Abstract
Background:
This study tested if the timing of meals, physical activity, light exposure, and sleep cluster within individuals and are associated with Body Mass Index (BMI) in a sample of free-living adults (n=125).
Methods:
Data were collected between November 2015 and March 2016 at UC San Diego, Children’s Hospital of Philadelphia, and Washington University in St. Louis. Height and weight were measured and BMI (kg/m2) was calculated. Sleep timing was estimated using actigraphy; and timing of meals, physical activity, and light exposure were self-reported using a smartphone application. General linear models estimated the mean BMI across time categories of behaviors, adjusting for covariates. A latent class analysis was used to identify patterns of timing variables that clustered within individuals and test for associations between identified patterns and BMI.
Results:
Later exposure to outdoor light was associated with a lower BMI (P-trend<0.01). The timing of other behaviors were not independently associated with BMI. The latent class analysis identified two distinct groups related to behavioral timing, reflecting an “early bird” and “night owl” phenotype. These phenotypes were not associated with BMI (P>0.05).
Conclusions:
Timing of exposures to light, meals, sleep, and physical activity were not strongly associated with BMI in this sample.
INTRODUCTION
The personal, economic, and public health costs of obesity have been well established. Obesity is associated with significant morbidity,1,2 mortality,3 and adds substantially to the national healthcare budget.4 Therefore, the development of effective strategies to prevent and treat it is a considerable public health priority.
Much of scientific research on the topic has focused on identifying what to eat or how much to exercise to control body weight. However, a small but growing body of evidence suggests the timing of behaviors is also important.5–10 The most robust literature on the topic has focused on meal timing, with both human and animal data suggesting that reducing food intake later in the day such as during the nighttime hours may help prevent and/or control obesity.5,8 Furthermore, epidemiologic and clinical data also suggest that the timing of other exposures such as light, sleep, and even timing of physical activity, may also influence weight control.6,7,10,11
A major limitation of research on the timing of behaviors and obesity is that studies to date have investigated the influence of the timing of one behavior in isolation. Yet, obesity-related behaviors may occur simultaneously within an individual, making inferences about associations challenging when only one behavior is examined without accounting for others. Person-centered modeling approaches, such as a latent class analysis (LCA), may be a useful analytic approach to address this issue of modeling co-occurring behaviors. Indeed, LCA has been successfully applied in a growing number of public health studies,12–15 but we are not aware of any research to date that has used this modeling strategy to understand if and how the timing of sleep, activity, meals, and light exposure cluster within an individual. We therefore tested if the timing of meals, light exposure, physical activity, and sleep were associated with BMI in a sample of healthy adults who recorded the timing of behaviors over multiple days using a novel smartphone application and actigraphy. We first used the traditional approach of examining individual exposures. We then used LCA to identify patterns of timing behaviors that tend to cluster together, and tested for associations between the patterns identified and BMI.
METHODS
INZEIT Study
The “Impact of Nocturnal Zeitgebers on Energy in TREC” (INZEIT) Study was funded by the National Cancer Institute’s Transdisciplinary Research in Energetics and Cancer (TREC) initiative.16 INZEIT enrolled and collected data on 133 participants during the winter season between November 2015 and March 2016 at three sites: University of California, San Diego (N=44), Children’s Hospital of Philadelphia (N=44), and Washington University in St. Louis (N=45). All study protocols and procedures were standardized across each site and were approved by site-specific Institutional Review Boards. Study staff at the Brigham and Women’s Hospital processed and managed all study data, and all participants provided written informed consent.
A convenience sample of individuals was identified by means of web advertisements, flyers, word of mouth, and by calling individuals who had previously consented to be contacted for research to solicit interest. Eligibility was assessed by telephone interview. Eligible participants had to be 21–60 years old, able to use and install a smartphone application, fluent in English, and able to attend an in-person visit. Participants were excluded if they had a skin disorder that prevented wearing an ActiGraph, were pregnant or breast-feeding, performed night shift work, or traveled across time zones within 30 days of data collection. In addition, participants were excluded if they had a serious or acute illness requiring bed-rest, were diagnosed with a sleep disorder, had a disability that limited or restricted mobility, or experienced persistent tremors.
All eligible participants were invited to attend an in-person study visit where they completed questionnaires and had their height and weight measured. A smartphone application was installed on their phone and they were provided with an ActiGraph to wear on their wrist for a seven-day period following the in-person assessments.
Measures
Actigraphy
Participants wore an ActiGraph GT3X+ device (ActiGraph, LLC) on their non-dominant wrist continuously for seven days, except during water-based activities or bathing. Data were downloaded using ActiLife software, and were visually screened for sufficient wear-time. For each day, we manually identified a main rest interval as the primary sleep period based on the app logs and observation of a sharp decrease/increase in activity. To be included, participants had to provide at least 4 days of recordings with >10 hours (600 minutes) recorded during wake time on each day and no more than 1 hour of nonvalid signal during sleep. Data meeting this criteria were processed to derive estimates of total sleep time (TST, hours per night) using the Cole-Kripke algorithm,17 as well as estimates of the times participants went to bed and woke up. Total activity, defined as the mean counts per minute (CPM) per wear-time on the vertical axis, was also computed for each participant.
PACO Smartphone Application
Participants used their smartphones to complete surveys during the same 7-day actigraphy period. The surveys were developed using the open source Personal Analytics Companion (PACO) application, which is a customizable smartphone application available on the AppStore and Google Play (https://www.pacoapp.com/). Participants were prompted at 9 AM, 1 PM, and 7 PM, to complete surveys about the timing of their sleep, physical activity, light exposure, meals, and bed/wake times. The surveys used were developed to mirror validated questionnaires assessing behavioral patterns and circadian timing.18,19 Upon receiving the prompts, the participants were instructed to complete the surveys at their earliest convenience. Response options were a list of ordered timing categories, ranging from earlier to later exposure; the specific timing categories for each exposure collected using this application are given in Table S1. Survey responses were uploaded to a study-specific database in real-time, and staff monitored completion. A minimum of 5 days of PACO survey data, matched to usable actigraphy data, was required for each participant.
PACO data were summarized across measurement days by computing the median timing category across days for each behavior except timing of physical activity. These person-level summary variables were then treated as ordered categorical variables (larger numbers corresponded to later exposure). In some instances, data were collapsed across time categories due to low cell sizes. Response options that did not correspond to an ordered timing category (e.g., “I wasn’t outdoors”) were treated as missing values because of our focus on the timing of exposures, and were not included as part of the summary measures. Because a large proportion of participants chose the response option “I did not do any moderate or vigorous activities” on questions about physical activity in the morning/evening, we could not summarize activity across measurement days without considerable missing data. Instead, the responses corresponding to physical activity timing were summarized as the proportion of measurement days that each participant was physically active in the morning (until 12 pm) or evening (after 7 pm).
Demographics
Age (y), race, ethnicity, sex, income (<$25,000; $25,000 - <$50,000; $50,000 - <$75,000; $75,000 - <$100,000; ≥$100,000), and education (Completed high school or less; Some college but no degree; Completed College; or Graduate or professional school) were self-reported.
Body Mass Index (BMI)
Height (m) and weight (kg) were measured by study staff at the clinic visit and used to calculate BMI (kg/m2). Those with a BMI ≥30kg/m2 were defined as obese.
Chronotype
Chronotype was assessed using the 19-item Horne-Ostberg Morningness Eveningness Questionnaire.18 Scores on this scale range from 16 to 86, with higher scores indicating greater morning preference. This scale has been shown to correlate with measures of the circadian timing system, such as body temperature.18,20
Statistical Analysis
Descriptive statistics characterized the study population by obesity status (obese [≥30kg/m2] vs. non-obese [<30kg/m2]). Differences in the characteristics of the sample by obesity status were examined using t-tests for continuous variables and chi-square/Fisher’s exact tests for categorical variables.
Bivariate correlations between BMI and the timing behaviors were calculated. The statistically significant correlations (P<0.05) were investigated in multivariable general linear models in order to evaluate whether the mean BMI was equal across timing categories, while controlling for potential confounders. Model 1 was adjusted for age, race/ethnicity (dichotomized as white/non-Hispanic vs. other), and education (dichotomized as college educated vs. not college educated). Model 2 was additionally adjusted for actigraphy-derived TST and total activity. Study site was examined as a potential confounder by adding it as a covariate in regression models; however, the addition of this variable did not materially change the associations reported and it was not included in final models. Trend tests were used to investigate whether there was a linear association between BMI and the timing categories. In addition, given the recent evidence that day-to-day variability in timing behaviors may be independent predictors of BMI,21 exploratory models also examined associations between the day-to-day variability in each exposure with BMI. For these exploratory models, the variability in the timing of each behavior was approximated as the difference between the maximum and the minimum timing category reported for each participant across measurement days.
To expand beyond modeling the timing of individual behaviors, we used a latent class analysis (LCA) to identify discrete patterns of timing behaviors that tended to cluster within an individual. The input variables in the LCA models included the timing behaviors that were statistically significantly correlated with BMI (i.e., timing of last exposure to outdoor light, last exposure to indoor (artificial) light, and first exposure to indoor light), as well the timing behaviors that were significantly modestly or strongly correlated (rs>0.5) with those three exposures (i.e., were co-occurring behaviors), which included the timing of last meal, bed time, and wake time. It is notable that these additional variables were added to the LCA model (timing of last meal, bed time, and wake time) because they were also borderline-significantly correlated with BMI (p<0.10). To reduce the number of latent class combinations, these timing (input) variables were dichotomized based on their distribution in our sample as follows: late last exposure to outdoor light (after 5pm [yes vs. no]); late first indoor light exposure (after 7:45 am [yes vs. no]); late bed time (after 11:30 pm [yes vs. no]); late wake time (after 7:24 am [yes vs. no]); and late last meal ([after 9 pm [yes vs. no]). It was difficult to pick a cut-point for late last indoor light exposure, given that over two-thirds of the participants in the study sample reported a median last exposure to indoor light between 10:15 pm and 12:45 am. We selected the more extreme cut-point for this exposure of after 12:45 am [yes vs. no]); however, sensitivity analyses were conducted to determine how selecting different cut-points for this exposure (and others) influenced the model fit and overall interpretation of the findings we report. The number of classes that adequately described the sample was selected using bootstrap likelihood ratio tests to compare models with an increasing number of classes.22 We then estimated associations between latent class membership and BMI by modeling class assignment as an independent variable in a general linear model with BMI as the outcome. This analysis was modeled with and without covariates, as done for the linear models described previously. All analyses were performed using SAS 9.4 (Cary, NC), and the LCA was conducted using PROC LCA,23,24 and the associated bootstrapping macro.22
RESULTS
Of the 133 participants recruited, 8 did not have complete PACO or actigraphy data and were excluded from this analysis. The final analytic sample included 125 adults. Participants had a mean (SD) age of 35.3 (11.0) years and a mean BMI of 27.6 (6.5) kg/m2 (Table 1). Most participants were non-Hispanic white (54%) and female (64%). Obese participants in our sample were older, more likely to be non-white, and were less educated. The obese participants also had a shorter mean TST (397.9 vs. 424.6 minutes per night). We also present descriptive statistics by study site as a supplementary table (Table S2).
Table 1.
Characteristics of the INZEIT Study Population by Obesity Status.
| All |
Non-Obese BMI <30 |
Obese BMI ≥ 30 |
P-value* | |
|---|---|---|---|---|
| N=125 | N=88 | N=37 | ||
| Age, mean (SD) | 35.3 (11.0) | 32.8 (9.6) | 41.0 (11.9) | <0.01 |
| White, non-Hispanic, n (%) | 68 (54.4) | 54 (61.4) | 14 (37.8) | 0.02 |
| Male, n (%) | 45 (36.0) | 35 (39.8) | 10 (27.0) | 0.18 |
| BMI (kg/m2) | 27.6 (6.5) | 24.2 (3.1) | 35.9 (4.8) | <0.01 |
| Education, n (%) | 0.02 | |||
| High school or less | 19 (15.2) | 9 (10.2) | 10 (27.0) | |
| Some college | 29 (23.2) | 18 (20.5) | 11 (29.7) | |
| Completed college | 53 (42.4) | 40 (45.5) | 13 (35.1) | |
| Graduate training | 24 (19.2) | 21 (23.9) | 3 (8.1) | |
| Income, n (%)1 | 0.47 | |||
| <$25,000 | 28 (24.8) | 23 (28.4) | 5 (15.6) | |
| $25,000 - <$50,000 | 36 (31.9) | 25 (30.9) | 11 (34.4) | |
| $50,000 - <$75,000 | 18 (15.9) | 11 (13.6) | 7 (21.9) | |
| $75,000 - <$100,000 | 18 (15.9) | 14 (17.3) | 4 (12.5) | |
| ≥$100,000 | 13 (11.5) | 8 (9.9) | 5 (15.6) | |
| Total Sleep Time, min/night, mean (SD) | 416.7 (53.1) | 424.6 (52.0) | 397.9 (51.4) | <0.01 |
| Activity, Axis-1 CPM2, mean (SD) | 1117.9 (371.9) | 1106.3 (365.9) | 1145.6 (389.7) | 0.59 |
| Horne-Ostberg Score3, mean (SD) | 56.0 (9.8) | 55.7 (9.7) | 56.9 (10.1) | 0.53 |
Abbreviations: CPM: counts per minute; MVPA: moderate-to-vigorous intensity physical activity.
Note: Participants were enrolled from November 2015 to March 2016 at UC San Diego, Children’s Hospital of Philadelphia, and Washington University in St. Louis.
P-value for between group comparisons using Chi-square/Fisher’s exact, or t-tests.
Missing data for n=12 participants.
Total activity derived from wrist actigraphy based on mean daily counts per minute on the vertical-axis.
Scores indicate diurnal preference; higher scores indicate greater preference for morning; data missing for one participant.
The timing of several behaviors were moderately-to-strongly correlated with one another (rs >0.5; Table 2): Later time of first exposure to indoor light was moderately correlated with later bed time (rs=0.52; P<0.01), and strongly correlated with later wake time (rs=0.71; P<0.01). Later time of last exposure to indoor light was moderately correlated with later time of last eating episode (rs=0.57; P<0.01), later bed time (rs=0.62; P<0.01), and later wake time (rs=0.54; P<0.01). Later time of last eating episode was moderately correlated with later bed time (rs=0.55; P<0.01) and later wake time (rs =0.56; P<0.01), and later wake time was strongly correlated with later bed time (rs=0.74; P<0.01).
Table 2.
Spearman Correlations Among Timing Variables and BMI among (n=125) Participants from the INZEIT Pilot Study.
| Time of First Exp. to Outdoor Light | Time of Last Exp. to Outdoor Light | Time of First Exp. to Indoor Light | Time of Last Exp. to Indoor Light | Time of First Eating Episode | Time of Last Eating Episode | Bed Time | Wake Time | Morning PA Proportion | Evening PA Proportion | BMI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time of First Exp. to Outdoor Light | 1.00 | −0.076 | 0.448*** | 0.298*** | 0.359*** | 0.438*** | 0.461*** | 0.464*** | −0.082 | 0.135 | −0.015 |
| Time of Last Exp. to Outdoor Light | 1.00 | −0.027 | 0.220** | −0.021 | 0.022 | 0.173 | 0.093 | −0.068 | −0.021 | −0.293*** | |
| Time of First Exp. to Indoor Light | 1.00 | 0.298*** | 0.393*** | 0.438*** | 0.520*** | 0.706*** | −0.117 | 0.051 | −0.202** | ||
| Time of Last Exp. to Indoor Light | 1.00 | 0.263*** | 0.570*** | 0.617*** | 0.538*** | −0.307*** | 0.232** | −0.213** | |||
| Time of First Eating Episode | 1.00 | 0.413*** | 0.372*** | 0.452*** | −0.174 | 0.044 | −0.059 | ||||
| Time of Last Eating Episode | 1.00 | 0.552*** | 0.562*** | −0.153 | 0.210** | −0.165 | |||||
| Bed Time | 1.00 | 0.737*** | −0.277*** | 0.110 | −0.168 | ||||||
| Wake Time | 1.00 | −0.276*** | 0.106 | −0.173 | |||||||
| Morning PA Proportion | 1.00 | 0.227** | 0.013 | ||||||||
| Evening PA Proportion | 1.00 | 0.041 | |||||||||
| BMI | 1.00 |
P<0.05;
P<0.01
Abbreviations: Exp.=exposure
Note: Participants were enrolled from November 2015 to March 2016 at UC San Diego, Children’s Hospital of Philadelphia, and Washington University in St. Louis.
Spearman correlations identified 3 timing behaviors that were significantly negatively correlated with BMI (P<0.05). As shown in Table 2, later exposure to outdoor light, later timing of last exposure to indoor light, and later timing of first exposure to indoor light were all negatively correlated with BMI. The correlation coefficients of these associations were weak to modest, ranging from r= −0.20 to rs=−0.29. None of the other timing behaviors were statistically significantly correlated with BMI.
Results of the multivariable general linear models are presented in Table 3, which shows the marginal (least-squares) means for BMI, by timing category of the individual behavior of interest. In model 1, the timing of last exposure to outdoor light remained negatively associated with BMI, after adjustment for age, race/ethnicity and education. Specifically, the model estimated that exposure to outdoor light was associated with an adjusted mean BMI ranging from 31.7 (Standard Error [SE]=3.0) in the earliest timing category (i.e., before 10 am), to 27.1 (SE=0.6) in the latest timing category (i.e., after 5 pm) (P-trend across timing categories: 0.006). Note that the least squares means approach assumes an equal proportion of the categorical exposure, at the mean age of 35.3 and equal proportion in race and education categories. These associations remained similar after adjustment for potential lifestyle confounders of TST and total activity (model 2). Timing of the other behaviors were not significantly associated with BMI in multivariable models. These associations remained similar when we modeled the timing of behaviors as binary variables using the cut-points selected for the LCA input variables (Table S3). Furthermore, day-to-day variability in the timing of each of the behaviors was not significantly associated with BMI (P>0.05, Table S4).
Table 3.
Marginal Mean BMI (SE) by Timing Category of Exposures in the INZEIT Pilot Study (n=125).
| Timing of Last Exposure to Outdoor Light | ||||||
| 8:00 AM - 10:00 AM (n=4) | 10:00 AM - 05:00 PM (n=30) | 05:00 – 10:00 PM (n=91) | P trend | |||
| Model 1 | 31.7 (3.0) | 30.3 (1.1) | 27.1 (0.6) | -- | -- | 0.006 |
| Model 2 | 31.5 (3.0) | 30.2 (1.1) | 27.1 (0.6) | -- | -- | 0.008 |
| Timing of Last Exposure to Indoor Light | ||||||
| 7:00 PM - 09:00 PM (n=5) |
09:00 – 10:15 PM (n=16) | 10:15 PM - 12:45 AM (n=86) | 12:45 – 2:00 AM (n=13) | 2:00 – 3:00 AM (n=5) | P trend | |
| Model 1 | 28.1 (2.8) | 30.3 (1.5) | 27.7 (0.7) | 28.7 (1.8) | 25.0 (2.7) | 0.24 |
| Model 2 | 27.9 (2.8) | 30.3 (1.5) | 27.6 (0.7) | 29.0 (1.8) | 24.9 (2.7) | 0.28 |
| Timing of First Exposure to Indoor Light | ||||||
| Before 6:30 AM (n=25) | 6:30 – 7:45 AM (n=48) | 7:45 – 9:45 AM (n=44) | After 9:45 AM (n=8) | P trend | ||
| Model 1 | 29.0 (1.3) | 27.9 (0.9) | 28.1 (1.0) | 25.7 (2.2) | -- | 0.32 |
| Model 2 | 28.8 (1.3) | 27.9 (0.9) | 28.1 (1.0) | 26.1 (2.3) | -- | 0.48 |
Note: Participants were enrolled from November 2015 to March 2016 at UC San Diego, Children’s Hospital of Philadelphia, and Washington University in St. Louis; models assume an equal proportion of the categorical covariates, and mean of continuous covariates.
Model 1: adjusted for age, race/ethnicity, and education.
Model 2: adjusted for same covariates in Model 1, in addition to total sleep time (min/night), and total activity derived from wrist actigraphy (vertical-axis counts per minute).
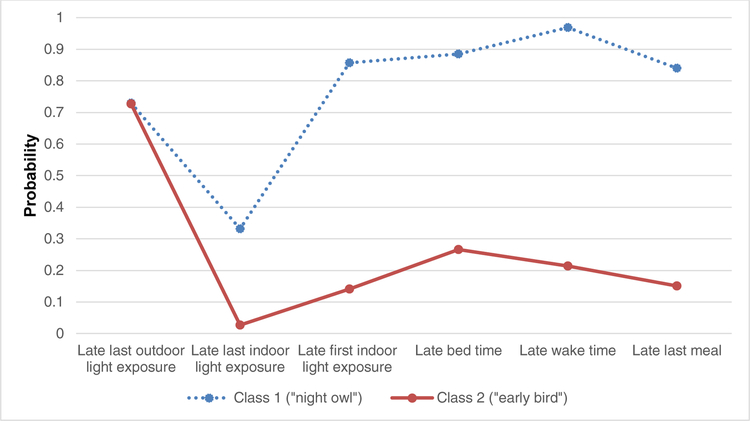
The results of the LCA are presented in Figure 1. We identified two distinct latent classes of timing of behaviors. The first class represents a population of participants with a later first and last exposure to indoor light, later last meal, and later bed and wake times (related to the concept of “night owl”). The second class represents a sample with an earlier first and last exposure to indoor light, earlier last meal, and earlier bed and wake times (“early bird”). It is notable that those classified as “early birds” based on timing of behaviors also had a higher mean Horne-Ostberg score (class 2=59.3 vs. class 1=50.6), indicating a greater morning preference. Sensitivity analyses confirmed that using different cut-points to define latent class categories did not meaningfully alter the findings we report; we also confirmed that removing the input variable of “late last exposure to outdoor light,” which did not appear to be discriminatory in our LCA models, or input variables that may not always be independent within an individual (e.g., bed times and last indoor light), did not materially change the latent subgroups identified or the overall findings we report.
Figure 1. Item Response Probabilities for 2-class Model: Probability of Reporting Engagement in Timing Behavior by Latent Class Membership.
Note: Participants were enrolled from November 2015 to March 2016 at UC San Diego, Children’s Hospital of Philadelphia, and Washington University in St. Louis.
In terms of the association between class membership and BMI, we observed the least squares mean BMI to be significantly lower for those belonging to class 1 (later timing) compared to class 2 (earlier timing) in unadjusted models: 25.8 (0.9) kg/m2 versus 28.7 (0.7) kg/m2, respectively (p=0.01). After adjustment for confounders, the association between class membership and BMI was attenuated and was no longer significant (Table 4).
Table 4.
Marginal Mean BMI (SE) by Latent Class Category in the INZEIT Pilot Study (n=125).
| Class 1: “Night Owl” N=47 |
Class 2: “Early Bird” N=78 |
P Value | |
|---|---|---|---|
| Unadjusted | 25.8 (0.9) | 28.7 (0.7) | 0.01 |
| Model 1 | 26.8 (0.9) | 28.8 (0.7) | 0.11 |
| Model 2 | 26.9 (0.9) | 28.7 (0.7) | 0.14 |
Note: Participants were enrolled from November 2015 to March 2016 at UC San Diego, Children’s Hospital of Philadelphia, and Washington University in St. Louis; models assume an equal proportion of the categorical covariates and mean of continuous covariates.
Model 1: adjusted for age, race/ethnicity, and education.
Model 2: adjusted for same covariates in Model 1, in addition to total sleep time (min/night), and total activity derived from wrist actigraphy (vertical-axis counts per minute).
In exploratory analyses, we found that 13 participants had a latent class membership that was discordant with their self-reported Horne-Ostberg chronotype score (i.e., a self-identified morningness vs eveningness preference). We therefore also tested if discordance between chronotype and behavioral timing profiles associated with BMI. We did not observe any BMI differences between the concordant and discordant groups.
DISCUSSION
This analysis is examined the associations between the timing of modifiable behaviors and BMI among adults in free-living settings. We build on previous studies, which have examined the timing of single behaviors in isolation, by recognizing and accounting for potential behavior timing co-occurrence by means of a latent class analysis approach – offering a novel contribution to the literature. When behaviors were examined separately, the timing of last exposure to outdoor light was the only behavior that was associated with BMI in multivariable models, such that a later exposure to outdoor light was associated with a lower BMI in our sample comprised mostly of younger adults with measurements occurring during winter months. However, when clusters of behavioral patterns were examined using a LCA approach, we found that timing patterns of behaviors were not independently associated with BMI.
The mechanisms through which the timing of light exposure, eating, physical activity, and sleep are hypothesized to influence body weight control involve circadian rhythms. Specifically, these behavioral exposures are powerful stimuli (i.e., “zeitgebers”) that provide input to the circadian timing system and help orient circadian rhythms within the 24-hour day.25 Exposure to these behaviors late at night or at irregular time intervals (i.e., out of synchronization with the 24-hour light/dark cycle), may alter the alignment between circadian rhythms in the suprachiasmatic nucleus and peripheral tissues. In effect, this misalignment can lead to less stable 24-hour rhythms, and has been experimentally shown to offset a number of metabolic processes including hormonal regulation and energy metabolism — which can predispose an individual to weight gain.25 For example, data from experimental trials in humans indicate that short-term, extensive misalignment of circadian rhythms results in unfavorable changes in leptin (a hormone that signals “fullness”), glucoregulation, as well as decreased energy expenditure.26,27 While there are data and sound biological mechanisms supporting causal associations between the timing of exposure to various zeitgebers and metabolic health in animal models and lab-based human studies, the epidemiological findings that are not subject to such extensive misalignment conditions are less clear.
The “night owl” behavior pattern, which was identified through LCA, had a lower score on the Horne-Ostberg scale, indicating a greater evening preference. However, we did not observe an association between “night owl” class membership and BMI. In contrast, many epidemiological studies in both younger and older adults have found evening chronotype to be associated with obesogenic behaviors,28 metabolic dysfunction,29 and increased BMI.30,31 For example, in a study of 1,620 adults aged 47–59 in the Korean Genome and Epidemiology Study, evening chronotype was associated with a 1.73-fold greater odds of having diabetes, a 1.74-fold greater odds of metabolic syndrome, and roughly a 3-fold increased odds of sarcopenia (all P<0.05)29. It is possible that our findings differed due to selection of a relatively healthy population who had a mean sleep duration of roughly 7 hours per night (median=7.1 hours), and which had few individuals with extreme chronotypes. Experimental studies show that metabolic effects of circadian misalignment are particularly pronounced when circadian misalignment is combined with sleep restriction.32 Our findings suggest that in individuals getting an average of almost 7 hours of sleep per night, and unselected based on extreme chronotypes, that evening preference and evening timing of activities is not associated with a higher BMI.
Despite the inconsistencies between our findings and previous studies noted above, our data are in agreement with findings from several genetic studies, which suggest that there is not a causal association between chronotype and BMI.33–35 For example, Jones and colleagues performed Mendelian randomization analyses using a genetic risk score of established chronotype and BMI variants and found no evidence that chronotype causally affects BMI or vice-versa.33 Taken together, the inconsistencies across previous epidemiologic and genetic research, combined with our study findings, underscore the fact that the links between chronotype and BMI are not completely understood. Thus, this remains an evolving research area.
The timing of later exposure to outdoor light was the only behavior that was associated with BMI after adjusting for potential confounding variables. The direction of this finding is unexpected, given that light is considered one of the strongest cues synchronizing circadian rhythms to the 24-hour day, and previous research examining the influence of the timing of light exposure on weight control have found that high-intensity light exposure earlier in the day is associated with a lower BMI.6 These discrepancies could be due to measurement error in the self-reported timing variables, as we discuss in more detail in the paragraph that follows. Furthermore, although our study distinguished between indoor and outdoor light, which is known to have somewhat distinct intensity ranges and may have different influences on the circadian system and health outcomes,36,37 it is important to point out that our study did not consider the precise intensity or duration of light exposure participants received. Future studies that consider the time, type, intensity, and duration of light, will be important for clarifying the association between timing of outdoor light exposure and BMI.
Limitations of this study include the small sample size, cross-sectional study design, and reliance on self-reported data for many of the timing behaviors assessed. However, timing of many of the behaviors was assessed using a smartphone application multiple times per day in an effort to minimize the potential for errors in the provided information due to participants not remembering. Furthermore, although our PACO survey questions were informed by validated surveys regarding behavior and circadian timing,18,19 they have not been validated under the research conditions and mobile tools described in this manuscript. We did not plan to use data collected using the actigraphy light sensor, given that all data were collected during winter months when sensors are known to be obstructed by jackets and heavy clothing.38,39 However, we did compare the consistency of the light sensor data to PACO responses using a random sample of actigraphy files that did not appear to be badly obstructed (i.e., light profiles that did not show long intervals of lux levels=0). In general, files were in reasonable agreement. For example, when we applied an “Actilux” threshold that has been shown to distinguish indoor from outdoor light using an ActiGraph,39 we found that PACO responses to the question about timing of last exposure to outdoor light converged with the actigraphy data roughly 70 percent of the time. In addition, although we made an effort to enroll participants whose data would not be confounded by shift work, sleep disorders, recent travel across time zones, or whose schedule may be offset by breastfeeding, we recognize that there are other circumstances that may have influenced participants’ timing behaviors in the short term (e.g., caring for non-breast-feeding infants, recent illness). Therefore, it is possible that data collected on some individuals in our study are not representative of their usual behavioral patterns. It is also important to note that there may be other unmeasured confounding variables such as employment status, which may alter the associations we report. Finally, as noted in the methods section, one variable in the main analysis had a response option that didn’t correspond to an ordered timing category (e.g., “I wasn’t outdoors”). We felt it was best to treat it as missing data, and removed this response option prior to computing summary variables across days for each participant. However, it is notable that this response option was infrequent (represents less than 4% of all measurement days), and thus we do not believe that excluding this information meaningfully influenced the derived summary variables or the associations we report.
Study strengths include the geographic diversity of participants that were enrolled at three centers across the country. In addition, we assessed the timing of behaviors using actigraphy and a novel smartphone application that enabled near real-time assessment of the primary study exposures. Finally, this is one of the first attempts that we are aware of to estimate latent classes of timing behaviors, which addresses issues of modeling co-occurring timing behaviors – a matter that has not been adequately addressed in the literature.
CONCLUSION
In summary, results of this study do not support the hypothesis that in healthy adults who on average sleep nearly 7 hours per night, the timing of behavioral zeitgebers such as light, meals, physical activity, and sleep, are strongly associated with BMI. Additional research is needed to address the potential heterogeneity of effects of timing of behaviors across diverse groups and seasons, and specifically address potential modification of effects by sleep duration and chronotype.
Supplementary Material
FUNDING SOURCE:
This work was supported by the NCI Centers for Transdisciplinary Research on Energetics and Cancer (TREC) (U01 CA116850, U54 CA155496, U54 CA155626, U54 CA155435, U54 CA155850). JAM was supported by NIH grant K01 HL123612 (NHLBI). CRM was supported by the National Cancer Institute under award numbers F31 CA183125, F32 CA220859, and an American Cancer Society Postdoctoral Fellowship under award number PF-17–231-01-CCE. PJ was supported by National Cancer Institute grant K99 CA201542. MQ was supported by a scholarship from the Tuebinger Program for the Advancement of Women in Science. SM was supported by NIH grant R24 HL114473 (NIH/NHLBI).
Footnotes
CONFLICT OF INTEREST: The authors declare no conflict of interest
REFERENCES
- 1.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature reviews Cancer. 2004;4(8):579–591. [DOI] [PubMed] [Google Scholar]
- 2.Eckel RH. Obesity and heart disease: a statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation. 1997;96(9):3248–3250. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. Jama. 2013;309(1):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trust for America’s Health and the Robert Wood Johnson Foundation. F as in Fat: How Obesity Threatens America’s Future. 2012. [Google Scholar]
- 5.Hatori M, Vollmers C, Zarrinpar A, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell metabolism. 2012;15(6):848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid KJ, Santostasi G, Baron KG, Wilson J, Kang J, Zee PC. Timing and intensity of light correlate with body weight in adults. PloS one. 2014;9(4):e92251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chomistek AK, Shiroma EJ, Lee IM. The Relationship Between Time of Day of Physical Activity and Obesity in Older Women. Journal of physical activity & health. 2016;13(4):416–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garaulet M, Gomez-Abellan P, Alburquerque-Bejar JJ, Lee YC, Ordovas JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. International journal of obesity. 2013;37(4):604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell metabolism. 2014;20(6):991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McFadden E, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ. The relationship between obesity and exposure to light at night: cross-sectional analyses of over 100,000 women in the Breakthrough Generations Study. American journal of epidemiology. 2014;180(3):245–250. [DOI] [PubMed] [Google Scholar]
- 11.Asarnow LD, McGlinchey E, Harvey AG. Evidence for a Possible Link between Bedtime and Change in Body Mass Index. Sleep. 2015;38(10):1523–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agrawal A, Lynskey MT, Madden PA, Bucholz KK, Heath AC. A latent class analysis of illicit drug abuse/dependence: results from the National Epidemiological Survey on Alcohol and Related Conditions. Addiction. 2007;102(1):94–104. [DOI] [PubMed] [Google Scholar]
- 13.Dierker LC, Vesel F, Sledjeski EM, Costello D, Perrine N. Testing the dual pathway hypothesis to substance use in adolescence and young adulthood. Drug and alcohol dependence. 2007;87(1):83–93. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy DE, Ebssa L, Witkiewitz K, Shiffman S. Repeated measures latent class analysis of daily smoking in three smoking cessation studies. Drug and alcohol dependence. 2016;165:132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leech RM, Worsley A, Timperio A, McNaughton SA. Temporal eating patterns: a latent class analysis approach. The international journal of behavioral nutrition and physical activity. 2017;14(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson RE, Colditz GA, Hu FB, et al. The 2011–2016 Transdisciplinary Research on Energetics and Cancer (TREC) initiative: rationale and design. Cancer causes & control : CCC. 2013;24(4):695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15(5):461–469. [DOI] [PubMed] [Google Scholar]
- 18.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International journal of chronobiology. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 19.Bajaj A, Rosner B, Lockley SW, Schernhammer ES. Validation of a light questionnaire with real-life photopic illuminance measurements: the Harvard Light Exposure Assessment questionnaire. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(7):1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostberg O Circadian rhythms of food intake and oral temperature in “morning” and “evening” groups of individuals. Ergonomics. 1973;16(2):203–209. [DOI] [PubMed] [Google Scholar]
- 21.Taylor BJ, Matthews KA, Hasler BP, et al. Bedtime Variability and Metabolic Health in Midlife Women: The SWAN Sleep Study. Sleep. 2016;39(2):457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dziak JJ, Lanza ST. LcaBootstrap SAS macro usersʹ guide (version 4.0). University Park: The Methodology Center, Penn State. [Google Scholar]
- 23.PROC LCA & PROC LTA (Version 1.3.2) [Software]. (2015). University Park: The Methodology Center, Penn State; Retrieved from http://methodology.psu.edu. [Google Scholar]
- 24.Lanza ST, Dzik JJ, Huang L, Wagner A, Collins LM. PROC LCA & PROC LTA users’ guide (Version 1.3.2). University Park: The Methodology Center Penn State; 2015. [Google Scholar]
- 25.Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiological reviews. 2013;93(1):107–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McHill AW, Melanson EL, Higgins J, et al. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(48):17302–17307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(11):4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleig D, Randler C. Association between chronotype and diet in adolescents based on food logs. Eating behaviors. 2009;10(2):115–118. [DOI] [PubMed] [Google Scholar]
- 29.Yu JH, Yun CH, Ahn JH, et al. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. The Journal of clinical endocrinology and metabolism. 2015;100(4):1494–1502. [DOI] [PubMed] [Google Scholar]
- 30.Arora T, Taheri S. Associations among late chronotype, body mass index and dietary behaviors in young adolescents. International journal of obesity. 2015;39(1):39–44. [DOI] [PubMed] [Google Scholar]
- 31.Culnan E, Kloss JD, Grandner M. A prospective study of weight gain associated with chronotype among college freshmen. Chronobiology international. 2013;30(5):682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buxton OM, Cain SW, O’Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4(129):129ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones SE, Tyrrell J, Wood AR, et al. Genome-Wide Association Analyses in 128,266 Individuals Identifies New Morningness and Sleep Duration Loci. PLoS genetics. 2016;12(8):e1006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lane JM, Vlasac I, Anderson SG, et al. Genome-wide association analysis identifies novel loci for chronotype in 100,420 individuals from the UK Biobank. Nature communications. 2016;7:10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu Y, Shmygelska A, Tran D, Eriksson N, Tung JY, Hinds DA. GWAS of 89,283 individuals identifies genetic variants associated with self-reporting of being a morning person. Nature communications. 2016;7:10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duffy JF, Czeisler CA. Effect of Light on Human Circadian Physiology. Sleep medicine clinics. 2009;4(2):165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boubekri M, Cheung IN, Reid KJ, Wang CH, Zee PC. Impact of windows and daylight exposure on overall health and sleep quality of office workers: a case-control pilot study. J Clin Sleep Med. 2014;10(6):603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flynn JI, Coe DP, Larsen CA, Rider BC, Conger SA, Bassett DR Jr, Detecting indoor and outdoor environments using the ActiGraph GT3X+ light sensor in children. Medicine and science in sports and exercise. 2014;46(1):201–206. [DOI] [PubMed] [Google Scholar]
- 39.Tandon PS, Saelens BE, Zhou C, Kerr J, Christakis DA. Indoor versus outdoor time in preschoolers at child care. American journal of preventive medicine. 2013;44(1):85–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.