Abstract
Depressive symptoms and frailty are each independently associated with morbidity and mortality in kidney transplant (KT) recipients. We hypothesized that having both depressive symptoms and frailty would be synergistic and worse than the independent effect of each. In a multicenter cohort study of 773 KT recipients, we measured the Fried frailty phenotype and the modified 18-question Center for Epidemiologic Studies-Depression Scale (CES-D). Using adjusted Poisson regression and survival analysis, we tested whether depressive symptoms (CES-D score > 14) and frailty were associated with KT length of stay (LOS), death-censored graft failure (DCGF), and mortality. At KT admission, 10.0% of patients exhibited depressive symptoms, 16.3% were frail, and 3.6% had both. Recipients with depressive symptoms were more likely to be frail (aOR = 3.97, 95% CI: 2.28–6.91, P < 0.001). Recipients with both depressive symptoms and frailty had a 1.88 times (95% CI: 1.70–2.08, P < 0.001) longer LOS, 6.20-fold (95% CI:1.67–22.95, P < 0.01) increased risk of DCGF, and 2.62-fold (95% CI:1.03–6.70, P = 0.04) increased risk of mortality, compared to those who were nonfrail and without depressive symptoms. There was only evidence of synergistic effect of frailty and depressive symptoms on length of stay (P for interaction < 0.001). Interventions aimed at reducing pre-KT depressive symptoms and frailty should be explored for their impact on post-KT outcomes.
Keywords: depressive symptoms, frailty, graft loss, kidney transplant recipients, kidney transplantation, mortality
1 |. INTRODUCTION
Frailty is a physical syndrome manifest by distinct vulnerability to stressors as the deterioration in physiologic reserve.1 The Fried physical frailty phenotype is characterized by weakness, low energy, unintentional weight loss, slowed gait, and low physical activity.1 Although the physical frailty phenotype was initially identified as a risk factor for adverse outcomes in community-dwelling older adults, it has since been identified as an important predictor of adverse outcomes in individuals with end-stage renal disease (ESRD) as well.2–5 For instance, an estimated 20% of kidney transplant (KT) recipients exhibited frailty at KT admission,6 and the clinical phenotype is a predictor of adverse short-and long-term post-KT outcomes such as longer length of hospital stay, delayed graft function, worse health-related quality of life, early hospital readmission, immunosuppression intolerance, and mortality.6–12 While previous studies have demonstrated that physical frailty and depressive symptoms commonly co-occur leading to adverse outcomes,13 the impact of this physical frailty phenotype on adverse KT outcomes among patients with and without mental health vulnerabilities, like depressive symptoms, is unclear.
Symptoms of depression include the frailty components of weight loss, low energy, and decreased physical activity.1,14 Among KT recipients, 18%−22% have depressive symptoms post-KT.15–17 These depressive symptoms are important markers of vulnerability and are independently associated with adverse outcomes after KT, including medication nonadherence, return to dialysis therapy, graft failure, as well as cardiovascular and all-cause mortality.15,18–22 Given that frail community-dwelling older adults with concurrent depression are more vulnerable to adverse outcomes,13 we hypothesized that depressive symptoms and frailty may have a synergistic association with adverse post-KT outcomes like length of stay, an important driver of subsequent mortality.7
We hypothesized that the presence of physical vulnerability captured by frailty status and mental health vulnerability measured by depressive symptoms synergistically impacts adverse KT outcomes. Using a prospective, multicenter cohort of KT recipients, the goals of this study were to estimate the prevalence of the co-occurrence of depressive symptoms and frailty at admission for KT, characterize the differences in depressive symptoms between frail and nonfrail recipients, and quantify the association between their co-occurrence and adverse outcomes after KT including length of stay (LOS), death-censored graft failure (DCGF), and mortality.
2 |. MATERIALS AND METHODS
2.1 |. Study population
This was a multicenter, prospective, longitudinal study of 773 adult (age 18 years and older) first-time KT recipients at the Johns Hopkins Hospital (N = 707) and the University of Michigan University Hospital (N = 66), from August 2009 to September 2017. KT candidates were enrolled at admission for KT, and the only inclusion criteria were speaking English; of those screened, <5% of all KTs did not meet this inclusion criteria. For the current analysis, we excluded participants who did not provide complete data on depressive symptoms (16.5%) or did not perform the frailty assessments (4.2%). Eligible participants who were excluded were similar to those who were included based on age (P = 0.86), sex (P = 0.11), and donor type (P = 0.34).
In this study, we measured the Fried physical frailty phenotype and Center for Epidemiologic Studies-Depression Scale (CES-D) at admission for KT as described below; these assessments were conducted as part of a research protocol for the cohort study. Recipient, donor, and transplant factors (age, sex, race, body mass index [BMI], time on dialysis, causes of ESRD, and donor type [living donor vs deceased donor]) were abstracted from medical charts. Obesity was defined as a BMI of ≥30 kg/m2. A modified Charlson comorbidity index (mCCI) adapted for patients with ESRD was calculated based on both abstracted and self-reported comorbidities at the time of admission for KT.23 The Johns Hopkins Institutional Review Board and the University of Michigan Institutional Board approved the study, and all participants provided written informed consent. This research is in adherence with the Declaration of Helsinki.
2.2 |. Frailty measurement
We assessed frailty at KT admission using the Fried frailty phenotype, a measure of physiologic reserve based on 5 components: slowed gait speed (walking time of 15 feet below an established cutoff by gender and height), weakness (grip strength below an established cutoff based on gender and BMI), exhaustion (self-report using two items from the CES-D), shrinking (self-report of unintentional weight loss of more than 10 pounds in the past year based on estimated “dry weight”), and low physical activity (kcal expended/wk below an established cutoff).1 Each of the five components was scored as either a 0 or 1 based on its absence or presence, respectively. The aggregate frailty score was calculated as the sum of the components on a scale from 0 to 5, with scores ≥3 categorized as frail. This scoring has previously been validated in older adults and in ESRD and KT populations.3,6–9,11,12,24–28
2.3 |. Depressive symptoms measurement
We ascertained depressive symptoms at KT admission using the CES-D, a 20-item questionnaire that queries depressive symptoms over the past week.14 Responses to each of the 20 questions are scored as a 0, 1, 2, or 3. This instrument has been previously validated in patients with ESRD 29–32 and identifies symptoms in the areas of depressed mood, guilt/worthlessness, helplessness/hopelessness, psychomotor retardation, loss of appetite, and sleep disturbance. The scale ranges from 0 to 60, with a score of ≥16 indicating depressive symptoms. In the analysis, we excluded two questions that overlapped with the exhaustion component of the Fried frailty phenotype (“Did you feel that everything you did was an effort?” and “Could you not get ‘going’?”). We modified the cutoff score to reflect the 2 omitted questions, resulting in a new depressive symptoms cutoff score of >14 out of a possible 54.
2.4 |. Depressive symptoms and frailty
KT recipients were categorized by their frailty/depressive symptoms status: (a) nonfrail/no depressive symptoms; (b) nonfrail/depressive symptoms; (c) frail/no depressive symptoms; or (d) frail/depressive symptoms. Recipient, donor, and transplant factors were summarized using means and standard deviations for normally distributed factors, medians and IQRs for non-normally distributed factors, and percentages for categorical variables. We tested whether recipient, donor, and transplant factors differed by frailty/depressive symptoms status using Fisher’s exact tests for categorical variables and Student t tests and Wilcoxon rank sum tests for continuous factors depending on their distribution. The association between frailty and depressive symptoms was quantified using adjusted logistic regression.
2.5 |. Depressive symptoms, frailty, and length of stay
We quantified the association between frailty/depressive symptoms status and LOS using a multilevel Poisson regression model [adjusted relative risk (aRR)] to account for the variation in LOS due to differences between the two hospitals.
2.6 |. Depressive symptoms, frailty, death-censored graft failure, and mortality
We quantified the association between frailty/depressive symptoms status and DCGF as well as mortality using adjusted Cox proportional hazards models. Cumulative incidences of DCGF and mortality were estimated using a Kaplan-Meier approach. For all models, proportional hazard assumptions were confirmed by visual inspection of the complementary log-log plots and Schoenfeld residuals.
2.7 |. Statistical analysis
Parsimonious models were adjusted for age, sex, and race. All other models were adjusted for age, sex, race, education, BMI, mCCI, causes of ESRD, time on dialysis, and donor type. As sensitivity analyses, we additionally adjusted for delayed graft function (DGF) in the analysis with LOS as the outcome. For all analyses, a P-value of <0.05 was considered statistically significant. All data were analyzed using Stata 14 (College Station, TX, USA).
3 |. RESULTS
3.1 |. Study population
Among 773 KT recipients, the mean age was 54 years (SD = 14 years; range: 19–86), 37.8% of KT recipients were female, 40.9% were African American, 41.4% attained a high school education or less, 35.3% were obese, 20.6% had diabetes, 40.8% spent >2 years on dialysis, and 38.0% received a live donor KT. The median Charlson comorbidity index score was 1 (IQR: 0–3).
3.2 |. Depressive symptoms
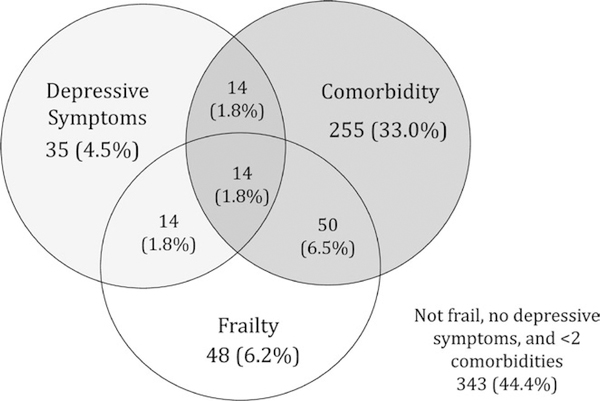
The median CES-D score was 4 (IQR: 1–9). About 10.0% of KT recipients had depressive symptoms (77 recipients). KT recipients with depressive symptoms were younger (median [IQR]: 50.0 [40.8, 58.7] vs 57.7 [45.9, 65.8], P < 0.001) and more likely to be female (53.3% vs 36.1%, P = 0.003; Table S5). There were no significant differences in BMI, cause of ESRD, donor type, education level, or time spent on dialysis between KT recipients with and without depressive symptoms (Figure 1).
FIGURE 1.
Prevalence and overlap of depressive symptoms, frailty, and comorbidity at the time of kidney transplantation (N = 773). The Fried frailty phenotype is scored on a scale ranging from 0 to 5 components, with the presence of ≥3 components representing frailty. Depressive symptoms were assessed using the modified CES-D, in which questions about exhaustion were removed to avoid overlap with the Fried frailty phenotype. The modified CES-D is scored on a scale from 0 to 54, with scores >14 indicating depressive symptoms. Comorbidity was defined as a score of ≥2 diseases on the modified Charlson comorbidity index for ESRD and KT recipients
3.3 |. Depressive symptoms and frailty
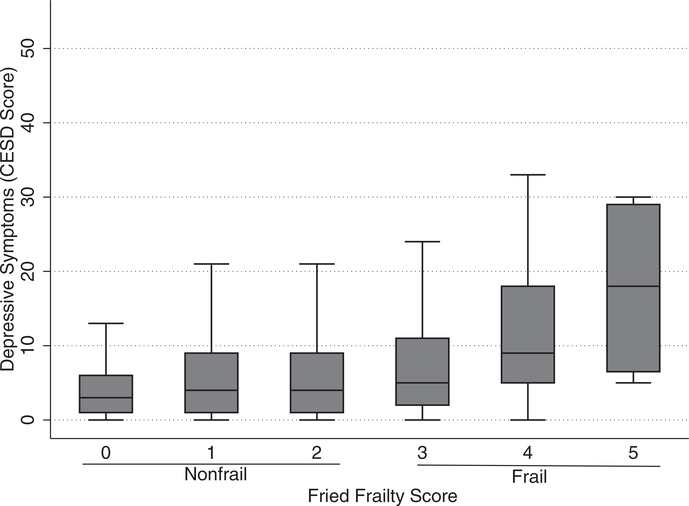
About 3.6% (28 recipients) of KT recipients exhibited both frailty and depressive symptoms (Table 1). The median CES-D score was 4 (IQR: 2–9) among nonfrail recipients and 8 (IQR: 4–16) among frail recipients. Additionally, CES-D scores increased monotonically with increasing frailty score (Figure 2). KT recipients who were frail and had depressive symptoms were more likely to be female (60.7% vs 35.8%, P = 0.02) and have a high school education or below (53.4% vs 37.6%, P = 0.02) compared to nonfrail KT recipients without depressive symptoms (Table 1). There were no differences in delayed graft function rates between the four frailty-depressive symptoms groups (P = 0.72). Depressive symptoms were independently associated with frailty (aOR = 3.97, 95% CI:2.28– 6.91, P < 0.001). Further, frail KT recipients were more likely than nonfrail KT recipients to report 9 of 18 depressive symptoms including inattention (30.2% vs 13.8%, P < 0.001), depressed mood (18.3% vs 5.1%, P < 0.001), and loss of appetite (23.8% vs 14.2%, P = 0.01; Table 2).
TABLE 1.
Characteristics of kidney transplant recipients, by depressive symptoms and frailty status (N = 773)
| No depressive symptoms, nonfrail (n = 598) | Depressive symptoms, nonfrail (n = 49) | No depressive symptoms, frail (n = 98) | Depressive symptoms, frail (n = 28) | |
|---|---|---|---|---|
| Age, median (IQR) | 56.6(44.9–65.1) | 49.0 (40.8–55.7) | 60.3 (51.3–68.3) | 53.4 (35.6–61.8) |
| Female, % | 35.8 | 49.0 | 37.8 | 60.7 |
| African American, % | 39.5 | 36.7 | 51.0 | 42.9 |
| Education level, % | ||||
| ≤High school | 37.6 | 44.9 | 52.0 | 53.4 |
| BMI, % | ||||
| Underweight | 2.3 | 2.0 | 2.0 | 14.3 |
| Normal | 29.3 | 24.5 | 24.5 | 25.0 |
| Overweight | 33.3 | 36.7 | 36.7 | 28.6 |
| Obese | 35.1 | 36.7 | 36.7 | 32.1 |
| Cause of ESRD, % | ||||
| Hypertension | 34.0 | 18.4 | 38.8 | 35.7 |
| Diabetes | 21.2 | 18.4 | 18.4 | 17.9 |
| Other | 44.8 | 63.3 | 42.9 | 46.4 |
| Living donor recipient, % | 39.1 | 44.9 | 28.6 | 35.7 |
| Delayed graft function, % | 22.8 | 20.4 | 27.6 | 25.0 |
| mCCI, median (IQR) | 1 (0–3) | 0(0–2) | 2(0–3) | 1.5 (0–2) |
| Time on dialysis, % | ||||
| Pre-emptive KT | 26.3 | 28.6 | 23.5 | 25.0 |
| ≤2 y | 32.9 | 24.5 | 24.5 | 21.4 |
| >2 y | 40.8 | 46.9 | 52.0 | 53.6 |
BMI, body mass index at KT; mCCI, modified Charlson comorbidity index; ESRD, end- stage renal disease, IQR, interquartile range
FIGURE 2.
Distribution of pre–kidney transplantation depressive symptoms score among frail and nonfrail kidney transplant recipients (N = 773). Fried frailty phenotype, scored on a scale ranging from 0 to 5 components, with the presence of ≥3 components representing frailty. The modified CES-D is scored on a scale from 0 to 54, with scores >14 indicating depressive symptoms. Depressive symptoms increase monotonically with frailty
TABLE 2.
Depressive symptoms reported by frail and nonfrail kidney transplant recipients (N = 773)
| Depressive symptoms questions | Nonfrail (n = 647) | Frail (n = 126) | P-value |
|---|---|---|---|
| Responding “Occasionally” or “Most of the Time,” (%) | |||
| Were you bothered by things that usually don't bother you? | 10.1 | 13.5 | 0.3 |
| Did you not feel like eating; your appetite was poor? | 14.2 | 23.8 | 0.01 |
| Did you feel that you could not shake off the blues? | 4.0 | 12.7 | <0.001 |
| Did you feel people were unfriendly? | 7.6 | 10.3 | 0.2 |
| Did you have trouble keeping your mind on what you were doing? | 13.8 | 30.2 | <0.001 |
| Did you feel depressed? | 5.1 | 18.3 | <0.001 |
| Did you feel sad? | 4.6 | 15.9 | <0.001 |
| Did you think your life had been a failure? | 1.7 | 6.4 | 0.01 |
| Did you feel fearful? | 6.2 | 14.3 | 0.01 |
| Was your sleep restless? | 32.6 | 41.3 | 0.07 |
| Did you have crying spells? | 3.9 | 4.8 | 0.08 |
| Did you talk less than usual? | 8.0 | 14.3 | 0.04 |
| Did you feel that people disliked you? | 1.4 | 2.4 | 0.4 |
| Did you feel lonely? | 5.1 | 12.7 | 0.004 |
| Responding “Rarely” or “Some of the Time,” a(%) | |||
| Did you feel you were just as good as other people? | 7.6 | 10.3 | 0.3 |
| Did you enjoy your life? | 3.3 | 2.4 | 0.8 |
| Were you happy? | 3.9 | 4.8 | 0.6 |
| Did you feel hopeful about the future? | 1.7 | 6.4 | <0.001 |
Row percentages are reported. The modified CES- D is an 18- item questionnaire that queries depressive symptoms from over the past week. Possible responses to the questions are “Rarely or none of the time,” “Some or a little of the time,” “Occasionally or a moderate amount of the time,” and “Most or all of the time.”
Responses were inversely scaled when calculating the total CES-D score.
3.4 |. Depressive symptoms, frailty, and length of stay
The median LOS was 10 days (IQR: 6–12). After adjusting for recipient, donor, and transplant factors, KT recipients with depressive symptoms who were concurrently frail had a 1.88 times (95% CI: 1.70–2.08) longer LOS; KT recipients with depressive symptoms but not frail had a 1.38 times (1.27–1.52) longer LOS. The co-occurrence of depressive symptoms and frailty had a synergistic effect on longer LOS (P for interaction < 0.001; Table 3). Among nonfrail KT recipients, each 10-point increase in the CES-D score was associated with 1.17 times (95% CI: 1.27–1.52) longer LOS. Among frail KT recipients, each 10-point increase in the CES-D score was associated with 1.23 times (95% CI: 1.16–1.31) longer LOS. The association between increased CES-D score and longer LOS differed significantly by frailty status (P for interaction = 0.009; Table 3).
TABLE 3.
Length of stay among kidney transplant recipients with depressive symptoms, by frailty status (N = 773)
| Length of Stay | ||||
|---|---|---|---|---|
| aRR (95% CI) |
||||
| Median days (IQR) | Parsimonious model | Fully adjusted model | P-value for interaction | |
| Depressive symptoms | ||||
| Nonfrail | 10 (7–14) | 1.38 (1.27, 1.52) | 1.38 (1.27, 1.52) | |
| Frail | 10 (7–16) | 1.85 (1.67, 2.05) | 1.88 (1.70, 2.08) | <0.001 |
| CES-D Score (10-point increase) | ||||
| Nonfrail | 1.11 (1.06, 1.16) | 1.17 (1.08, 1.27) | ||
| Frail | 1.22 (1.15, 2.29) | 1.23 (1.16, 1.31) | 0.009 | |
The parsimonious models were adjusted for age, sex, and race. The fully adjusted models were adjusted for age, sex, race, education, BMI, cause of ESRD, time on dialysis, modified Charlson comorbidity index, and donor type. Additionally, the P-values for the interactions were based on the fully adjusted models.
3.5 |. Depressive symptoms, frailty, and death-censored graft failure
For KT recipients with depressive symptoms and frailty, DCGF at 5-years post-KT was 19.1%, compared to 8.5% for nonfrail KT recipients without depressive symptoms (Table 4). After adjusting for recipient, donor, and transplant factors, KT recipients with depressive symptoms and frailty were at a 6.20-fold (95% CI: 1.67–22.95, P < 0.01) increased risk of DCGF compared to nonfrail KT recipients without depressive symptoms (Table 4). The association between depressive symptoms and increased risk of DCGF did not differ by frailty status (P for interaction = 0.67).
TABLE 4.
Cumulative incidence and risk of death-censored graft failure and mortality among kidney transplant recipients, by depressive symptoms and frailty status (N = 773)
| aHR (95% CI) |
P-value for interaction | |||||
|---|---|---|---|---|---|---|
| i-y | 3-y | 5-y | Parsimonious model | Fully adjusted model | ||
| Death-censored graft failure | ||||||
| No depressive symptoms, Nonfrail | 1.3 | 4.4 | 8.5 | Ref | Ref | |
| Depressive symptoms, Nonfrail | 6.8 | 6.8 | 6.8 | 2.17 (0.65, 7.23) | 3.16 (0.90, 11.04) | |
| No depressive symptoms, Frail | 1.1 | 6.1 | 8.7 | 1.14 (0.47, 2.77) | 0.97 (0.37, 2.75) | |
| Depressive symptoms, Frail | 0 | 19.1 | 19.1 | 4.26 (1.23, 14.80) | 6.20 (1.67, 22.95) | 0.67 |
| CES-D score (10-point increase) | 1.69 (1.04, 2.77) | 1.86 (1.10, 3.14) | 0.28 | |||
| Mortality | ||||||
| No depressive Symptoms, Nonfrail | 3.6 | 10.7 | 14.1 | Ref | Ref | |
| Depressive Symptoms, Nonfrail | 4.6 | 11.4 | 20.2 | 1.57 (0.57, 4.36) | 1.92 (0.68, 5.38) | |
| No depressive Symptoms, Frail | 4.2 | 11.7 | 16.1 | 0.90 (0.49, 1.64) | 0.93 (0.49, 1.76) | |
| Depressive Symptoms, Frail | 3.7 | 9.4 | 27.5 | 2.51 (1.00, 6.31) | 2.62 (1.03, 6.70) | 0.61 |
| CES-D score (10-point increase) | 1.51 (1.06, 2.17) | 1.45 (0.98, 2.14) | 0.82 | |||
Cumulative incidences are expressed as % and estimated using a Kaplan- Meier approach. All Cox proportional hazards models were adjusted for age, sex, race, education, BMI, cause of ESRD, time on dialysis, modified Charlson comorbidity index, and donor type.
3.6 |. Depressive symptoms, frailty, and mortality
For KT recipients with depressive symptoms and frailty, mortality at 5-years post-KT was 27.5%, compared to 14.1% for nonfrail KT recipients without depressive symptoms. After adjusting for recipient, donor, and transplant factors, KT recipients with depressive symptoms and frailty were at a 2.62-fold (95% CI: 1.03–6.70, P = 0.04) higher risk of mortality compared to nonfrail KT recipients without depressive symptoms (Table 4). The association between depressive symptoms and higher risk of mortality did not differ by frailty status (P for interaction = 0.61).
4 |. DISCUSSION
In this prospective, multicenter study of 773 KT recipients, 10.0% of KT recipients had depressive symptoms and 16.3% of KT recipients were frail. We found that the presence of depressive symptoms was associated with a 3.97-fold higher likelihood of being frail. The co-occurrence of depressive symptoms and frailty was observed in 3.6% of KT recipients and was associated with a synergistically longer length of stay. Additionally, frail recipients who had depressive symptoms experienced a 6.20-fold increased risk of DCGF and a 2.62-fold increased risk of mortality, compared to KT recipients who were not frail and did not exhibit depressive symptoms. However, we did not find a synergistic effect between frailty and depressive symptoms on the longer-term outcomes of DCGF and mortality.
Our finding of depressive symptoms among 10% of KT recipients was lower than previously reported estimates of 21%−27% among nondialysis chronic kidney disease 33 and 26% among maintenance hemodialysis patients.34 The prevalence of depressive symptoms is likely lower in KT recipients because these patients complete an extensive mental health screening prior to clearance for KT. Additionally, we found KT recipients who were younger, female, and frail were more likely to have depressive symptoms. This is consistent with a study of post-KT Medicare claims which reported higher rates of diagnosed depression among female and younger KT recipients.21 Notably, we report the novel finding of increased depressive symptoms reported among frail KT recipients, suggesting that frail recipients are not only physically vulnerable but often experience comorbid depressive symptoms, which likely puts them on an accelerated path to adverse health outcomes.
We have extended previous findings of a 4%−16% co-occurrence of depressive symptoms and frailty among community-dwelling older adults to a surgical population of KT recipients and report a slightly lower prevalence.13,35–38 Additionally, the co-occurrence of depressive symptoms and frailty was observed in KT recipients of all ages, but was most common in those who were younger, female, and had less than a high school education. Importantly, the co-occurrence of depressive symptoms and frailty was associated with significantly increased risk of DCGF and mortality, but we did not observe a synergistic effect of the two conditions on these long-term outcomes potentially due to a lack of power.
Prior prospective studies have identified frailty as a predictor of longer LOS7; however, our study is the first to show a similar impact of pre-KT depressive symptoms on LOS. Our finding of a 1.38 times longer LOS among KT recipients with depressive symptoms is consistent with a previous report of a 1.25 times longer LOS among liver transplant recipients with pre-liver transplant depressive symptoms.39 This finding suggests a significant impact of pre-KT depressive symptoms on immediate perioperative outcomes. We also found that the presence of both frailty and depressive symptoms at KT admission synergistically increased LOS.
This study has several important strengths including prospective measurement of depressive symptoms as well as frailty ascertained at KT admission in a large multicenter cohort study. To assess depressive symptoms, we used the CES-D based on the screening tool’s validation for use in ESRD patients and ease of administration.29–32 The CES-D tool’s wide use also allows direct comparison with studies of the relationship between frailty and depressive symptoms among older adults and other chronic illness populations. To avoid measuring the same exposures, the overlapping exhaustion component between the CES-D and the Fried frailty phenotype was mitigated by omitting the two exhaustion questions from the total CES-D score.38 Additionally, the CES-D may have utility in clinical settings by allowing transplant centers to monitor psychological symptoms outside of a formal diagnosis of depression, as well as identify KT recipients with more mild depressive symptoms. However, our study does have some limitations. Although this study sample included over 700 KT recipients, insufficient power may have hindered our ability to detect all interactions of frailty and depressive symptom as well as some of the marginal effects (ie, for those with no depressive symptoms and frailty) with adverse post-KT outcomes. While we did not enroll all KT recipients at these centers, our response rate was approximately 65%. It is unlikely that there was a selection bias because the cohort was representative of the KT population at these centers, and there are no systematic differences in those who were enrolled and not enrolled that would distort the associations between depressive symptoms, frailty, and these adverse outcomes. Furthermore, participants reported their depressive symptoms in the prior 2 weeks at admission for KT and there may be recall bias at this time. Additionally, there was no information on the use of antidepressants in this cohort. Finally, the cross-sectional ascertainment of frailty and depressive symptoms prohibits examination of temporality between the onset of depressive symptoms and development of frailty.
In conclusion, the co-occurrence of depressive symptoms and frailty was associated with a synergistically longer length of hospital stay, as well as an increased risk of DCGF and mortality. However, the synergistic effect of frailty and depressive symptoms only impacts short-term outcomes like LOS; frailty and depressive are both, separately, associated with long-term outcomes but do not have a synergistic effect long term. Our study provides preliminary evidence for a critical need of pre-KT screening for both depressive symptoms and frailty to better identify KT candidates at higher risk for adverse post-KT outcomes. Given the elevated risk of adverse outcomes among frail KT recipients with depressive symptoms, consideration of the overlap of physical and mental health vulnerabilities may be an important aspect of care for this patient population. Depressive symptoms and frailty assessment at KT admission may have the potential to identify KT recipients at higher risk of adverse outcomes and may also provide further insights into the mechanisms leading to these adverse outcomes. Exploring interventions aimed at reducing the burden of both depressive symptoms and frailty, such as pre-habilitation programs, may be warranted in order to improve post-KT outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by NIH grants R01AG042504 (PI: Segev), R01AG055781 (PI: McAdams-DeMarco), R01DK114074 (PI: McAdams-DeMarco), K23DK097184 (PI: Crews), F32AG053025 (Haugen), and K24DK101828 (PI: Segev). Mara McAdams-DeMarco was also supported by the Johns Hopkins University Claude D. Pepper Older Americans Independence Center (P30AG021334) and the National Institute on Aging (K01AG043501).
Funding information
Claude Pepper Older Americans Independence Center, Johns Hopkins, Grant/Award Number: P30AG021334, National Institute on Aging, Grant/Award Number: F32AG053025, K01AG043501, R01AG042504 and R01AG055781·, National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: K23DK097184, K24DK101828 and R01DK114074; NIH
Footnotes
CONFLICT OF INTEREST
The authors of this manuscript declare no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sciw. 2001;56(3):M146–M157. [DOI] [PubMed] [Google Scholar]
- 2.McAdams-Demarco MA, Law A, Salter, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61(6):896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAdams-Demarco MA, Tan J, Salter ML, et al. Frailty and cognitive function in incident hemodialysis patients. Clin J Am Soc Nephrol. 2015;10(12):2181–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172(14):1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansen KL, Dalrymple LS, Delgado C, et al. Association between body composition and frailty among prevalent hemodialysis patients: a US renal data system special study. J Am Soc Nephrol. 2014;25(2):381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAdams-Demarco MA, Law A, King E, et al. Frailty and mortality in kidney transplant recipients. Am J Transplant. 2015;15(1):149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAdams-DeMarco MA, King EA, Luo X, et al. Frailty, length of stay, and mortality in kidney transplant recipients. Ann Surg. 2016;266(6):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garonzik-Wang JM, Govindan P, Grinnan JW, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. 2012;147(2):190–193. [DOI] [PubMed] [Google Scholar]
- 9.McAdams-DeMarco MA, Olorundare IO, Ying H, et al. Frailty and postkidney transplant health-related quality of life. Transplantation. 2018;102(2):291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty and early hospital readmission after kidney transplantation. Am J Transpl. 2013;13(8):2091–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAdams-DeMarco MA, Law A, Tan J, et al. Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation. 2015;99(4):805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAdams-DeMarco MA, Ying H, Olorundare I, et al. Individual frailty components and mortality in kidney transplant recipients. Transplantation. 2017;101(9):2126–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makizako H, Shimada H, Doi T, et al. Physical frailty predicts incident depressive symptoms in elderly people: prospective findings from the obu study of health promotion for the elderly. J Am Med Dir Assoc. 2015;16(3):194–199. [DOI] [PubMed] [Google Scholar]
- 14.Radloff LS. A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 15.Novak M, Molnar MZ, Szeifert L, et al. Depressive symptoms and mortality in patients after kidney transplantation: a prospective prevalent cohort study. Psychosom Med. 2010;72(6):527–534. [DOI] [PubMed] [Google Scholar]
- 16.Szeifert L, Molnar MZ, Ambrus C, et al. Symptoms of depression in kidney transplant recipients: a cross-sectional study. Am J Kidney Dis. 2010;55(1):132–140. [DOI] [PubMed] [Google Scholar]
- 17.Troen AM, Phil D, Scott T, et al. HHS Public Access. 2015;22(2):1–17. [Google Scholar]
- 18.Brown PJ, Rutherford BR, Yaffe K, et al. The depressed frail phenotype: the clinical manifestation of increased biological aging. Am J Geriatr Psychiatry. 2016;24(11):1084–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jindal RM, Neff RT, Abbott KC, et al. Association between depression and nonadherence in recipients of kidney transplants: analysis of the united states renal data system. Transplant Proc. 2009;41(9):3662–3666. [DOI] [PubMed] [Google Scholar]
- 20.Zelle DM, Dorland HF, Rosmalen JGM, et al. Impact of depression on long-term outcome after renal transplantation. Transplant J. 2012;94(10):1033–1040. [DOI] [PubMed] [Google Scholar]
- 21.Dobbels F, Skeans MA, Snyder JJ, Tuomari AV, Maclean JR, Kasiske BL. Depressive disorder in renal transplantation: an analysis of medicare claims. Am J Kidney Dis. 2008;51(5):819–828. [DOI] [PubMed] [Google Scholar]
- 22.Amanda Dew M, Rosenberger EM, Myaskovsky L, et al. Depression and anxiety as risk factors for morbidity and mortality after organ transplantation: a systematic review and meta-analysis 1 HHS public access. Transplantation. 2015;100:988–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemmelgarn BR, Manns BJ, Quan H, Ghali WA. Adapting the charlson comorbidity index for use in patients with ESRD. Am J Kidney Dis. 2003;42(1 SUPPL. 2):125–132. [DOI] [PubMed] [Google Scholar]
- 24.McAdams-DeMarco MA, Isaacs K, Darko L, et al. Changes in frailty after kidney transplantation. J Am Geriatr Soc. 2015;63(10):2152–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAdams-DeMarco A, Suresh S, Law A, et al. Frailty and falls among adult patients undergoing chronic hemodialysis: a prospective cohort study. BMC Nephrol. 2013;14:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAdams-DeMarco MA, Konel J, Warsame F, et al. Intradialytic cognitive and exercise training may preserve cognitive function. Kidney Int Reports. 2018;3(1):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nastasi AJ, McAdams-DeMarco MA, Schrack J, et al. Pre-kidney transplant lower extremity impairment and post-kidney transplant mortality. Am J Transplant. 2018;18(1):189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmussen SVP, Konel J, Warsame F, et al. Engaging clinicians and patients to assess and improve frailty measurement in adults with end stage renal disease. BMC Nephrol. 2018;19:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hedayati SS, Bosworth HB, Kuchibhatla M, Kimmel PL, Szczech LA. The predictive value of self-report scales compared with physician diagnosis of depression in hemodialysis patients. Kidney Int. 2006;69(9):1662–1668. [DOI] [PubMed] [Google Scholar]
- 30.Santos PR, Capote Júnior JRFG, Cavalcante Filho JRM, Ferreira TP, dos Santos Filho JNG, da Silva OS. Religious coping methods predict depression and quality of life among end-stage renal disease patients undergoing hemodialysis: a cross-sectional study. BMC Nephrol. 2017;18(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paterson TSE, O’Rourke N, Elmer E, Shapiro RJ, Thornton WL. The composition and structure of depressive symptomatology in renal disease. Can J Behav Sci. 2011;43(4):318–327. [Google Scholar]
- 32.Stanfill A, Hathaway D, Bloodworth R, Cashion A. A prospective study of depression and weight change after kidney transplant. Prog Transplant. 2016;26(1):70–74. [DOI] [PubMed] [Google Scholar]
- 33.Palmer S, Vecchio M, Craig JC, et al. Prevalence of depression in chronic kidney disease : systematic review and meta-analysis of observational studies. Kidney Int. 2013;84(1):179–191. [DOI] [PubMed] [Google Scholar]
- 34.Fan L, Sarnak MJ, Tighiouart H, Drew DA, Kantor AL. Depression and all-cause mortality in hemodialysis patients. Am J Nephrol. 2014;40(12):12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ni Mhaolain AM, Fan CW, Romero-Ortuno R, et al. Frailty, depression, and anxiety in later life. Int Psychogeriatr. 2012;24(8):1265–1274. [DOI] [PubMed] [Google Scholar]
- 36.Buigues C, Padilla-Sánchez C, Garrido JF, Navarro-Martínez R, Ruiz-Ros V, Cauli O. The relationship between depression and frailty syndrome: a systematic review. Aging Ment Health. 2015;19(9):762–772. [DOI] [PubMed] [Google Scholar]
- 37.St John PD, Tyas SL, Montgomery PR. Depressive symptoms and frailty. Int J Geriatr Psychiatry 2013;28(6):607–614. [DOI] [PubMed] [Google Scholar]
- 38.Feng L, Nyunt MSZ, Feng L, Yap KB, Ng TP. Frailty predicts new and persistent depressive symptoms among community-dwelling older adults: Findings from singapore longitudinal aging study. J Am Med Dir Assoc. 2014;15(1):76.e7–76.e12. [DOI] [PubMed] [Google Scholar]
- 39.Rogal SS, Mankaney G, Udawatta V, et al. Pre-transplant depression is associated with length of hospitalization, discharge disposition, and survival after liver transplantation. PLoS ONE. 2016;11(11):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.