY. Whether these results apply to the United States remains unknown. This study hypothesizes that higher drug prices produce worse cost-effectiveness for adding capecitabine and bevacizumab maintenance therapy to standard mCRC treatment in the United States.
Methods
Cost Model
The study involves cost modeling based on publicly available data and the aggregate deindentified results of the CAIRO3 RCT. No institutional board approval or patient consent were required. A Markov model summing costs and life-years accrued for maintenance therapy and observation was constructed using 2018 costs in R software, version 3.4.3 with the heemod package.7 The model assumes that patients with mCRC who meet the CAIRO3 RCT inclusion criteria move through 6 possible states: alive with disease (AWD), treatment complications (TC), treatment discontinued for complications, progression, progression with complications, and dead (eFigure 1 in the Supplement).4 In the model, no patients in the observation group discontinue treatment because the RCT does not mention dropout in this arm.4 Patients begin in the AWD state in the first Markov iteration (or Markov cycle) and progress to other states based on assigned transition probabilities derived from CAIRO3 RCT data (eMethods in the Supplement).4,8 The Markov iteration length is 9 weeks, the interval between status assessments in the CAIRO3 RCT.4 The model runs for 29 iterations, corresponding to 60 months of follow-up. Patients accrue costs in the AWD and TC states.
Costs
The costs were calculated from a Medicare payer’s perspective based on part B and D reimbursement. Chemotherapy drug costs ($76.66/10 mg for bevacizumab and $1.67/150 mg or $5.74/500 mg for capecitabine) were obtained from the Medicare 2018 Average Sales Price Drug Pricing File9 and included the Medicare-allowed markup of 4.3%.10 Costs for administering maintenance bevacizumab (including infusion and additional office visit payments) were obtained from the Centers for Medicare and Medicaid Services Physician Fee Schedule.11 Costs per 9-week Markov iteration are 3 times those for each 3-week chemotherapy cycle (Table 1). Frequency-weighted complication costs for grade 3 to 4 complications were estimated based on the Medicare Part D costs for 90-day supplies of treatment drugs (per-unit costs: amlodipine-benazepril for hypertension, $0.74; clobetasol emollient for hand-foot syndrome, $7.66; gabapentin for neuropathy, $0.17).12 This cost was charged once on entry to the TC state and was adjusted upward to account for patients entering the progression with complications state directly, without first entering the TC state. Costs to all progression and dead states were $0. Costs for care after progression and for other routine care (office visits, imaging) were assumed to be nonincremental between groups and were not included in the study.
Table 1. Costs and Quality-Adjusted Life-Years per 9-Week Markov Iteration for Each Model State.
| State | Observation Arm | Maintenance Arm | ||
|---|---|---|---|---|
| Costs/9 wk, $ | QALY/9 wk | Costs/9 wk, $ | QALY/9 wk | |
| Alive with disease | 0 | 0.1332 | 19 803.03 | 0.1268 |
| Treatment complications | 100.43a | 0.1332 | 20 178.34a | 0.1268 |
| Treatment discontinued for complications | NA | NA | 0 | 0.1268 |
| Progression | 0 | 0.1159 | 0 | 0.1095 |
| Progression with complications | 0 | 0.1159 | 0 | 0.1095 |
| Dead | 0 | 0 | 0 | 0 |
Abbreviation: QALY, quality-adjusted life-years.
Complication costs are charged on patient entry into the treatment complications state and are $0 for subsequent Markov iterations for patients who remain in this state. Total complication costs include medications to treat complications, with weights proportional to the incidence of different complication types in each group (shown in 3-week costs). This total is adjusted to account for costs among the proportion of patients progressing directly to progression with complications states in each treatment arm (represented in 9-week costs).
Utility and ICER
Quality of life was modeled as maintenance QoL (0.735) and observation QoL (0.772), with progression decreasing QoL by 0.1.4,13 The primary outcome was the ICER, or the difference between maintenance and observation arms in total costs divided by total QALYs.
Simplifying assumptions are that patients all begin in the AWD state and move on to death only through a progressive disease state. Patients with complications progress with the same probability as those without them. The model does not permit multiple complications (sums of rates of individual complications in the CAIRO3 RCT approximate total complications)4 and considers only grade 3 to 4 complications.
Sensitivity Analyses
Both deterministic (univariable) and probabilistic (multivariable) sensitivity analyses were performed. Deterministic sensitivity analyses varied transition probabilities and QoL by 25%. Probabilistic sensitivity analyses varied inputs simultaneously based on probability distributions with 2500 resamplings (eTable in the Supplement).
Results
Costs and Utilities
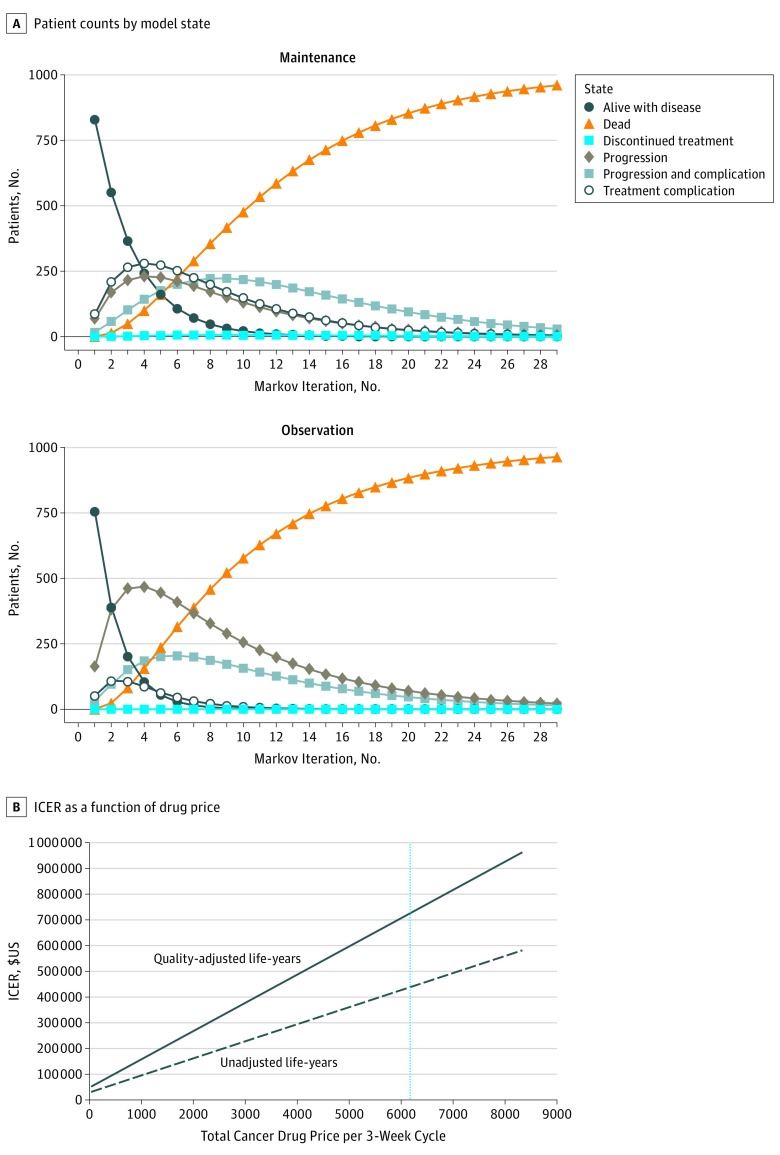
Assigned Markov transition probabilities yielded survival outcomes and complication rates closely approximating those reported for the CAIRO3 RCT (Figure 1A).4
Figure 1. Patient Counts and Incremental Cost-effectiveness Ratios (ICERs).
A, The model begins with 1000 patients in the alive-with-disease state. Their progression through the other model states by model iteration is indicated for the maintenance and observation treatment groups. The CAIRO3 randomized clinical trial4 reported times to first progression event of 8.5 and 4.1 months in the maintenance and observation groups, respectively, which correspond to 4.11 and 1.98 Markov iterations, while overall survival times of 21.6 and 18.1 months correspond to 10.44 and 8.74 iterations. B, ICERs per quality-adjusted life-year (solid line) and per unadjusted life-year (dashed line) are shown. The vertical line indicates the current price ($6173) paid by Medicare. For a willingness to pay of $200 000 per unadjusted life-year, the corresponding total drug cost would be approximately $2500 per 3-week cycle.
Total incremental costs per 3-week maintenance chemotherapy cycle were $6601.01. Drug costs per cycle were $6173.35 (Table 2). Total weighted costs for complication treatment were $62.05 for observation and $311.99 for maintenance therapy, with the difference owing to the higher costs of treating palmar-plantar erythrodysesthesia compared with hypertension and neuropathy. Accrued QALYs decrease on progression but are higher for observation group patients in all states owing to the CAIRO3 RCT finding of slightly improved QoL during observation.4
Table 2. Costs for Each 3-Week Chemotherapy Cycle .
| Cost Type | Item | Costs/3 wk, $ |
|---|---|---|
| Chemotherapya | Bevacizumab | 5596.95 |
| Capecitabine | 576.40 | |
| Administrationb | NA | 427.66 |
| Complicationsc | Observation | 62.05 |
| Maintenance | 311.99 |
Assumes a 1.86-m2, 82-kg patient.
Based on 1 hour or less administration time every 3 weeks.
Complication costs include medication for neuropathy, hand-foot syndrome, and hypertension, but not hospitalizations because nearly all grade 3 to 4 complications reported in the CAIRO3 randomized clinical trial could be managed in the outpatient setting.4
Total Treatment Cost
After 29 Markov iterations, virtually all patients had exited cost- and utility-accruing states (Figure 1A). Mean maintenance therapy costs totaled $105 239 per patient. Average life-years accrued were 1.97, with 1.34 QALYs gained. For the observation group, mean costs were $21.10 per patient, with 1.73 life-years gained (1.20 QALYs). Thus, patients receiving maintenance therapy lived, on average, 2.88 months longer than patients in the observation group, with an incremental cost of $105 217. This yielded an ICER of $438 394 per life-year, ($36 533/mo), and $725 601 per QALY ($60 467/quality–adjusted life-month) for maintenance therapy over observation. Assuming a willingness to pay equal to the US median annual household income ($59 039 per unadjusted life-year), cost-effectiveness would be achieved if the total antineoplastic drug costs were $452 per 3-week chemotherapy cycle (7.3% of the $6173 January 2018 cost) (Figure 1B).
Sensitivity Analyses
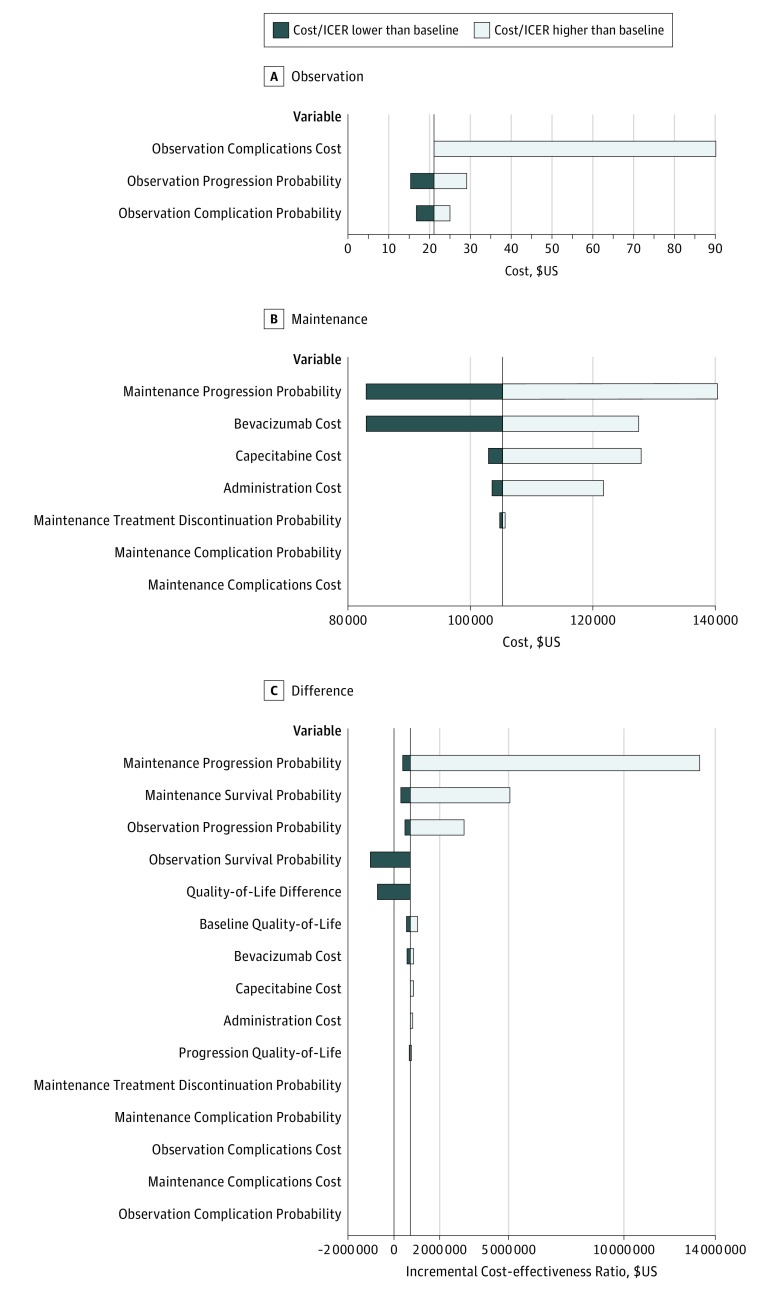
Deterministic sensitivity analyses found that observation costs were most sensitive to complication costs (Figure 2A). In the maintenance therapy arm, the transition probability for progression and costs of capecitabine and bevacizumab were the most influential factors (Figure 2B). In the deterministic sensitivity analysis, mean observation costs per person varied between $15.31 (high rate of progression) and $90.26 (high complication costs) and between $82 970 (low capecitabine and bevacizumab price or high rate of progression) and $140 349 (low rate of progression) for the maintenance therapy arm.
Figure 2. Deterministic Sensitivity Analysis Tornado Plots Showing the Range of Model Outputs .
A and B, Plots show mean per-patient cost ranges for the observation and maintenance therapy arms when each variable is tested at the high and low values listed in the eTable in the Supplement. Variables to which model costs were not sensitive are not shown. C, Plot shows the range in incremental cost-effectiveness ratio (ICER). The vertical lines in each plot indicate baseline values found in the model ($21.10 per patient for observation costs, $105 239 per patient for maintenance costs, and $725 601 per quality-adjusted life-year gained for ICER). Observation costs were most influenced by complications, while the transition probability for progression and costs of bevacizumab and capecitabine most influenced maintenance costs. Transition probabilities and quality-of-life (QoL) values create the greatest ICER differences. Changes to drug costs represent the most influential modifiable variables. Observation and maintenance QoL values are calculated by adding quality-of-life difference to baseline QoL, which is midway between the baseline values for patients in the observation and maintenance therapy groups. On progression, progression QoL is subtracted from baseline QoL. This allowed varying QoL assumptions for both treatment arms simultaneously.
The ICERs showed sensitivity to variation in transition probabilities for progression and survival and to assigned QoL (Figure 2C). Maximum ICER occurs if the maintenance therapy arm progression transition probability increases by 25% to 0.21 per Markov iteration. Assuming more patients progress and therefore fewer QALYs accumulate, the incremental cost of an additional QALY increases to $13 304 631. Conversely, if the chance of progressing to death on observation drops to 0.09 per Markov iteration, the ICER falls to −$1 018 985, meaning observation conferred longer life than maintenance therapy regardless of costs. Among potentially modifiable costs, antineoplastic drug prices exert the greatest influence on cost-effectiveness. With a capecitabine and bevacizumab cost of $4198 per 3-week chemotherapy cycle, ICER falls to $572 036 per QALY, whereas at $6996 per cycle, it increases to $879 166 per QALY.
A probabilistic sensitivity analysis varying all inputs simultaneously demonstrated a wide range of possible ICERs (eFigure 2A in the Supplement). Some resamplings had a negative ICER (361 of 2500, 14.4%), indicating longer life with observation. Covariance showed sensitivity of the model to drug and administration costs for the maintenance therapy arm, and complications in the observation arm. The QALYs accrued were sensitive to transition probability and QoL value assumptions (eFigure 2B in the Supplement). The probability of cost-effectiveness in a probabilistic sensitivity analysis varying only transition probability and QoL assumptions was 50% at a willingness to pay $777 220 per QALY (95% ICER range, $410 718-$1 467 550/QALY) (eFigure 3 in the Supplement).
Discussion
From a Medicare payer’s perspective, maintenance capecitabine and bevacizumab for mCRC is not cost-effective. With incremental costs of $105 217 per patient and minimal gains in incremental QALYs (1.7 quality–adjusted life-months), maintenance therapy had an ICER of $725 601 per QALY. Drug prices remain the most important modifiable determinant of cost-effectiveness. The current incidence of mCRC in the United States is 7.74 per 100 000 population at risk14; therefore, if capecitabine and bevacizumab maintenance therapy were universally implemented at current Medicare prices, it would add $2.53 billion per year to health care costs.
Existing investigations of bevacizumab for mCRC also report high ICERs. A study evaluating the cost-effectiveness of FOLFIRI (ie, folinic acid, fluorouracil, and irinotecan hydrochloride) and bevacizumab vs FOLFIRI alone after first-line treatment with FOLFOX (ie, folinic acid, fluorouracil, and oxaliplatin) and bevacizumab reported ICERs of $571 240 per QALY for first-line treatment and $364 083 per QALY for second-line treatment.15 A cost-effectiveness analysis performed by Franken et al6 found an ICER of €175 452 per QALY (about $216 500) for maintenance capecitabine and bevacizumab treatment, partly owing to higher mean incremental QALYs (0.21 vs 0.14). These Dutch investigators had patient-level data, allowing granular cost and transition probability estimates, precise QoL values for progressive and salvage therapy states, and costs for second-line and salvage therapy.6 Our study used average QoL with a progression penalty, potentially leading to differences in total QALYs.4,13 With many options available for therapy upon progression in the United States, our model assumed these treatments would be, on average, equivalent between the 2 study arms and therefore considered the cost difference between capecitabine and bevacizumab maintenance therapy and observation. The results from a Dutch population cannot be generalized to the US health care market; however, the current study offers a new perspective on costs for the Medicare population.
Sensitivity analyses tested drug administration costs (range, $962-$4400) and capecitabine costs ($1297-$6000 per Markov iteration), at values higher than those in the model, accounting for cost variation in real-world practice. For example, Centers for Medicare and Medicaid Services data yielded administration costs of $427.66 per 3-week chemotherapy cycle (Table 2).11 Although similar costs were reported elsewhere,15 payment data from the authors’ home institutions priced these services in the $2000 to $4500 range, suggesting that actual costs may trend higher. Similarly, capecitabine prices varied from $7721 per 9 weeks in a 2015 preliminary version of this work to $1729 currently.
Advanced treatments offering modest benefits at high costs are neither new, nor limited to mCRC,16 and Medicare allocates substantial sums to treatments not yet proven effective.17 Medicare spending for capecitabine and bevacizumab maintenance therapy, even at cost-ineffective ICER values, could be substantial. At current prices, cancer care represents an unsustainable burden to US health care,2,3,18,19,20 and strong reasons exist to reduce costs. A recent survey of Medicare patients with cancer found that many patients pay more than 60% of their income toward out-of-pocket (including copay) treatment expenses.21 High costs drive bankruptcies, use of inferior treatments, nonadherence, and worse survival.2,19,21 This study helps avoid “financial toxicity,”22 by identifying capecitabine and bevacizumab maintenance treatment as an intervention with low cost-effectiveness.
Rather than rejecting capecitabine and bevacizumab maintenance therapy altogether, payers could insist on lower drug prices. In the United States, the capecitabine and bevacizumab maintenance therapy ICER of $725 601 per QALY is more than 3.3 times that in the Netherlands. If the higher incremental QALY of 0.21 is used (as reported in a Dutch population),6 the cost-effectiveness of the maintenance therapy in the United States is still 2.3 times worse ($501 033/QALY). Differences in capecitabine and bevacizumab drug costs in the United States and the Netherlands explain this discrepancy. Medicare payments of $6173.35 per 3-week chemotherapy cycle are 2.3 times those in the Netherlands ($2671). Using the capecitabine and bevacizumab costs from the Netherlands in the US model results in a cost-effectiveness of $341 271 per QALY (with incremental QALYs of 0.14) or $235 651 per QALY (with the Dutch incremental QALY value of 0.21). If capecitabine and bevacizumab can be profitably supplied to the Netherlands at half its US cost, this price could represent a benchmark for future negotiations by US payers. Furthermore, our model suggests a target total drug cost ($452 per 3-week capecitabine and bevacizumab cycle) to achieve an ICER of $59 039 per unadjusted life-year, which could inform indication-specific or pathway-based pricing (Figure 1B).16,23,24
Limitations
Strengths of this study include its Medicare payer perspective. Medicare represents the largest US cancer care payer, covering one-third of US cancer costs and 57% of treated patients,25 and publicly available Medicare cost data permit accurate price estimates. A limitation of the study is that these results cannot be generalized to other insurers. An additional limitation concerns progression and survival assumptions. This model accurately represents patient outcomes from the CAIRO3 RCT; however, individuals eligible for clinical trials do not represent all patients in actual practice. Because sensitivity analyses identified transition probability assumptions as critically important to ICER, small differences in rates of progression or survival in patients receiving capecitabine and bevacizumab maintenance therapy will greatly influence its cost-effectiveness. Future study should investigate outcomes.
Conclusions
Capecitabine and bevacizumab maintenance treatment for mCRC is associated with high incremental costs and a 1.7 quality-adjusted life-month incremental benefit. The Medicare payer’s perspective ICER of $725 601 per QALY is far higher than previously estimated for a Dutch cohort, and supports cost-ineffectiveness of capecitabine and bevacizumab maintenance treatment at current US prices. Sensitivity analyses identify drug prices as the best target for improving cost-effectiveness.
eMethods.
eTable. Sensitivity analysis inputs.
eFigure 1. Markov model diagrams showing progression through different model states for patients undergoing maintenance therapy (A) and observation (B).
eFigure 2. Probabilistic sensitivity analysis varying all model inputs simultaneously according to the distributions specified in eTable.
eFigure 3. Incremental utilities and probability of cost-effectiveness.
eReferences.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Prasad V, De Jesús K, Mailankody S. The high price of anticancer drugs: origins, implications, barriers, solutions. Nat Rev Clin Oncol. 2017;14(6):381-390. doi: 10.1038/nrclinonc.2017.31 [DOI] [PubMed] [Google Scholar]
- 3.Tefferi A, Kantarjian H, Rajkumar SV, et al. In support of a patient-driven initiative and petition to lower the high price of cancer drugs. Mayo Clin Proc. 2015;90(8):996-1000. doi: 10.1016/j.mayocp.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simkens LH, van Tinteren H, May A, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015;385(9980):1843-1852. doi: 10.1016/S0140-6736(14)62004-3 [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network NCCN guidelines. Colon cancer. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed December 21, 2017.
- 6.Franken MD, van Rooijen EM, May AM, et al. Cost-effectiveness of capecitabine and bevacizumab maintenance treatment after first-line induction treatment in metastatic colorectal cancer. Eur J Cancer. 2017;75:204-212. doi: 10.1016/j.ejca.2017.01.019 [DOI] [PubMed] [Google Scholar]
- 7.Filipovic-Pierucci A, Zarka K, Wiener M, et al. Package heemod: Markov models for health economic evaluations. https://cran.microsoft.com/web/packages/heemod/heemod.pdf. Accessed December 14, 2017.
- 8.Beck JR, Pauker SG, Gottlieb JE, Klein K, Kassirer JP. A convenient approximation of life expectancy (the “DEALE”). II. use in medical decision-making. Am J Med. 1982;73(6):889-897. doi: 10.1016/0002-9343(82)90787-2 [DOI] [PubMed] [Google Scholar]
- 9.Centers for Medicare and Medicaid Services Jan 2018. ASP pricing file. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-drugs/McrPartBDrugAvgSalesPrice/2018ASPFiles.html. Accessed January 12, 2018.
- 10.Polite B, Conti RM, Ward JC. Reform of the buy-and-bill system for outpatient chemotherapy care is inevitable: perspectives from an economist, a realpolitik, and an oncologist. Am Soc Clin Oncol Educ Book. 2015:e75-e80. doi: 10.14694/EdBook_AM.2015.35.e75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Medicare and Medicaid Services Physician fee schedule. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/index.html. Accessed January 12, 2018.
- 12.Centers for Medicare and Medicaid Services 2015. Medicare drug spending data. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Information-on-Prescription-Drugs/2015MedicareData.html. Accessed March 7, 2018.
- 13.Quidde J, Hegewisch-Becker S, Graeven U, et al. Quality of life assessment in patients with metastatic colorectal cancer receiving maintenance therapy after first-line induction treatment: a preplanned analysis of the phase III AIO KRK 0207 trial. Ann Oncol. 2016;27(12):2203-2210. doi: 10.1093/annonc/mdw425 [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Surveillance, epidemiology, and end results program. SEER explorer. https://seer.cancer.gov/explorer/application.php. Accessed March 13, 2018.
- 15.Goldstein DA, Chen Q, Ayer T, et al. First- and second-line bevacizumab in addition to chemotherapy for metastatic colorectal cancer: a United States-based cost-effectiveness analysis. J Clin Oncol. 2015;33(10):1112-1118. doi: 10.1200/JCO.2014.58.4904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bach PB. Indication-specific pricing for cancer drugs. JAMA. 2014;312(16):1629-1630. doi: 10.1001/jama.2014.13235 [DOI] [PubMed] [Google Scholar]
- 17.Beaver JA, Howie LJ, Pelosof L, et al. A 25-year experience of US food and drug administration accelerated approval of malignant hematology and oncology drugs and biologics: a review. JAMA Oncol. 2018;4(6):849-856. doi: 10.1001/jamaoncol.2017.5618 [DOI] [PubMed] [Google Scholar]
- 18.Tangka FK, Trogdon JG, Richardson LC, Howard D, Sabatino SA, Finkelstein EA. Cancer treatment cost in the United States: has the burden shifted over time? Cancer. 2010;116(14):3477-3484. doi: 10.1002/cncr.25150 [DOI] [PubMed] [Google Scholar]
- 19.Abboud C, Berman E, Cohen A, et al. ; Experts in Chronic Myeloid Leukemia . The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood. 2013;121(22):4439-4442. doi: 10.1182/blood-2013-03-490003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramsey SD, Ganz PA, Shankaran V, Peppercorn J, Emanuel E. Addressing the American health-care cost crisis: role of the oncology community. J Natl Cancer Inst. 2013;105(23):1777-1781. doi: 10.1093/jnci/djt293 [DOI] [PubMed] [Google Scholar]
- 21.Narang AK, Nicholas LH. Out-of-pocket spending and financial burden among medicare beneficiaries with cancer. JAMA Oncol. 2017;3(6):757-765. doi: 10.1001/jamaoncol.2016.4865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Souza JA, Conti RM. Mitigating financial toxicity among US patients with cancer. JAMA Oncol. 2017;3(6):765-766. doi: 10.1001/jamaoncol.2016.4850 [DOI] [PubMed] [Google Scholar]
- 23.Chandra A, Garthwaite C. The economics of indication-based drug pricing. N Engl J Med. 2017;377(2):103-106. doi: 10.1056/NEJMp1705035 [DOI] [PubMed] [Google Scholar]
- 24.Polite BN, Page RD, Nabhan C. Oncology pathways-preventing a good idea from going bad. JAMA Oncol. 2016;2(3):297-298. doi: 10.1001/jamaoncol.2015.5778 [DOI] [PubMed] [Google Scholar]
- 25.Lee JA, Roehrig CS, Butto ED. Cancer care cost trends in the United States: 1998 to 2012. Cancer. 2016;122(7):1078-1084. doi: 10.1002/cncr.29883 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable. Sensitivity analysis inputs.
eFigure 1. Markov model diagrams showing progression through different model states for patients undergoing maintenance therapy (A) and observation (B).
eFigure 2. Probabilistic sensitivity analysis varying all model inputs simultaneously according to the distributions specified in eTable.
eFigure 3. Incremental utilities and probability of cost-effectiveness.
eReferences.