Abstract
Achieving transplant tolerance remains the ultimate goal in the field of organ transplantation. We demonstrated previously that ablation of the transcription factor interferon regulatory factor 4 (IRF4) in T cells induced heart transplant acceptance by driving allogeneic CD4+ T cell dysfunction. Herein, we showed that heart‐transplanted mice with T cell‐specific IRF4 deletion were tolerant to donor‐specific antigens and accepted the subsequently transplanted donor‐type but not third‐party skin allografts. Moreover, despite the rejection of the primary heart grafts in T cell-specific Irf4 knockout mice under immune checkpoint blockade, the establishment of donor‐specific tolerance in these mice was unhindered. By tracking alloantigen‐specific CD4+ T cells in vivo, we revealed that checkpoint blockade restored the expression levels of the majority of wild‐type T cell‐expressed genes in Irf4‐deficient T cells on day 6 post‐heart grafting, indicating the initial reinvigoration of Irf4‐deficient T cells. Nevertheless, checkpoint blockade did not restore cell frequency, effector memory cell generation, and IFN‐γ/TNF‐α production of Irf4−/− alloreactive T cells at day 30 post‐heart grafting. Hence, targeting IRF4 represents a potential therapeutic strategy for driving intrinsic T cell dysfunction and achieving alloantigen‐specific transplant tolerance.
Keywords: animal models: murine, basic (laboratory) research/science; cellular biology; graft survival; immunobiology; organ transplantation in general; T cell biology; tolerance
1 |. INTRODUCTION
Organ transplantation is a life‐saving treatment for patients with end‐stage organ failure, but long‐term graft survival is limited by immune rejection and side effects of immunosuppressive drugs.1 Allogeneic T cell response plays a decisive role in transplant rejection. The activation signals through T cell receptor (TCR), along with costimulation and cytokine inputs, mediate T cell response to organ transplants.2–4 Hence, for more effective control of transplant immunity, it is essential to define the key regulators that link the TCR and other signals to the functional fates of alloreactive T cells.
Interferon regulatory factor 4 (IRF4) is a member of the IRF family of transcription factors. IRF4 is preferentially expressed in hematopoietic cells and plays critical roles in the differentiation and function of T cells, B cells and dendritic cells.5–7 We found that IRF4 was promptly induced in T cells upon TCR signaling through the MEK1/2 pathway. IRF4 ablation in T cells resulted in progressive establishment of CD4+ T cell dysfunction and induced heart allograft acceptance in mice. The dysfunctional state of Irf4−/− T cells was initially reversible by blocking programmed death‐ligand 1 (PD‐L1) and cytotoxic T‐lymphocyte‐associated protein 4 (CTLA‐4), but it progressively developed into a terminal/irreversible dysfunctional state.8 Therefore, the TCR‐IRF4 axis governs the functional versus dysfunctional fates of alloreactive T cells.
Recent reports showed that in patients with stable function of transplanted kidneys but also developed metastatic cancer, programmed cell death protein 1 (PD‐1) blockade was used to treat cancer. Unfortunately, PD‐1 blockade triggered acute rejection of the transplanted kidneys.9,10 Therefore, irreversible dysfunction of alloreactive T cells was not established in those kidney recipients, though they were under chronic immunosuppression. Low dose of FK506, the mainstay immunosuppressant, has even been shown to inhibit the terminal differentiation of T cell dysfunction.11 Hence, in the context of achieving stable transplant tolerance, it would be essential to define the irreversible state of T cell dysfunction.
Herein, we investigated the irreversible dysfunctional state of Irf4−/− T cells. Irf4fl/flCd4‐Cre heart recipients were treated with checkpoint blockade on days 0, 3, and 5, and transplanted again with donor‐type skin grafts on day 30 post‐heat grafting. All heart allografts were acutely rejected due to checkpoint blockade‐mediated reinvigoration of Irf4−/− T cells.8 Strikingly, those Irf4fl/flCd4‐Cre recipients with rejected heart allografts still permanently accepted the subsequent donor‐type skin grafts, indicating that checkpoint blockade‐reinvigorated alloreactive Irf4−/− T cells become re‐dysfunction within 30 days. Indeed, initial checkpoint blockade did not restore cell frequency, effector memory cell generation, and IFN‐γ/TNF‐α production of alloreactive Irf4−/− T cells at day 30 post‐heart grafting. Taken together, ablation of IRF4 drives terminal T cell dysfunction, and targeting IRF4 represents a potential therapeutic strategy for achieving transplant tolerance.
2 |. MATERIALS AND METHODS
2.1 |. Mice
Cd4‐Cre, Irf4flox/flox, B6.SJL CD45.1 congenic, TEa TCR transgenic,8 BALB/c, and C57BL/6 (B6) mice were purchased from Jackson Laboratory (Bar Harbor, MA). Irf4flox/flox mice were crossed to Cd4‐Cre mice to create Irf4fl/flCd4‐Cre mice. TEa mice were crossed to Irf4−/− mice to create Irf4−/− TEa mice. TEa mice were crossed to CD45.1 congenic mice to create (TEa × CD45.1) F1 mice, in which leucocytes are CD45.1+CD45.2+. All animal experiments in this study were approved by the Houston Methodist Animal Care Committee in accordance with institutional animal care and use guidelines.
2.2 |. Murine heterotopic heart transplantation
Hearts from BALB/c donors were transplanted into 8‐10‐week‐old male Irf4fl/flCd4‐Cre or CD45.1+ congenic mice by a previously described method.8 Some recipient mice were ip injected with 400 μg Rat IgG, or 200 μg anti‐PD‐L1 (clone 10F.9G2) plus 200 μg anti-CTLA‐4 (clone 9D9) mAbs (Bio‐X‐Cell, West Lebanon, NH) on days 0, 3, 5 post‐heart grafting.
2.3 |. Murine skin transplantation
BALB/c and C3H ear skin allografts were transplanted onto BALB/c heart transplanted Irf4fl/flCd4‐Cre mice by a previously described method.12 More than 80% necrosis of the donor skin tissue was considered as rejection.
2.4 |. Irf4 transduction and adoptive transfer of Irf4−/− CD4+ T cells
BALB/c splenic dendritic cells (DCs) were isolated by using the Pan Dendritic Cell Isolation Kit (Miltenyi Biotec, San Diego, CA). Irf4−/− CD4+ T cells were activated for 3 days with BALB/c splenic DCs and 100 IU IL‐2 (PeproTech, Rocky Hill, NJ), and then transduced with IRF4‐GFP or GFP‐Ctrl retrovirus as previously described.8 Cells were cultured for 1 day after transduction, and then adoptively transferred into Irf4 fl/flCd4‐Cre mice on day 1 post‐heart transplantation.
2.5 |. Adoptive transfer of TEa cells and microarray analysis
Microarray was performed by the Genomic and RNA Profiling Core at Baylor College of Medicine and data generated has been deposited in NCBI’s Gene Expression Omnibus with accession number GSE111757. TCR(Vα2+Vβ6+)CD45.2+CD4+ TEa cells were isolated from splenocytes of WT TEa or Irf4−/− TEa mice by a FACSAria flow cytometer (BD Biosciences, San Jose, CA). B6.SJL CD45.1+ congenic mice were adoptively transferred with either 5 × 106 CD45.2+ WT TEa or 5 × 106 CD45.2+ Irf4−/− TEa cells on day ‐1, and transplanted with BALB/c hearts on day 0. CD45.1+ mice transferred with CD45.2+ Irf4−/− TEa cells were ip injected with either 400 μg Rat IgG or 200 μg anti‐PD‐L1 plus 200 μg anti‐CTLA‐4 mAbs on days 0, 3, 5. On day 6, adoptively transferred CD45.2+ TEa cells were sorted for microarray analysis, using a previously described method.8
2.6 |. Tracking of adoptively transferred TEa cells
CD45.1+ congenic mice were adoptively transferred with mixed splenocytes containing 7.5 × 106 CD45.1+CD45.2+ WT TEa cells (from [TEa × CD45.1]F1 mice) and 7.5 × 106 CD45.2+ Irf4−/− TEa cells (from Irf4−/− TEa mice) on day ‐1, and transplanted with BALB/c hearts on day 0. Some CD45.1+ recipient mice were also ip injected with 200 μg anti‐PD‐L1 plus 200 μg anti‐CTLA‐4 mAbs on days 0, 3, 5. TEa cells in peripheral blood and spleens of transplant recipients were analyzed on the LSRFortessa flow cytometer (Beckton Dickinson, Franklin Lakes, NJ). Fluorochrome‐conjugated antibodies were purchased from BioLegend (San Diego, CA) or Thermo Fisher Scientific (Waltham, MA). Zombie Aqua Fixable Viability Kit was purchased from BioLegend. Intracellular staining method was previously described.8
2.7 |. Statistical analysis
Data were represented as mean ± SD and analyzed with Prism version 7.0a (GraphPad Software, San Diego, CA). The P values of skin graft survival were determined by the Mann‐Whitney test. Other measurements were performed using unpaired Student’s t test. Differences were considered significant when P < .05.
3 |. RESULTS
3.1 |. Ablation of IRF4 in T cells abrogates their ability to reject donor‐type skin grafts in heart transplanted recipients
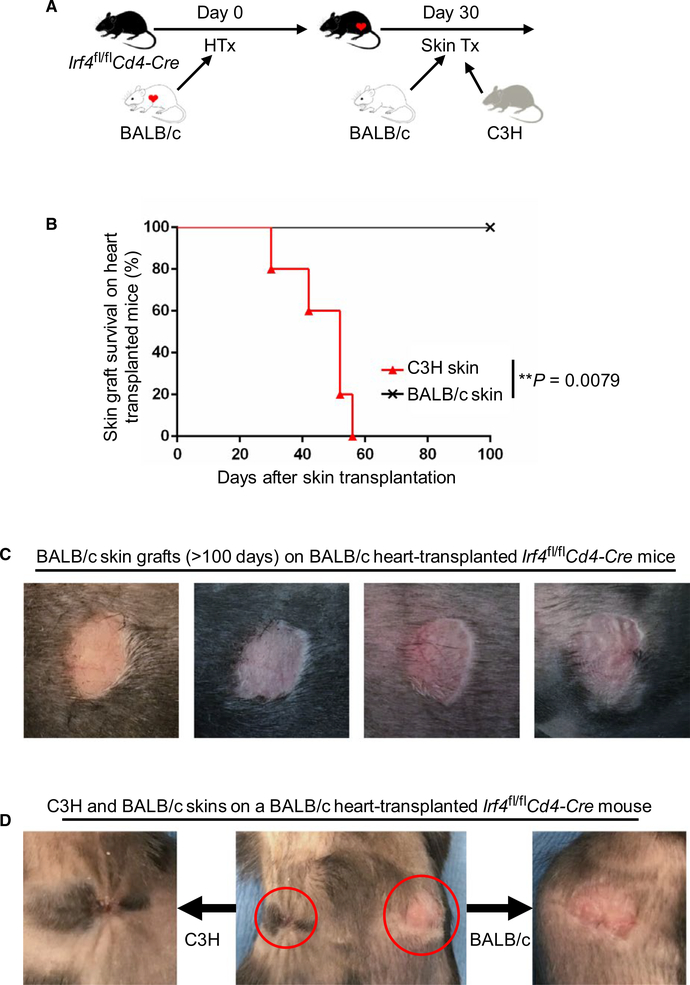
We have previously shown that alloreactive T cell dysfunction was achieved in Irf4fl/flCd4‐Cre mice after heart transplantation.8 To determine whether induced T cell dysfunction affects the survival of subsequently transplanted skin allografts, Irf4fl/flCd4‐Cre recipients were first transplanted with BALB/c hearts and then transplanted with BALB/c and C3H skin allografts 30 days later. All heart allografts were permanently accepted as previously described.8 Importantly, none of the heart‐transplanted Irf4fl/flCd4‐Cre mice rejected the subsequently transplanted BALB/c skins (mean survival time [MST] of >100 days; n = 5) (Figure 1). By contrast, all C3H skins were rejected within 60 days (MST = 46.4 ± 10.53 days; n = 5) (Figure 1). Irf4fl/flCd4‐Cre mice without BALB/c heart transplantation were also capable of rejecting BALB/c skin grafts (MST = 31.0 ± 8.16 days; n = 4) (data not shown). Hence, ablation of IRF4 in T cells abrogated their ability to reject heart allografts and subsequently transplanted donor‐type skins.
FIGURE 1.
IRF4 deletion in T cells induces transplant tolerance in heart graft recipients. Irf4fl/flCd4‐Cre mice were transplanted with BALB/c hearts. Thirty days later, recipients were transplanted again with BALB/c and C3H skins (n = 5). A, Schematic of the experimental design. B, The percentage of skin allograft survival after skin transplantation on BALB/c heart‐transplanted Irf4fl/flCd4‐Cre recipients. **P = .0079; Mann‐Whitney test. C, Representative images of accepted BALB/c skin allografts (>100 days) on BALB/c heart‐transplanted Irf4fl/flCd4‐Cre recipients. D, A representative image of C3H (left) and BALB/c (right) skin allografts on a BALB/c heart‐transplanted Irf4fl/flCd4‐Cre recipient
3.2 |. Adoptive transfer of IRF4 re‐introduced Irf4−/− CD4+ T cells inhibits the induction of transplant tolerance in mice with T cell‐specific IRF4 deletion
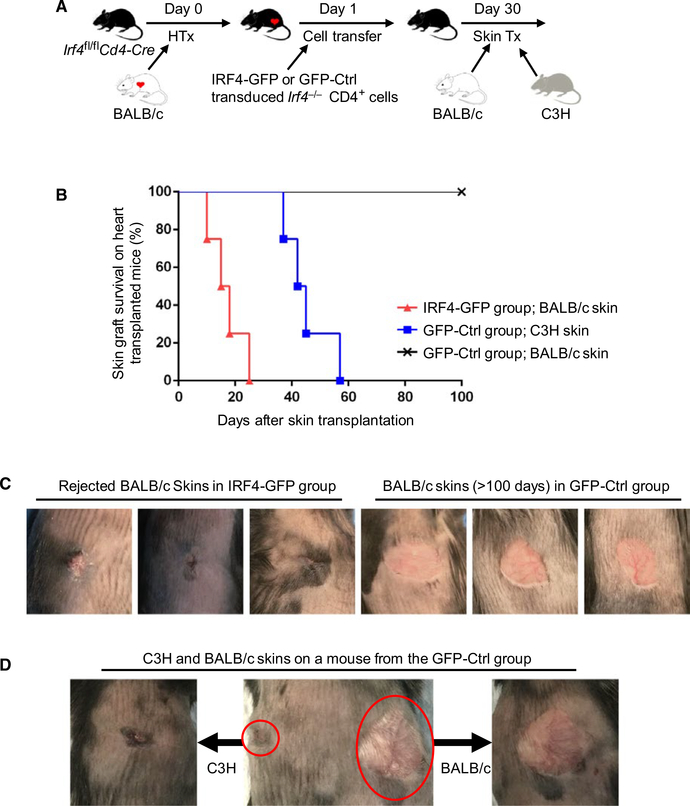
One approach that has been applied in restoring heart transplant rejection in Irf4fl/flCd4‐Cre mice was to transfer IRF4 re‐introduced Irf4−/− CD4+ T cells. Irf4−/− CD4+ T cells were stimulated in vitro with allogenic BALB/c splenic DCs and IL‐2 for 3 days, followed by transduction with IRF4‐GFP or GFP‐Ctrl retrovirus for 1 day. Irf4fl/flCd4‐Cre recipients injected with one million IRF4 re‐introduced, but not GFP‐Ctrl transduced, Irf4−/− CD4+ T cells acutely rejected heart allografts within 6 days, as previously described.8 Recipient mice were transplanted again with skin allografts 30 days after heart grafting. As shown in Figure 2, BALB/c skins were acutely rejected on heart‐transplanted recipients that were transferred with IRF4 re‐introduced Irf4−/− CD4+ T cells (IRF4‐GFP cell transfer group; BALB/c skin; MST = 17.0 ± 6.27 days; n = 4), but were accepted on heart‐transplanted recipients that were transferred with GFP‐Ctrl transduced Irf4−/− CD4+ T cells (GFP‐Ctrl cell transfer group; BALB/c skin; MST of >100 days; n = 4) (Figure 2). C3H skins were rejected within 60 days on heart‐transplanted recipients that were transferred with GFP‐Ctrl transduced Irf4−/− CD4+ T cells (GFP‐Ctrl cell transfer group; C3H skin; MST = 45.3 ± 8.50 days; n = 4) (Figure 2). Therefore, adoptive transfer of IRF4 re‐introduced, but not GFP‐Ctrl transduced, Irf4−/− CD4+ T cells inhibit the induction of transplant tolerance in heart‐transplanted Irf4fl/flCd4‐Cre recipients.
FIGURE 2.
Adoptive transfer of IRF4 re‐introduced Irf4−/− CD4+ T cells breaks transplant tolerance in Irf4‐deficient mice. Irf4−/− CD4+ T cells were stimulated with allogenic BALB/c splenic DCs and IL‐2 for 3 days, followed by transduction with IRF4‐GFP or GFP‐Ctrl retrovirus for 1 day. Irf4fl/flCd4‐Cre mice were transplanted with BALB/c hearts on day 0 and adoptively transferred with one million IRF4‐GFP or GFP‐Ctrl transduced Irf4−/− CD4+ T cells on day 1. Thirty days later, recipient mice in the IRF4‐GFP group were transplanted again with BALB/c skins (n = 4), whereas recipients in the GFP‐Ctrl group were transplanted with both C3H and BALB/c skins (n = 4). A, Schematic of the experimental design. B, The percentage of skin allograft survival after skin transplantation on BALB/c heart‐transplanted Irf4fl/flCd4‐Cre recipients that had been adoptively transferred with IRF4‐GFP or GFP‐Ctrl transduced Irf4−/− CD4+ T cells. C, Representative images of rejected (left 3 panels) and accepted (right 3 panels) BALB/c skins on BALB/c heart transplanted Irf4 fl/flCd4‐Cre recipients that had been adoptively transferred with IRF4‐GFP and GFP‐Ctrl transduced Irf4−/− CD4+ T cells, respectively. D, A representative image of C3H (left) and BALB/c (right) skin allografts on a BALB/c heart‐transplanted Irf4 fl/flCd4‐Cre mouse that had been adoptively transferred with GFP‐Ctrl transduced Irf4−/− CD4+ T cells
3.3 |. Immune checkpoint blockade induces heart transplant rejection but does not prevent the later establishment of transplant tolerance in mice with T cell‐specific IRF4 deletion
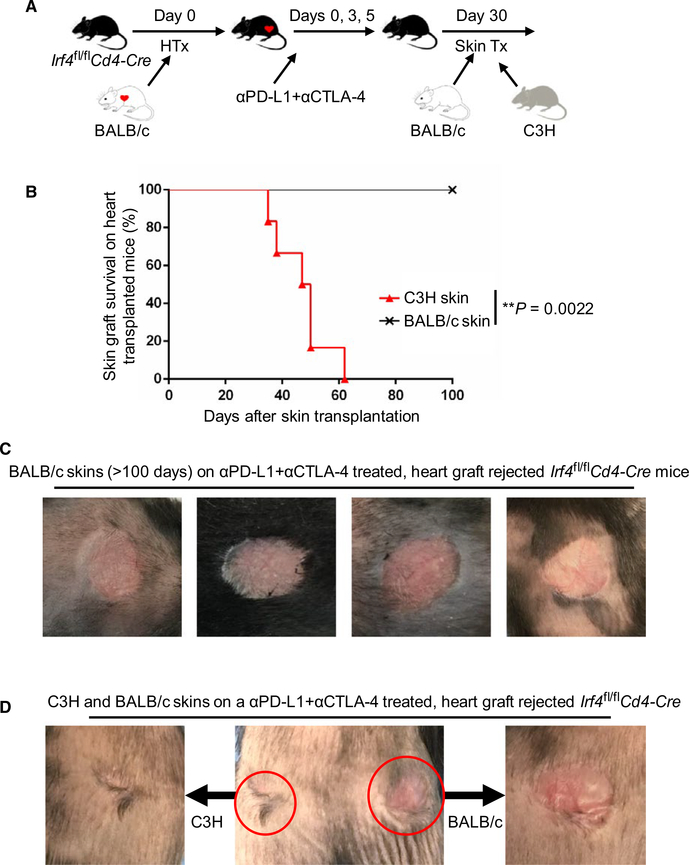
Checkpoint blockade restored acute heart transplant rejection in Irf4fl/flCd4‐Cre mice8. Herein we investigated the influence of initial checkpoint blockade‐mediated heart allograft rejection on the survival of subsequently transplanted skins. We transplanted BALB/c hearts into Irf4fl/flCd4‐Cre mice and treated them with anti‐PD‐L1 and anti‐CTLA‐4 mAbs on days 0, 3, and 5 post‐heart grafting. All heart allografts were acutely rejected within 8 days as previously described.8 Recipients with rejected heart allografts were then transplanted again with skin allografts 30 days after heart grafting. Strikingly, all BALB/c skins were accepted on those recipients (MST of >100 days; n = 6), while all C3H skins were rejected within 60 days (MST = 47.0 ± 9.67 days; n = 6) (Figure 3). Therefore, checkpoint blockade induced acute heart transplant rejection in Irf4fl/flCd4‐Cre recipients, but did not prevent the later establishment of transplant tolerance.
FIGURE 3.
Checkpoint blockade does not prevent the establishment of transplant tolerance in Irf4‐deficient mice. Irf4fl/flCd4‐Cre mice were transplanted with BALB/c hearts on day 0 and treated with anti‐PD‐L1 and anti‐CTLA‐4 (αPD-L1 + αCTLA‐4) mAbs on days 0, 3, and 5 to trigger heart graft rejection. Recipients with rejected heart allografts were then transplanted again with BALB/c and C3H skin allografts 30 days after heart grafting (n = 6). A, Schematic of the experimental design. B, The percentage of skin allograft survival after skin transplantation on αPD-L1 + αCTLA‐4 treated, BALB/c heart graft rejected Irf4 fl/flCd4‐Cre mice. **P = .0022; Mann‐Whitney test. C, Representative images of accepted BALB/c skin allografts (>100 days) on αPD‐L1 + αCTLA‐4 treated, BALB/c heart graft rejected Irf4 fl/flCd4‐Cre recipients. D, A representative image of C3H (left) and BALB/c (right) skin allografts on a αPD‐L1 + αCTLA‐4 treated, BALB/c heart graft rejected Irf4fl/flCd4‐Cre mouse
3.4 |. Identification of un‐restored gene expressions in Irf4‐deficient alloreactive T cells upon checkpoint blockade
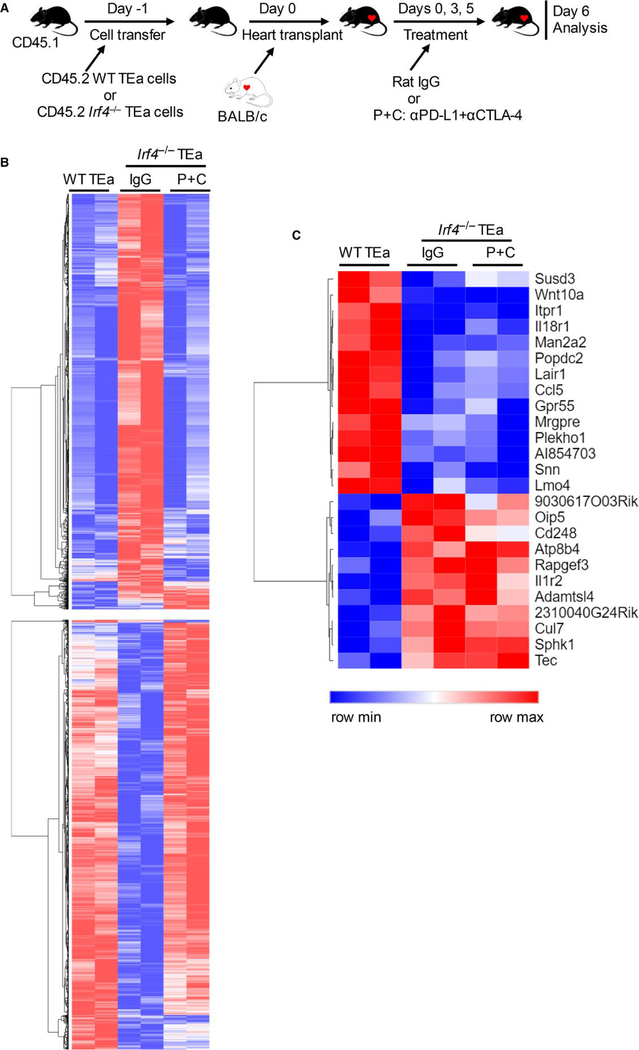
TCR‐transgenic TEa CD4+ T cells (B6 background) recognize a BALB/c I‐Eα allopeptide presented by B6 antigen presenting cells, and were used to assess the effects of checkpoint blockade on Irf4‐deficient alloreactive T cells.8 Herein, we compared the gene expression profiles between WT and Irf4−/− TEa cells following heart transplantation and checkpoint blockade. CD45.1+ B6 mice were adoptively transferred with either CD45.2+ WT TEa or CD45.2+ Irf4−/− TEa cells on day‐1, and transplanted with BALB/c hearts on day 0. Recipients transferred with Irf4−/− TEa cells were further treated with rat IgG or anti‐PD‐L1 plus anti‐CTLA‐4 mAbs (P+C group) on days 0, 3, and 5. Adoptively transferred CD45.2+ TEa cells were isolated from splenocytes on day 6 by flow cytometry sorting (Figure 4A). RNA was isolated and gene expression profiles were determined by microarray analysis. Differentially expressed genes between adoptively transferred WT TEa and Irf4−/− TEa cells (IgG group) are shown in Figure 4B. Importantly, checkpoint blockade (Irf4−/− TEa; P+C group) restored the expression levels of the majority of WT TEa cell‐expressed genes in Irf4−/− TEa cells (Figure 4B), which may explain why checkpoint blockade robustly reversed the initial dysfunction of Irf4−/− T cells. Some of the unrestored genes in Irf4−/− TEa cells following checkpoint blockade were shown in Figure 4C. It would be interesting to further determine whether these unrestored genes are responsible for the reinvigorated Irf4‐deficient T cells to become re‐dysfunction.
FIGURE 4.
Identification of un‐restored genes in alloreactive Irf4−/− CD4+ T cells upon checkpoint blockade. CD45.1+ B6 mice were adoptively transferred with CD45.2+ WT or Irf4−/− TEa cells on day ‐1, and transplanted with BALB/c hearts on day 0. Recipients transferred with Irf4−/− TEa cells were further treated with rat IgG or anti‐PD‐L1 plus anti‐CTLA‐4 mAbs (P+C group) on days 0, 3, and 5. Adoptively transferred CD45.2+ TEa cells were isolated from splenocytes on day 6 by flow cytometry sorting. RNA was isolated for microarray analysis. A, Schematic of the experimental design. B and C, Heat maps showing the normalized gene expression scores from indicated groups. Two RNA samples of each group were obtained from two independent experiments. Each RNA sample was isolated from pooled TEa cells from three (WT TEa group and Irf4−/− TEa P+C group) or five (Irf4−/− TEa IgG group) recipient mice
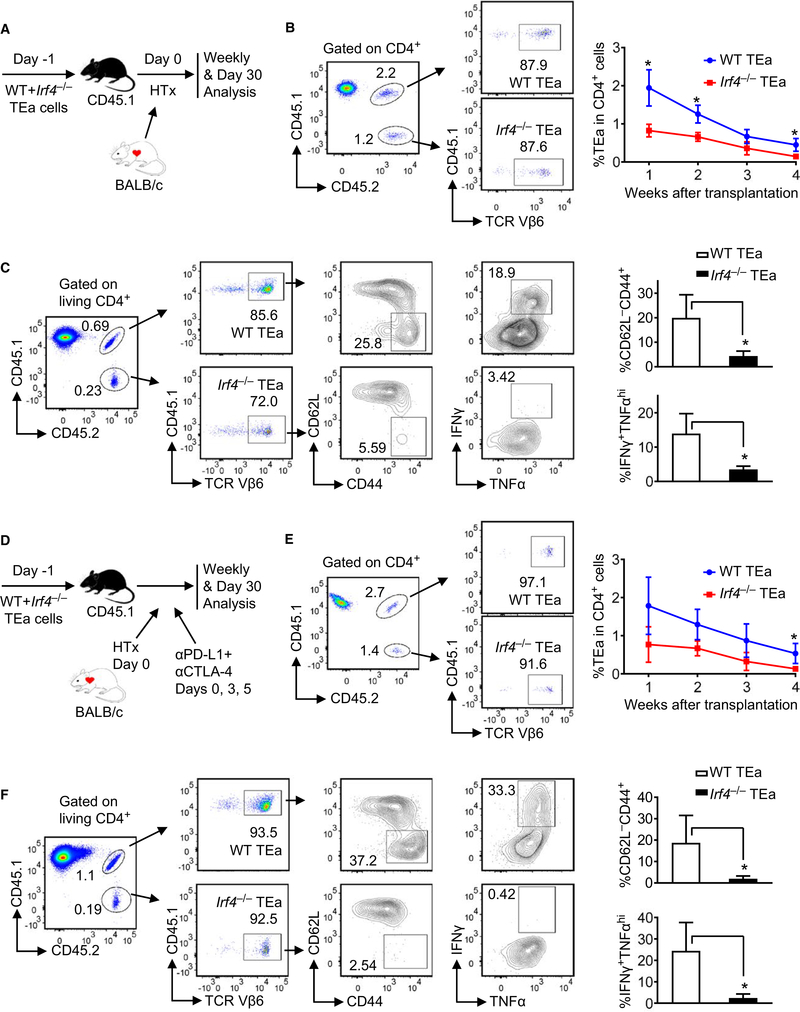
3.5 |. Checkpoint blockade does not restore effector memory cell generation from Irf4‐deficient alloreactive T cells
To track the fate of Irf4−/− alloreactive T cells, CD45.1+ B6 mice were adoptively transferred with mixed splenocytes containing a 1:1 ratio of CD45.1+CD45.2+ WT TEa (from [TEa × CD45.1]F1 mice) and CD45.2+ Irf4−/− TEa cells (from Irf4−/− TEa mice) 1 day prior to BALB/c heart transplantation (Figure 5A). TEa cell frequencies in peripheral blood were assessed weekly post‐grafting. Flow cytometry plots in Figure 5B show the gating strategy detecting co‐transferred CD45.1+CD45.2+TCR Vβ6+ WT TEa and CD45.1−CD45.2+TCR Vβ6+ Irf4−/− TEa cells in peripheral blood at 1 week post‐grafting. Both WT TEa and Irf4−/− TEa cell frequencies were gradually decreased in peripheral blood (Figure 5B, line graph). On day 30 post‐grafting, splenocytes of transplant recipients were harvested and analyzed. The percentage of Irf4−/− TEa cells among CD4+ splenocytes was significantly lower than that of WT TEa cells (Figure S1A, i). Flow cytometry plots in Figure 5C show the gating strategy detecting TEa cell populations, and the percentages of CD62L−CD44+ effector memory and IFN‐γ+TNF‐αhi cells within WT TEa (top plots) and Irf4−/− TEa (bottom plots) cell populations, respectively. Compared to WT TEa cells, Irf4−/− TEa cells exhibited significantly lower frequencies of effector memory and IFN‐γ/TNF‐α producing cells (Figure 5C, bar graphs). Percentages of IL‐2+, PD‐1+, and CX3CR1+, as well as mean fluorescence intensity (MFI) of CCR2 and CCR7 were not significantly different between WT and Irf4−/− TEa cells in spleens when n = 3 per group (Figure S1A, ii‐vi).
FIGURE 5.
Checkpoint blockade does not restore effector memory cell generation from alloreactive Irf4−/− CD4+ T cells. CD45.1+ B6 mice were adoptively transferred with mixed splenocytes containing a 1:1 ratio of CD45.1+CD45.2+ WT TEa and CD45.2+ Irf4−/− TEa cells on day ‐1, transplanted with BALB/c hearts on day 0, and left untreated (A‐C) or treated with αPD‐L1 + αCTLA‐4 (D‐F) on days 0, 3, and 5. A, Schematic of the experimental design. B, Flow cytometry plots display the gating strategy detecting co‐transferred CD45.1+CD45.2+TCR Vβ6+ WT TEa and CD45.1−CD45.2+TCR Vβ6+ Irf4−/− TEa cells in peripheral blood at 1 week post‐grafting. The line graph shows WT Tea and Irf4−/− TEa cell frequencies in peripheral blood weekly after transplantation. C, Splenocytes were analyzed on day 30 post‐grafting. Shown are the gating strategy detecting TEa cell populations, and the percentages of CD62L−CD44+ and IFN‐γ+TNF‐αhi cells within WT TEa and Irf4−/− TEa cell populations. D, Schematic of the experimental design, with αPD‐L1 + αCTLA‐4 treatment. E, WT Tea and Irf4−/− TEa cell frequencies in peripheral blood at 1 week post‐grafting (flow cytometry plots) and weekly after transplantation (line graph). F, The percentages of CD62L−CD44+ and IFN‐γ+TNF‐αhi cells within WT TEa and Irf4−/− TEa cells in spleens at day 30 post‐grafting. Data are mean ± SD (n = 3). *P < .05
To track the fate of Irf4−/− alloreactive T cells following checkpoint blockade, CD45.1+ B6 mice received cell transfer and heart transplantation as mentioned above, and treated with anti‐PD-L1 plus anti‐CTLA‐4 mAbs on days 0, 3, and 5 post‐grafting (Figure 5D). Both WT TEa and Irf4−/− TEa cell frequencies remained gradually declined in peripheral blood despite of checkpoint blockade (Figure 5E). On day 30 post‐grafting, the percentage of Irf4−/− TEa cells among CD4+ splenocytes was significantly lower than that of WT TEa cells (Figure S1B, i). Irf4−/− TEa cells displayed significantly lower frequencies of effector memory and IFN‐γ/TNF‐α producing cells than those of WT TEa cells (Figure 5F). Percentages of IL‐2+, perforin/granzyme B+, PD‐1+, and CX3CR1+, as well as MFI of CCR2, CCR7, TIM‐3, and LAG‐3 were not significantly different between WT and Irf4−/− TEa cells in spleens when n = 3 per group (Figure S1B, ii‐ix). Of note, CX3CR1 has been reported as a marker for anti‐PD‐1 therapy‐responsive T cells,13 whereas CCR2 expressed on T cells has been shown to modulate the effector/regulatory T cell ratio.14 Taken together, checkpoint blockade does not restore cell frequency, effector memory cell generation, and IFN‐γ/TNF‐α production of Irf4−/− TEa cells at day 30 post‐grafting.
4 |. DISCUSSION
Sixty‐five years ago, Medawar et al demonstrated that mice injected at birth with allogeneic cells were subsequently able to accept skin allografts from the same donor strain.15 Since then transplant tolerance was further achieved in adult animals through various approaches.16,17 Deletion, anergy, and Treg suppression of alloreactive T cells contribute to transplant tolerance.18–21 We have previously shown that ablation of IRF4 induced allogeneic T cell dysfunction.8 Loss of IRF4 expression has also been shown to be associated with tumor‐specific T cell dysfunction.11 Herein, we demonstrated that ablation of IRF4 in T cells induced robust transplant tolerance in heart allograft recipients, resulting in the acceptance of secondary donor‐type skin allografts. We noticed that Irf4fl/flCd4‐Cre mice were capable of rejecting primary skin allografts and secondary third‐party skin allografts. It is possible that heart and skin allografts exhibit different capabilities in driving the dysfunctional differentiation of Irf4‐deficient T cells.
Checkpoint blockade induced acute heart transplant rejection in Irf4fl/flCd4‐Cre recipients.8 Our previous mechanistic studies revealed that checkpoint blockade restored cell number, proliferation (indicated by Ki67), metabolic activation (indicated by CD98 and CD71), and IFN‐γ production of Irf4−/− TEa cells on day 6 post-heart grafting.8 In this study, we compared the gene expression profiles between WT TEa and Irf4−/− TEa cells in heart recipients on day 6, and found that checkpoint blockade also restored many other genes in Irf4−/− TEa cells, including Tbx21, Il12rb1, Il2rb, Il2rg, Il10rb, Il15ra, and Il21r (data not shown). Hence, restoring some cytokine responses may be another essential mechanism by which checkpoint blockade transiently reinvigorate Irf4−/− T cells. Nevertheless, checkpoint blockade did not prevent the later establishment of transplant tolerance. Hence, similar to the re‐exhaustion of reinvigorated T cells in a chronic infection model,22 reinvigorated Irf4‐deficient T cells also became re‐dysfunction in our model. We identified un‐restored gene expressions in Irf4−/− TEa cells upon checkpoint blockade. For instance, regardless with or without checkpoint blockade, Irf4−/− TEa cells exhibited down-regulated expressions of Il18r1 (encoding IL‐18Rα) and Wnt10a, as well as up‐regulated expression of Il1r2 (encoding IL‐1R2). IL‐18Rα has been shown to be down‐regulated on exhausted CD8+ T Cells, rendering them unresponsive to inflammatory cytokines.23 IL‐1R2 is a negative regulator of IL‐1 signaling,24 whereas Wnt signaling is essential for T cell memory.25 It remains to be determined the role of these un‐restored genes in establishing the irreversible state of Irf4−/− T cell dysfunction.
It is a challenge to determine whether transplant tolerance has been achieved in the clinic, and how to proceed if it has not. Successful weaning from immunosuppression may be an indication of transplant tolerance, but it remains unclear how to stratify transplant recipients according to their likelihood of being able to discontinue immunosuppression. Moreover, transplant tolerance can be achieved and maintained after rejection, as demonstrated in murine and larger animal models.26–30 Hence, there is a fundamental need to reveal the determinants of T cell fate in transplant tolerance. The current works identified IRF4 as a key determinant governing transplant tolerance, which will advance the understanding and further characterization of tolerogenic T cell fate.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the startup fund from Houston Methodist Research Institute (to W.C.) and the US National Institutes of Health grant (#NIH R01AI132492 to W.C.). The authors thank the Flow Cytometry Core at Houston Methodist and the Genomic and RNA Profiling Core at Baylor College of Medicine for excellent services.
Funding information
Houston Methodist Research Institute; National Institute of Allergy and Infectious Diseases, Grant/Award Number: R01AI132492
Abbreviations:
- B6
C57BL/6
- CTLA‐4
cytotoxic T‐lymphocyte‐associated protein 4
- Ctrl
control
- DCs
dendritic cells
- HTx
heart transplantation
- IRF4
interferon regulatory factor 4
- MST
mean survival time
- PD‐1
programmed cell death protein 1
- PD‐L1
programmed death‐ligand 1
- TCR
T cell receptor
- Tx
transplantation
- WT
wild‐type
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Sawinski D, Trofe‐Clark J, Leas B, et al. Calcineurin inhibitor minimization, conversion, withdrawal, and avoidance strategies in renal transplantation: a systematic review and meta‐analysis. Am J Transplant. 2016;16(7):2117–2138. [DOI] [PubMed] [Google Scholar]
- 2.Li XC, Rothstein DM, Sayegh MH. Costimulatory pathways in transplantation: challenges and new developments. Immunol Rev. 2009;229(1):271–293. [DOI] [PubMed] [Google Scholar]
- 3.Miyahara Y, Khattar M, Schroder PM, et al. Anti‐TCRbeta mAb induces long‐term allograft survival by reducing antigen‐reactive T cells and sparing regulatory T cells. Am J Transplant. 2012;12(6):1409–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W, Ghobrial RM, Li XC. The evolving roles of memory immune cells in transplantation. Transplantation. 2015;99(10):2029–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochiai K, Maienschein‐Cline M, Simonetti G, et al. Transcriptional regulation of germinal center B and plasma cell fates by dynamical control of IRF4. Immunity. 2013;38(5):918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huber M, Lohoff M. IRF4 at the crossroads of effector T‐cell fate decision. Eur J Immunol. 2014;44(7):1886–1895. [DOI] [PubMed] [Google Scholar]
- 7.Vander Lugt B, Khan AA, Hackney JA, et al. Transcriptional programming of dendritic cells for enhanced MHC class II antigen presentation. Nat Immunol. 2014;15(2):161–167. [DOI] [PubMed] [Google Scholar]
- 8.Wu J, Zhang H, Shi X, et al. Ablation of transcription factor IRF4 promotes transplant acceptance by driving allogenic CD4(+) T cell dysfunction. Immunity. 2017;47(6):1114–1128 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alhamad T, Venkatachalam K, Linette GP, Brennan DC. Checkpoint inhibitors in kidney transplant recipients and the potential risk of rejection. Am J Transplant. 2016;16(4):1332–1333. [DOI] [PubMed] [Google Scholar]
- 10.Lipson EJ, Bagnasco SM, Moore J, et al. Tumor regression and allograft rejection after administration of anti‐PD‐1. N Engl J Med. 2016;374(9):896–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philip M, Fairchild L, Sun L, et al. Chromatin states define tumour‐specific T cell dysfunction and reprogramming. Nature. 2017;545(7655):452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrod KR, Cahalan MD. Murine skin transplantation. J Vis Exp. 2008;11:e634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan Y, Cao S, Liu X, et al. CX3CR1 identifies PD‐1 therapy‐responsive CD8 + T cells that withstand chemotherapy during cancer chemoimmunotherapy. JCI Insight. 2018;3(8):e97828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakos E, Thaiss CA, Kramer MP, et al. CCR2 regulates the immune response by modulating the interconversion and function of effector and regulatory T cells. J Immunol. 2017;198(12):4659–4671. [DOI] [PubMed] [Google Scholar]
- 15.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172(4379):603–606. [DOI] [PubMed] [Google Scholar]
- 16.Larsen CP, Elwood ET, Alexander DZ, et al. Long‐term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381(6581):434–438. [DOI] [PubMed] [Google Scholar]
- 17.Wekerle T, Kurtz J, Ito H, et al. Allogeneic bone marrow transplantation with co‐stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat Med. 2000;6(4):464–469. [DOI] [PubMed] [Google Scholar]
- 18.Monk NJ, Hargreaves RE, Marsh JE, et al. Fc‐dependent depletion of activated T cells occurs through CD40L‐specific antibody rather than costimulation blockade. Nat Med. 2003;9(10):1275–1280. [DOI] [PubMed] [Google Scholar]
- 19.Daley SR, Cobbold SP, Waldmann H. Fc‐disabled anti‐mouse CD40L antibodies retain efficacy in promoting transplantation tolerance. Am J Transplant. 2008;8(11):2265–2271. [DOI] [PubMed] [Google Scholar]
- 20.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193(11):1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuber J, Sykes M. Mechanisms of mixed chimerism‐based transplant tolerance. Trends Immunol. 2017;38(11):829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pauken KE, Sammons MA, Odorizzi PM, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD‐1 blockade. Science. 2016;354(6316):1160–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingram JT, Yi JS, Zajac AJ. Exhausted CD8 T cells downregulate the IL‐18 receptor and become unresponsive to inflammatory cytokines and bacterial co‐infections. PLoS Pathog. 2011;7(9):e1002273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters VA, Joesting JJ, Freund GG. IL‐1 receptor 2 (IL‐1R2) and its role in immune regulation. Brain Behav Immun. 2013;32:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gattinoni L, Zhong XS, Palmer DC, et al. Wnt signaling arrests effector T cell differentiation and generates CD8 + memory stem cells. Nat Med. 2009;15(7):808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller ML, Alegre ML, Chong AS. Transplantation tolerance after allograft rejection. Curr Opin Organ Transplant. 2017;22(1):64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada Y, Nadazdin O, Boskovic S, et al. Repeated injections of IL‐2 break renal allograft tolerance induced via mixed hematopoietic chimerism in monkeys. Am J Transplant. 2015;15(12):3055–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scalea JR, Okumi M, Villani V, et al. Abrogation of renal allograft tolerance in MGH miniature swine: the role of intra‐graft and peripheral factors in long‐term tolerance. Am J Transplant. 2014;14(9):2001–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young JS, Daniels MD, Miller ML, et al. Erosion of transplantation tolerance after infection. Am J Transplant. 2017;17(1):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller ML, Daniels MD, Wang T, et al. Spontaneous restoration of transplantation tolerance after acute rejection. Nat Commun. 2015;6:7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.