Abstract
AIM:
To analyse the change in size on follow-up of hepatic adenomas (HAs) and adenomatosis, and to investigate the relationship of imaging features with size change.
MATERIALS AND METHODS:
The study included 44 patients (142 lesions) who underwent magnetic resonance imaging (MRI) or computed tomography (CT) for diagnosis and follow-up of HA. The imaging features and percentage change in maximum tumour dimension were observed over a follow-up duration of up to 139 months.
RESULTS:
With an average follow-up of 43 months, 37% lesions decreased in size, 58% were stable, 4% increased; one lesion regressed completely. Adenomas were stratified into size groups (<3, 3–5, and ≥5 cm). Size change among the three groups was similar (p>0.05). Percent size change was different for lesions followed for ≤12 months (−7.2%) compared with lesions followed for 13e60 months (−20.5%), and those followed for ≥60 months (−23.5%; p<0.05); there was no difference between lesions followed for 13–60 months and ≥60 months (p=0.523). Baseline size and percent size change was similar between the hepatocyte nuclear factor 1α-inactivated HA (HA-H) and inflammatory HA (HA-I) subtype (p>0.05).
CONCLUSION:
Most adenomas were either stable or regressed on follow-up. Size change was independent of baseline size. After an initial size decrease within 5 years, no further size reduction was noted on extended follow-up. The percent size change in the HA-H and HA-I subtype was similar.
Introduction
Hepatic adenoma (HA) is the third most common benign solid liver tumour after haemangioma and focal nodular hyperplasia. HA is usually found in young women aged between 20–40 years and increased prevalence is seen in women with a history of prolonged oral contraceptive (OCP) intake.1 The estimated incidence of HA is 3 per million per year and increases to 30–40 per million among long-term OCP users.2 Hepatic adenomatosis3 is defined by the presence of 10 or more HA involving both hepatic lobes. It is not associated with a specific HA subtype4 and its imaging features are similar to those of solitary HA.5
HA are often asymptomatic but can be associated with a risk of rupture, haemorrhage, and malignant transformation into hepatocellular carcinoma (HCC). The reported incidence of macroscopic haemorrhage and malignant transformation in adenomas <5 cm is 5% and 2%, respectively; the risk increases to 25% and 9%, respectively, for adenomas ≥5 cm.6 Hepatic adenomatosis can be seen in either sex with or without oral contraceptive use5; other risk factors for adenoma include glycogen storage disease and hepatic steatosis.7 In addition, adenoma formation may be associated with germ-line HNF1A (hepatocyte nuclear factor 1α) gene mutations.5,8,9 Risk of complication in both HA and adenomatosis is related to the size of the largest lesion and underlying histopathological subtype.10 As HA are associated with OCP use, they have been reported to regress or disappear after OCP withdrawal,2,11–15 although this may not be true for hepatic adenomatosis.16
With recent recognition of HA as a complex entity, there has been an increasing interest in categorising subtypes of adenomas. Based on the molecular characteristics, HA can be divided into four subtypes1: HNF1A-inactivated HA (HA-H),2 β-catenin-activated HA (HA-B),3 inflammatory HA (HA-I), and4 unclassified HA (HA-U).17 Studies have emphasised the importance of this histopathological classification of HA on the patient management.18–20 Based on studies21,22 suggesting that HA-B subtype may demonstrate a high risk of malignant transformation, authors have proposed that this subgroup be surgically resected regardless of its size at presentation.19 On the other hand, HA-H subtype demonstrates a much lower risk of malignant transformation in comparison to other subtypes. Therefore, surgical resection or even biopsy can be avoided in favour of imaging follow-up if this subtype can be recognised, particularly for small HAs (<5 cm) in women.19 For HA-I subtype, biopsy to rule out coexisting β-catenin mutation (that is associated with increased malignant potential) is suggested.19 Authors have also shown the successful role of MRI in this subtype characterisation of HA.23,24 About 30–35% of HA are of the HA-H subtype, and are characterised by steatosis within the lesion.17,19
Size is an important factor in the management of adenoma as it relates to risk of complications and malignant transformation; however, the natural history of adenoma size is not well described. As such, the objective of the current study was to analyse the change in size on follow-up of hepatic adenomas and adenomatosis and the relationship of imaging features with the size change.
Materials and methods
Patient population
This was a retrospective, institutional review-board approved, Health Insurance Portability and Accountability Act-compliant study with waived patient consent. Review of the medical records identified 44 patients (age range, 20–58 years; mean age, 41 years) between October 2004 and May 2016, including 27 patients with HA (nine patients with solitary HA; 18 patients with multiple HA) and 17 patients with hepatic adenomatosis. Among patients with more than five HA, up to five lesions with at least ≥1 month follow-up were included to reduce selection bias; thus a total of 142 lesions were evaluated. Pathological diagnosis along with histological subtype was established for 67 lesions in 21 patients. For remaining lesions, diagnosis was made by characteristic imaging findings. All patients underwent imaging with computed tomography (CT) and/or magnetic resonance imaging (MRI) for diagnoses and follow-up. Nineteen patients (n=61) underwent both MRI and CT, 22 patients (n=73) had MRI only, whereas three patients (n=8) had CT only. The protocol used for MRI and CT studies performed is shown in Table 1.
Table 1.
Imaging protocol.
| MRI parameters (Magnetom Avanto, Siemens 1.5 T) | T1WI | T2WI | Chemical T1WI shift | DWI |
|---|---|---|---|---|
| Repetition time (ms) | 5.77 | 4500 | 166 | 3000 |
| Echo time (ms) | 2.77 | 92 | 4.4/2.2 | 69 |
| Section thickness (mm) | 2.5 | 8 | 8 | 8 |
| Matrix size | 192×160 | 256×256 | 192×160 | 128×128 |
| Intersection gap (mm) | 2 | 2 | 2 | 2 |
| Receiver bandwidth (kHz) | 64 | 32 | 64 | 64 |
| b-Values (s/mm2) | NA | NA | NA | 0 & 750 |
| Contrast agent used | Gadopentate dimeglumine | NA | NA | NA |
| (intravenous) | (Magnevist, Bayer, NJ) | |||
| Dosage (mmol/kg) | 0.1 | NA | NA | NA |
| Phases | Hepatic arterial: 20 s | NA | NA | NA |
| Portal venous: 70 s | ||||
| Delayed: 3 min | ||||
| Field of view (mm) | 320–400 | 320–400 | 320–400 | 320–400 |
| CT parameters | Unenhanced CT (Sensation 64 scanner, Siemens) | Contrast enhanced CT | ||
| Peak tube voltage (kVp) | 120 | 120 | ||
| Tube current (mA) | 250 | 250 | ||
| Detector collimation (mm) | 0–6 | 0–6 | ||
| Section thickness (mm) | 5 | 5 | ||
| Interval (mm) | 5 | 5 | ||
| Agent used | NA | Visipaque 320 or Omnipaque 350 (iodinated contrast, GE healthcare, Waukesha WI, USA) | ||
| Dosage (ml) | NA | 100–120 | ||
| Phases | NA | Arterial: 25–30 s | ||
| Portal venous: 60 s | ||||
MRI, magnetic resonance imaging; WI, weighted imaging; DWI, diffusion-weighted image; NA, not applicable; CT, computed tomography.
Imaging analysis
MRI and CT images were analysed by one experienced abdominal radiologist with 7 years of experience in abdominal MRI. Each lesion was evaluated for signal intensity (SI) or density (relative to surrounding liver parenchyma), homogeneity, presence of capsule, and presence of fat or haemorrhage within the lesion and enhancement characteristics. All region of interest (ROI) measurements were performed using circular ROIs measuring at least 1 cm2 with the aim of covering maximum area of the lesion while avoiding peripheral 1 mm of the lesion to avoid volume averaging. Homogeneity was defined as uniform SI or density. Heterogeneity was defined as any difference in SI or density within the lesion on T1- or T2-weighted imaging (WI) or CT images. Capsule was defined as a thin low-SI or low density rim surrounding all or a part of the lesion. The dropout of signal on chemical-shift images or fat-suppression sequence was interpreted as intralesional fatty infiltration. Haemorrhage was defined as areas of high-SI on T1- and T2WI with or without low-SI on T2WI suggesting haemosiderin deposition, or hyper-density on unenhanced CT.
The longest dimension of the lesion was measured at baseline and follow-up images. All the lesion measurements were performed on contrast-enhanced images in the hepatic arterial or portal venous phase using CT/MRI T1W three-dimensional (3D) fat-suppressed spoiled gradient-cho images. As HAs demonstrate variable enhancement profiles, it was decided not to use the same enhancement phase images for all lesions. Rather, the arterial or portal phases were chosen by the reader on a case-by-case basis according to the phase that best allowed the lesion delineation. Percent change in tumour’s maximum dimension was calculated using the following formula:
An increase or decrease of ≥20% in the longest baseline diameter was considered as a change in lesion size.
Histopathological analysis
All histopathology slides were reviewed by one expert who determined adenoma subtype. Specimens were analysed for the presence of fat infiltration, haemorrhage, calcification as well as molecular and histological features such as arrangement of hepatocytes, portal veins, bile ducts, the presence of steatosis, inflammatory cell infiltrates, sinusoidal dilatation, and other relevant pathological characteristics.
Statistical analysis
To assess the relationship between change in size and lesion characteristics, lesions were divided into subgroups: <3 cm classified as group 1; 3–5 cm as group 2; and ≥5 cm as group 3. Similarly, based on fat infiltration, lesions were divided into lesions with versus without fat infiltration. Lesions were also divided according to the duration of follow-up: ≤12, 13–60, and ≥60 months. Based on histopathological subtypes, lesions were divided into HA-H and HA-I subtypes. Lastly, adenomas and adenomatosis were also compared for change in size measurements. A t-test was applied when comparing size changes in the different groups. A two-sample paired t-test was applied for comparison between baseline size and size on last imaging. Statistical analysis was performed with a software package (SPSS 21.0; SPSS, Chicago, IL, USA). A p-value of <0.05 was considered statistically significant.
Results
Demographic information (Table 2)
Table 2.
Demographic information.
| Age, mean (SD, range), years | ||
| All patients | 41 (10, 20–58) | |
| Sex, n (%) | ||
| Female | 43 (98) | |
| Male | 1 (2) | |
| Ethnicity, n (%) | ||
| White | 32 (73) | |
| African American | 10 (23) | |
| Asia | 2 (4) | |
| Diagnosis, n (%) | Patient | Lesion |
| Solitary adenoma | 9 (20) | 9 (6) |
| Multiple adenomas | 18 (41) | 52 (37) |
| Adenomatosis | 17 (39) | 81 (57) |
| Total number | 44 | 142 |
| Size | Patient (lesion) | Follow-up, mean (range), months |
| <3 cm | 28 (80) | 46 (1–139) |
| 3–5 cm | 17 (34) | 40 (1–108) |
| ≥5 cm | 18 (28) | 40 (1–108) |
| History of OCPs/hormone | Patient (lesion) | |
| Taking and discontinuation | 35 (122) | |
| Taking without termination | 1 (5) | |
| NA | 8 (15) | |
SD, standard deviation; OCP, oral contraceptive pills; NA, not applicable.
The sole male patient had type I glycogen storage disease. Twenty-seven patients were asymptomatic while 17 presented with abdominal pain.
Morphologic findings and global change in size
Morphological features of the lesions are shown in Table 3 and Figs 1–3. Size change is shown in Table 4. The mean size of the 142 lesions on baseline imaging was 3.2 cm (standard deviation [SD], 2.4; range, 0.4–13.4). After follow-up, 53 (37%) of 142 lesions regressed in size, 82 (58%) lesions were stable, and six (4%) lesions grew in size (Electronic Supplementary Material Fig. S1); one lesion disappeared on follow-up scans. For the lesions that demonstrated an increase in size, there were no associated changes in the imaging features/signal characteristics. One hundred and seventeen out of 142 lesions were followed up after patients discontinued using OCPs or terminated hormone therapy. Out of 53 lesions that had size regression, 51 (96%) were among patients who had discontinued OCP use. One lesion disappeared after discontinuing use of OCP. Similarly, out of 77 lesions that were stable, 60 (78%) were associated with discontinuation of OCP use and termination of hormone therapy. Out of six lesions that increased in size, five had history of OCP discontinuation.
Table 3.
Lesion characteristics (n).
| Bleeding (mean size=7.4 cm) | 8 (6) |
| Capsule | 36 (26) |
| Heterogeneousa | 15 (11) |
| Homogeneous | 127 (89) |
| Total number | 142 |
| Fat infiltration | 43 (32) |
| Total number | 134 |
| TI weighted | |
| Hyperintensity | 4 (3) |
| Iso-intensity | 118 (88) |
| Hypointensity | 12 (9) |
| Total number | 134 |
| T2 weighted | |
| Hyperintensity | 90 (67) |
| Iso-intensity | 37 (28) |
| Hypointensity | 7 (5) |
| Total number | 134 |
| Dynamic enhanced MRI | |
| Wash out | 70 (53) |
| Persistent enhancement | 49 (37) |
| No enhancement | 14 (10) |
| Total number | 133 |
| DW (b = 750 s/mm2) | |
| Hyperintensity | 66 (63) |
| Iso-intensity | 36 (34) |
| Hypointensity | 3 (3) |
| Total number | 105 |
Numbers in parentheses are percentages.
Heterogeneity was defined as any difference in SI or density within the lesion on T1WI or T2WI or CT images.
Figure 1.
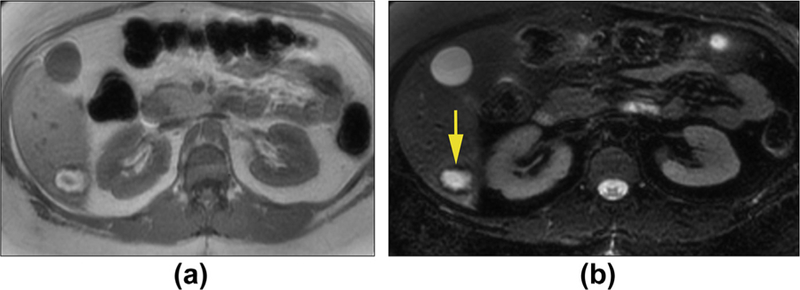
Hepatic adenoma with haemorrhage in a 39-year-old woman. Axial T1WI in-phase (a) and T2WI with fat suppression (b) show a mass that is hyperintense with low signal in the periphery. These findings are consistent with bleeding within the lesion and rim of haemosiderin deposition.
Figure 3.
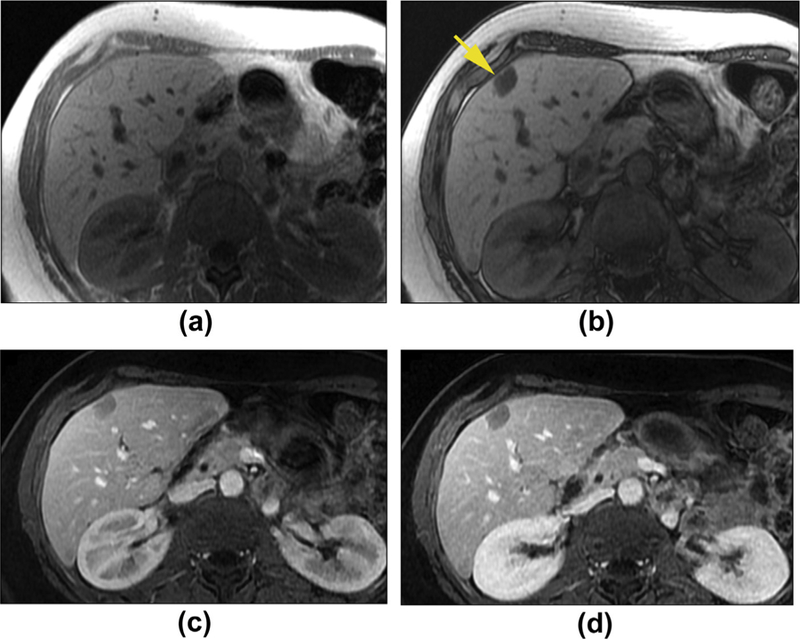
Hepatic adenoma with fat infiltration in a 49-year-old woman. Axial T1W in-phase (a) and out-phase (b) images show a mass with signal drop on out-phase image (arrow), which is consistent with a lipid-containing lesion. The capsule is noted as a rim of low signal intensity around the lesion on in-phase image (a). Axial enhanced T1WI in the hepatic late arterial phase (c) and delayed phase (d) shows the lesion to have minimal enhancement. Of note, the late arterial phase image acquisition might be the reason for the apparent lack of hypervascularity in this lesion.
Table 4.
Change in size over time in adenomas.
| Groups (cm) | Size at baseline (cm) | Total (n) | Size at follow-up (cm) | Mean size change (%) | Disappear (%) | Decrease (%) | Stable (%) | Increase (%) | Mean duration (months) |
|---|---|---|---|---|---|---|---|---|---|
| <3 cm | 1.6±0.7 (0.4–2.3) | 80 | 1.4±0.7 (0–4.7) | −12.3±27.8 (−100 to 79) | 1 | 24 (26.3) | 51 (63.8) | 4 (5) | 47±35 (1–139) |
| 3–5 cm | 3.7±0.7 (3–4.9) | 34 | 3±1.1 (1.4–6.8) | −18.1±30.8 (−64 to 94) | 0 | 19 (55.9) | 13 (38.2) | 2 (5.9) | 35±34 (1–108) |
| ≥5 cm | 7.7±2.2 (5.1–13.4) | 28 | 5.8±2.7 (1.9–13.4) | −24.4±24 (−70 to 5) | 0 | 10 (43.5) | 18 (64.2) | 0 (0) | 40±35 (1–108) |
| Global | 3.2±2.4 (0.4–13.4) | 142 | 2.5±2.1 (0–13.4) | −15.76±28.16 (−100 to 94) | 1 | 53 (37%) | 82 (58) | 6 (4) | 43±35 (1–139) |
Table 4 shows the size change characteristics in lesions based on baseline size groups. The follow-up duration among the three groups was not significantly different (p=0.239). Size change in the three groups did not differ (p=0.315 between group 1 and group 2, p=0.06 between group 1 and group 3 and p=0.337 between group 2 and group 3). One lesion in group 1 disappeared completely on follow-up scans.
Size change based on fat content, follow-up duration, and pathological subtypes
Table 5 shows the size change characteristics in lesion groups based on fat content. HA lesions with fat infiltration on imaging showed a significantly less decrease in size as compared to those without fat infiltration (−2.9±19.1% versus −24.1±26.9%; p≤0.0001), despite a similar follow-up duration (42±31.8 months versus 42.5±42.7 months; p=0.942). Among the 44 patients (n=142), the number of lesions with imaging follow-up of <12 months, between 13 and 60 months, and ≥60 months was 41, 67, and 34, respectively. Mean percent size change for the various follow-up duration categories were −7.2%, −20.5%, and −23.5%, respectively. The percent size change was different for lesions followed for ≤12 months (−7.2%) compared with lesions followed for 13–60 months (−20.5%), as well as those lesions followed for ≥60 months (−23.5%; p=0.005 and 0.003, respectively); there was no difference in percent size change among lesions followed for 13−60 months versus ≥60 months (p=0.523).
Table 5.
Size change based on fat content.
| Variables | With fatty infiltration | Without fatty infiltration | p-Value |
|---|---|---|---|
| Number of lesions (n) | 43 | 91 | NA |
| Size at baseline (cm) | 3.6±2.4 (0.5–11) | 2.6±2.5 (0.4–13.4) | NA |
| Size at follow-up (cm) | 2.6±2 (0–10.8) | 2.5±2.5 (0.4–13.4) | NA |
| Mean Size change (%) | −2.9±19.1 | −24.1±26.9 | ≤0.0001 |
| Mean Duration (months) | 42±31.8 (4–108) | 42.5±42.7 (1–139) | 0.942 |
Numbers in parentheses are range.
In 21 patients (n=67 lesions), diagnosis of adenoma along with histological subtype was established at histopathology after biopsy or surgery; 12 (n=35) patients had the HA-H subtype, eight (n=31 lesions) patients had the HA-I subtype, and one (n=1 lesion) patients had the HA-U subtype. Mean baseline size of 35 lesions with the HA-H subtype was 2.6 cm (SD, 2.7) with a mean follow-up duration of 63 months (SD, 43). The mean size of this subtype on last follow-up was 2.5 cm (SD, 2.8; mean size change −4.7%). Mean baseline size of 31 lesions under HA-I subtype was 3.1 cm (SD, 1.7). After a follow-up of 33 months (SD, 23), mean size was 2.4 cm (SD, 1.4; mean size change −17.1%). HA-H and HA-I subtype adenomas had comparable difference in their baseline size (p=0.32) and size change (p=0.11); however, the follow-up duration between the two subtypes was different (p=0.002).
Size change in adenomas versus adenomatosis
Mean size of the 61 adenomas was 3.1 cm (SD, 2.9) and decreased to 2.7 cm (SD, 2.7) after a mean follow-up duration of 38 months (SD, 4). Mean percent size change was −12.2%. Among the patients with the diagnosis of adenomatosis, mean size of the 81 lesions was 3.5 cm (SD, 2) and decreased to 2.4 cm (SD, 1.5), demonstrating 18.6% reduction after a mean follow-up of 46 months (SD, 35). The lesions in adenoma and adenomatosis group were comparable with regards to baseline size, follow-up duration and size change (p=0.885, 0.192, 0.191, respectively).
Other findings
Overall, eight (5.6%) of 142 lesions presented with imaging evidence of bleeding. The mean size of HA lesions with bleeding was 7.4 cm (SD, 3.3; range, 2.3–11.7 cm). Six (4%) of 142 lesions increased in size after follow-up, three of these six lesions were proven to be HA-H subtype and two were HA-I subtype. One patient (n=1) continued to be on oral contraceptives during follow-up. Four lesions in one patient resected after imaging evidence of bleeding demonstrated evidence of well-differentiated HCC arising within the HA (Electronic Supplementary Material Fig. S2). The size of these four HA lesions were 11.7, 11, 7.4, and 6.2 cm.
Discussion
This single institution study was undertaken to help suggest future guidelines regarding the frequency and duration of follow-up of patients with hepatic adenoma and adenomatosis. The results of the present study demonstrate that most HA lesions remained stable or decreased in size, likely secondary to OCP discontinuation. The findings of this study suggest that in non-surgical cases of HA at presentation, there may be a less need for frequent and extended imaging follow-up.
General morphological findings and correlation with histopathology
Haemorrhage in HA has been reported in 21–40% of cases25 with larger size (≥5 cm) and hormone intake was reported to be associated with higher risk of bleeding.25–28 The incidence of haemorrhage was much lower in the present study (n=8; 5.6%); all lesions were >5 cm. MRI images depict fat in 35–77% of adenomas10,15,29; in the current study fatty infiltration was noted in 43 (32%) of 134 lesions, comparable to that reported in the literature. Most adenomas that were heterogeneous in appearance in the current study were likely due to areas of haemorrhage or fatty infiltration. Erik et al.15 and Luigi et al.29 reported that 17% and 25% of HA had a capsule, similar to the current study (26%).
Malignant transformation of adenoma has been reported in about 5–10% of cases.18,27,30,31 In the present study, out of the six lesions that increased in size, five were proven at histopathology to be benign: three were HA-H subtype of adenoma and two were the HA-I subtype. One patient (n=1) continued on oral contraceptives during follow-up. Therefore, none of these six lesions appeared to show malignant transformation to HCC; however, four haemorrhagic lesions from a symptomatic patient underwent surgical resection and revealed well-differentiated HCC at histopathology. HA-B lesions have a higher risk of malignant transformation, but represent only 10–15% of HA.10 In the present study, 22 patients (n=67) had histopathological analysis for subtype of the lesions after biopsy or surgery; 35 lesions were HA-H, while 31 lesions were HA-I and one lesion was HA-U. Thus, among the lesions that were investigated at histopathology in the present study, HA-H and HA-I subtypes were more common, including the five lesions that demonstrated size increase on follow-up.
In the current series, most HA were isointense relative to liver on T1WI and moderately hyperintense on T2WI, conforming to previous reports.15,32,33 According to Luigi et al.,8 most HA were hyperintense to the liver on the arterial phase and became isointense on venous phase and delayed phase images. The lesions also appeared homogeneous in enhancement on venous and delayed phase images, with hyperintense capsule. In the present study, 51% of the lesions demonstrated washout on the venous and delayed phase imaging while 38% lesions showed persistent enhancement on these phases, similar to prior studies.8 Imaging findings depend on tumour characteristics and underlying liver parenchyma, and HA-H, HA-I type have some specific MRI patterns.34 The fat infiltration sets HA-H apart from the other HA subtypes, and patchy CD34 immunostaining in HA-H is indicative of sinusoidal capillarization, which is the reason for the signal dropout on opposed-phase, enhancement on arterial phase and washout in venous and delayed phase imaging. In contrast, HA-I subtype demonstrate arterial enhancement that persists on both venous and delayed phase imaging due to sinusoidal dilation.35–37 The present findings support these differences between HA-H and HA-I subtypes. Out of 43 adenomas with fat infiltration on MRI, 22 (51%) lesions were proven at histopathology to be HA-H subtype. Out of 65 lesions that had washout characteristic, 30 lesions had histopathological diagnosis with 23 (77%) demonstrating HA-H subtype. Out of 49 lesions that demonstrated persistent type enhancement characteristic, 24 lesions had histopathological diagnosis; all were proven to be HA-I subtype.
Global change in size
Overall, 53 (37%) of 142 lesions regressed in size, 77 (58%) lesions were stable while one lesion disappeared. Several case reports have described HA regression or disappearance after OCP withdrawal,11,13,38 was similar to the present observations. Ninety-six percent of the HA lesions that demonstrated size regression and the one lesion that disappeared on follow-up were among patients who had discontinued OCP use. Surgical resection is typically recommended for larger HAs (>5 cm). In the present study, baseline lesion size had no impact on the percent size change of HA; eight (29%) out of 28 lesions with baseline size >5 cm changed to the smaller size group, 19 were stable while only one lesion demonstrated increase in size; however, it is still necessary to follow-up HA >5 cm because of the increased risk of bleeding and rupture in large HAs.
Size change in fat containing versus non-fat-containing adenoma
Fat infiltration is one of the key characteristics of HA with 43 (30%) lesions demonstrating this feature. The present study indicated that fat infiltration may play an important role in size change of HA with lesions having fat infiltration demonstrating significantly less size decrease than those without fat infiltration (p≤0.0001). The reasons for this observation are not clear.
Size change based on follow-up duration
The present study demonstrated that the percent size change was different among lesions followed for up to 1 year versus lesions that were followed for >1 year, with most HA showing stability during the first year of follow-up while demonstrating decrease in size after 1 year. On the other hand, there was no difference in size change among lesions followed for 1–5 years versus those that were followed for >5 years. This may suggest that if the HA remains stable or decreases in size during 5 years of follow-up after discontinuing OCPs, it may continue to do so in the future; however, this needs to be confirmed on further studies with longer follow-up durations.
Size change in HA-H subtype versus HA-I subtype
Fat infiltration is a characteristic of HA-H subtype and as the present results show that fat infiltration was associated with slower regression of lesions, HA-H subtype adenomas would seem to regress more slowly than other subtypes; however, in the present study, the size change was not significantly different between HA-H and HA-I subtypes. This may be related to different follow-up durations in the two subtypes. The HA-I subtype lesions were observed for a mean duration of 33 months (SD, 23) with a mean size change of −17.1%. The HA-H subtype lesions were followed for a mean duration of 63 months (SD, 43), with a mean size change of −4.7%. If both subtypes were followed for a similar duration, it is possible that more significant differences in size changes could have been observed.
Size change in adenomas versus adenomatosis
According to some studies, hepatic adenomatosis does not have a predilection for female gender5,8; however, among the 17 adenomatosis patients included in the current study, only one patient was male. In addition, according to some previous literature, hepatic adenomatosis has been described to be less often associated with steroid or oral contraceptive use; however, in the present study, out of 81 adenomatosis lesions, 76 lesions were associated with history of oral contraceptive intake. In the present series, 71 out of these 76 adenomatosis lesions were associated with discontinuation of OCP intake and 31 (43.6%) of these lesions decreased in size. In addition, no significant difference in size change was observed between adenomas and adenomatosis.
The present study has limitations. First, this is a single centre retrospective study. Second, histopathology confirmation was not available for all the HA lesions; however, HA is a benign disease with typical imaging features and a biopsy may not be clinically indicated. Last, although the present study cohort was followed up over a mean duration of 43 months, there were three lesions with a relatively short follow-up of 1 month. The small follow-up duration may make a clinically relevant conclusion difficult for these few lesions.
In conclusion, most non-surgical adenomas were either stable or regressed in size on average follow-up of 43 months suggesting a reduced need for extended or frequent imaging follow-up in these patients. Additionally, size change was independent of baseline HA size, but related to fat infiltration within the HA lesion. After an initial decrease in size within 5 years of HA detection, no further size reduction was noted on extended follow-up. Finally, the percent size change in the HA-H subtype and HA-I subtype was not different. Future studies with larger and possibly multi-institutional databases may be needed to confirm the present findings in order to help fulfil the need for definitive guidelines regarding the imaging follow-up of hepatic adenoma and adenomatosis.
Supplementary Material
Figure 2.
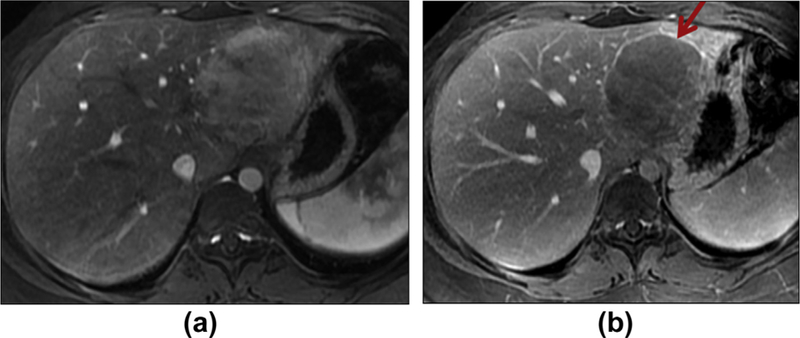
Hepatic adenoma in a 20-year-old woman. Axial enhanced T1WI in the hepatic arterial phase (a) shows an enhancing mass. Delayed phase of enhancement (b) shows washout and rim-like enhancement (arrow).
Acknowledgements
The authors thank Sepideh Besharati, Department of Pathology, Johns Hopkins Medical Institutions, for providing help with the histopathological correlation for the manuscript.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.crad.2018.06.015.
References
- 1.De Kock I, Mortele KJ, Smet B, et al. Hepatic adenomatosis: MRI imaging features. JBR-BTR 2014;97(2):105–8. [DOI] [PubMed] [Google Scholar]
- 2.Rooks JB, Ory HW, Ishak KG, et al. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA 1979;242(7):644–8. [PubMed] [Google Scholar]
- 3.Flejou J-F, Barge J, Menu Y, et al. Liver adenomatosis: an entity distinct from liver adenoma? Gastroenterology 1985;89(5):1132–8. [PubMed] [Google Scholar]
- 4.Frulio N, Chiche L, Bioulac-Sage P, et al. Hepatocellular adenomatosis: what should the term stand for? Clin Res Hepatol Gastroenterol 2014;38(2):132–6. [DOI] [PubMed] [Google Scholar]
- 5.Raman SP, Hruban RH, Fishman EK. Hepatic adenomatosis: spectrum of imaging findings. Abdom Imag 2013;38(3):474–81. [DOI] [PubMed] [Google Scholar]
- 6.Chun YS, Parker RJ, Inampudi S, et al. Imaging surveillance of hypervascular liver lesions in non-cirrhotic patients. J Gastrointest Surg 2016;20(3):564–7. [DOI] [PubMed] [Google Scholar]
- 7.Furlan A, van der Windt DJ, Nalesnik MA, et al. Multiple hepatic adenomas associated with liver steatosis at CT and MRI: a caseecontrol study. AJR Am J Roentgenol 2008;191(5):1430–5. [DOI] [PubMed] [Google Scholar]
- 8.Grazioli L, Federle MP, Ichikawa T, et al. Liver adenomatosis: clinical, histopathologic, and imaging findings in 15 patients. Radiology 2000;216(2):395–402. [DOI] [PubMed] [Google Scholar]
- 9.Ulu EM, Uyusur A, Ekici Y, et al. Multidetector CT findings of spontaneous rupture of hepatic adenoma in a patient with hepatic adenomatosis. Diagn Interv Radiol 2009;15(2):135–8. [PubMed] [Google Scholar]
- 10.Thapar M, Grapp O, Fisher C. Management of hepatic adenomatosis. Curr Gastroenterol Rep 2015;17(3):12. [DOI] [PubMed] [Google Scholar]
- 11.Buhler H, Pirovino M, Akobiantz A, et al. Regression of liver cell adenoma. A follow-up study of three consecutive patients after discontinuation of oral contraceptive use. Gastroenterology 1982;82(4):775–82. [PubMed] [Google Scholar]
- 12.Kawakatsu M, Vilgrain V, Erlinger S, et al. Disappearance of liver cell adenoma: CT and MRI imaging. Abdom Imag 1997;22(3):274–6. [DOI] [PubMed] [Google Scholar]
- 13.Aseni P, Sansalone CV, Sammartino C, et al. Rapid disappearance of hepatic adenoma after contraceptive withdrawal. J Clin Gastroenterol 2001;33(3):234–6. [DOI] [PubMed] [Google Scholar]
- 14.Arrive L, Flejou JF, Vilgrain V, et al. Hepatic adenoma: MRI findings in 51 pathologically proved lesions. Radiology 1994;193(2):507–12. [DOI] [PubMed] [Google Scholar]
- 15.Paulson EK, McClellan JS, Washington K, et al. Hepatic adenoma: MRI characteristics and correlation with pathological findings. AJR Am J Roentgenol 1994;163(1):113–6. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro A, Burgart LJ, Nagorney DM, et al. Management of liver adenomatosis: results with a conservative surgical approach. Liver Transpl Surg 1998;4(5):388–98. [DOI] [PubMed] [Google Scholar]
- 17.Zu cman-Rossi J, Jeannot E, Nhieu JT, et al. Genotypeephenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology 2006;43(3):515–24. [DOI] [PubMed] [Google Scholar]
- 18.Bioulac-Sage P, Laumonier H, Couchy G, et al. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology 2009;50(2):481–9. [DOI] [PubMed] [Google Scholar]
- 19.Nault JC, Bioulac-Sage P, Zu cman-Rossi J. Hepatocellular benign tumorsfrom molecular classification to personalized clinical care. Gastroenterology 2013;144(5):888–902. [DOI] [PubMed] [Google Scholar]
- 20.Katabathina VS, Menias CO, Shanbhogue AK, et al. Genetics and imaging of hepatocellular adenomas: 2011 update. RadioGraphics 2011;31(6):1529–43. [DOI] [PubMed] [Google Scholar]
- 21.Evason KJ, Grenert JP, Ferrell LD, et al. Atypical hepatocellular adenoma-like neoplasms with beta-catenin activation show cytogenetic alterations similar to well-differentiated hepatocellular carcinomas. Hum Pathol 2013;44(5):750–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farges O, Ferreira N, Dokmak S, et al. Changing trends in malignant transformation of hepatocellular adenoma. Gut 2011;60(1):85–9. [DOI] [PubMed] [Google Scholar]
- 23.Ronot M, Bahrami S, Calderaro J, et al. Hepatocellular adenomas: accuracy of magnetic resonance imaging and liver biopsy in subtype classification. Hepatology 2011;53(4):1182–91. [DOI] [PubMed] [Google Scholar]
- 24.van Aalten SM, Thomeer MG, Terkivatan T, et al. Hepatocellular adenomas: correlation of MRI imaging findings with pathological subtype classification. Radiology 2011;261(1):172–81. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal S, Agarwal S, Arnason T, et al. Management of hepatocellular adenoma: recent advances. Clin Gastroenterol Hepatol 2015;13(7):1221–30. [DOI] [PubMed] [Google Scholar]
- 26.Bioulac-Sage P, Rebouissou S, Thomas C, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology 2007;46(3):740–8. [DOI] [PubMed] [Google Scholar]
- 27.Cho SW, Marsh JW, Steel J, et al. Surgical management of hepatocellular adenoma: take it or leave it? Ann Surg Oncol 2008;15(10):2795–803. [DOI] [PubMed] [Google Scholar]
- 28.Deneve JL, Pawlik TM, Cunningham S, et al. Liver cell adenoma: a multicenter analysis of risk factors for rupture and malignancy. Ann Surg Oncol 2009;16(3):640–8. [DOI] [PubMed] [Google Scholar]
- 29.Grazioli L, Federle MP, Brancatelli G, et al. Hepatic adenomas: imaging and pathological findings. RadioGraphics 2001;21(4):877–92. discussion 92–94. [DOI] [PubMed] [Google Scholar]
- 30.Foster JH, Berman MM. The malignant transformation of liver cell adenomas. Arch Surg 1994;129(7):712–7. [DOI] [PubMed] [Google Scholar]
- 31.Barthelmes L, Tait IS. Liver cell adenoma and liver cell adenomatosis. HPB (Oxford) 2005;7(3):186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rummeny E, Weissleder R, Stark DD, et al. Primary liver tumors: diagnosis by MRI imaging. AJR Am J Roentgenol 1989;152(1):63–72. [DOI] [PubMed] [Google Scholar]
- 33.Nokes SR, Baker ME, Spritzer CE, et al. Hepatic adenoma: MRI appearance mimicking focal nodular hyperplasia. J Comput Assist Tomogr 1988;12(5):885–7. [PubMed] [Google Scholar]
- 34.Laumonier H, Bioulac-Sage P, Laurent C, et al. Hepatocellular adenomas: magnetic resonance imaging features as a function of molecular pathological classification. Hepatology 2008;48(3):808–18. [DOI] [PubMed] [Google Scholar]
- 35.Dhingra S, Fiel MI. Update on the new classification of hepatic adenomas: clinical, molecular, and pathological characteristics. Arch Pathol Lab Med 2014;138(8):1090–7. [DOI] [PubMed] [Google Scholar]
- 36.Cristiano A, Dietrich A, Spina JC, et al. Focal nodular hyperplasia and hepatic adenoma: current diagnosis and management. Updates Surg 2014;66(1):9–21. [DOI] [PubMed] [Google Scholar]
- 37.Shanbhogue A, Shah SN, Zaheer A, et al. Hepatocellular adenomas: current update on genetics, taxonomy, and management. J Comput Assist Tomogr 2011;35(2):159–66. [DOI] [PubMed] [Google Scholar]
- 38.Steinbrecher UP, Lisbona R, Huang SN, et al. Complete regression of hepatocellular adenoma after withdrawal of oral contraceptives. Dig Dis Sci 1981;26(11):1045–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


