Abstract
The role of infection in cerebrovascular disease is complex and remains incompletely understood. Over the last 5 years, investigators have made notable inroads in untangling this thorny topic. In this review, we examine these recent developments, concentrating on four aspects of the relationship between infection and stroke. We first discuss specific infectious agents as direct causes of stroke, focusing on recent work implicating herpesviruses and HIV in cerebral vasculopathy. We then discuss systemic infection of any type as a stroke trigger, focusing on the relationship of infection to timing of acute stroke, both in children and adults, as well as the role of vaccination in stroke prevention. We examine the evidence for chronic infection or “infectious burden” as a stroke risk factor. Finally, we discuss recent work on infection as a risk factor for increased morbidity after stroke, possible mechanisms mediating this effect, and the evidence for prophylactic antibiotics.
Keywords: Stroke, Infection, Herpesvirus, HIV, Vaccination, Infectious burden
Introduction
The relationship between infection and stroke may have been suspected as early as the second century A.D., when Galen described “apoplexy” as due to “some inflammatory disease that exists in the head” [1]. Pierre Marie and Sigmund Freud both observed a connection between acute infectious diseases and stroke in children in the late 1800s [2, 3]. Syphilis was also well known to cause arteritis by this time; Sir William Osler recommended mercurials in the treatment of “the thrombosis that follows syphilitic disease of the [cerebral] arteries,” commenting that “practically these are the only cases of hemiplegia in which we see satisfactory results from treatment” [4]. Evidence for the connection of various infectious agents with systemic atherosclerosis was reviewed in 1911 by Channing Frothingham, an internist at the Peter Bent Brigham Hospital [5]. In 1921, pathologist William Ophuls of Stanford published a study of 500 consecutive autopsies in JAMA, with the striking finding that systemic arteriosclerosis, including of the cerebral arteries, appeared far earlier and more extensively in the patients with a history of chronic systemic infection and inflammation [6].
In 1978, Fabricant and investigators experimentally infected chickens with an avian herpesvirus and produced atherosclerosis, sparking reneweHIV increases the risk of both ischemicd interest in the subject [7]. In that same year, a case series of 64 ischemic strokes in young people noted a seasonal variation in patients lacking traditional vascular risk factors; the authors postulated a possible role of systemic infection in stroke pathogenesis in the young [8], but other similar studies at the time found no such connection [9, 10]. Despite multiple case reports of patients (both children and adults) with strokes directly caused by various infections, it was not until the late 1980s with the advent of AIDS that investigators began to explore in more detail the role of infection (both common and opportunistic) in cerebrovascular disease [11].
The twenty-first century has brought exciting advances in our understanding of the complex interactions between infection and stroke (Table 1). Increasingly, we have learned that viral infections such as herpesviruses and HIV appear to play a role in cerebral arteriopathy. We have found that systemic infections, even minor ones, may trigger acute stroke in patients with vascular risk factors, raising the question of whether immunizations might be useful as a stroke prevention strategy. We have explored the role of chronic, low-grade infection or “infectious burden” in creating an inflammatory milieu that predisposes patients to stroke. Finally, an increased national focus on patient outcomes has led to new evidence that post-stroke infectious complications factor prominently in stroke morbidity and mortality. In this review, we will discuss these developments, with special attention to new findings in the last 4 years.
Table 1.
Proposed mechanisms of stroke pathogenesis in connection with infection
| Mechanism of pathogenesis | Examples |
|---|---|
| Direct invasion of arterial wall, endotheliopathy | Syphilis, VZV, HSV, HIV, parvovirus B19 |
| Acceleration of atherosclerosis through induction of cytokines (TNF-alpha, interleukin 2) in response to specific antigenic stimulus | Herpesviruses, Chlamydia pneumoniae |
| Acute systemic infection as stroke trigger (platelet activation, dehydration, infection-induced cardiac arrhythmias) | Influenza, upper respiratory infections, urinary tract infections |
| Chronic inflammation due to multiple infections (infectious burden) | Periodontal infection, Chlamydia pneumoniae, herpesviruses |
| Post-stroke infection due to stroke-induced reduction in cell mediated immunity; increased antigen presentation leading to autoimmune inflammatory response against damaged brain tissue → poor stroke recovery, worse functional outcomes | Urinary tract infections, pneumonia, hospital acquired line infections |
Infectious Causes of Stroke
We now know that a multitude of infections can directly cause stroke, including bacterial (syphilis and tuberculosis are classic examples), fungal (cryptococcus, aspergillus, mucormycosis), parasitic [12] (most commonly neurocysticercosis), and numerous viruses (Table 2). To review all the infectious causes of stroke is beyond the scope of this paper. Instead, we concentrate on recent updates in our understanding of the role of viral infections in stroke.
Table 2.
Selected organisms implicated in stroke pathogenesis
| Organism | Infection | Mechanism |
|---|---|---|
| Bacterial infections | ||
| Treponema pallidum | Neurosyphilis | Vasculitis/arteritis |
| Mycobacterium tuberculosis | Tuberculous meningitis | Arteritis; meningitis |
| Chlamydia pneumoniae | Acute or chronic respiratory infections | Accelerated atherogenesis, enhanced platelet aggregation |
| Helicobacter pylori | Gastritis, peptic ulcer disease | Enhanced platelet aggregation, prothrombotic state |
| Porphyromonas gingivalis (and other periodontal pathogens) | Periodontal disease | Chronic inflammation due to infectious burden; prothrombotic state |
| Parasitic infections | ||
| Trypanosoma cruzi | Chagas disease, Heart failure | Cardioembolism |
| Taenia solium | Neurocysticercosis | Arachnoiditis/small artery vasculitis; direct compression of large arteries by cysts |
| Plasmodium falciparum | Cerebral malaria | Occlusion of cerebral arteries by infected erythrocytes |
| Echinococcus granulosis | Cardiac hydatidosis; cerebral cystic echinococcosis | Cardioembolism; arterial compression from cerebral cysts |
| Schistosoma mansoni | Schistosomiasis | Microembolic borderzone infarction |
| Toxocara canis | Toxocariasis | Arachnoiditis; vasculitis |
| Spirometra species (tapeworm) | Cerebral sparganosis | Vasculitis |
| Trichinella spiralis | Neurotrichinelliasis | Microinfarction due to direct obstruction of small vessels with larvae; vasculitis |
| Fungal infections | ||
| Cryptococcus | Systemic and CNS infections (usually immunocompromised) | Meningitis; vasculitis |
| Aspergillus | Systemic and CNS infections | Arteritis, vasculopathy |
| Mucorales (including Rhizopus, Mucor, etc.) | Mucormycosis | Vascular invasion of fungus, aneurysmal dilatation, vascular necrosis |
| Viral infections | ||
| Human immunodeficiency virus (HIV) | HIV disease/AIDS | Vasculopathy; susceptibility to opportunistic CNS infections |
| Cytomegalovirus | Often asymptomatic, latent; occasional mononucleosis-like syndrome | Inflammatory response with accelerated atherogenesis |
| Varicella zoster virus | Chickenpox, shingles | Vasculitis/vasculopathy |
| Herpes simplex virus (types 1 and 2) | Oral and genital infections | Vasculopathy; possible stroke trigger in young people |
| Parvovirus B19 | “Fifth disease” | Possible arteriopathy |
Herpesviruses
Herpesviruses are ubiquitous, and many have a known neurotropic tendency, as well as the ability to remain latent in sensory ganglia and other cells for life. The common herpesvirus cytomegalovirus (CMV) was first suggested in the 1990s to be associated with increased atherogenesis, particularly coronary artery disease and carotid disease [13–15]. Proposed mechanisms included endothelial dysfunction and impaired vascular reactivity [16], induction of pro-inflammatory cytokines [17], and a T cell-mediated inflammatory response [18]. It remained unclear if CMV played a direct causal role or was simply more likely to attack atherosclerotic arteries [19]. Furthermore, multiple other studies refuted this association of CMV (among other infectious agents) with atherogenesis [20–22]. Some studies indicated that CMV infection might both predispose to and trigger cardiovascular events in certain vulnerable hosts (such as transplant patients, AIDS patients, or patients with end stage renal disease) [18, 23, 24], but others found no association [25].
Mounting evidence now points toward a strong positive association and possible causal relationship between CMV and atherogenesis. A systematic review and meta-analysis in 2012, including 9000 cases and 8608 controls from 55 case–control studies (6 of which were prospective), found that evidence of CMV (either by PCR or ELISA testing) increased the odds of coronary disease [26•]. In the population-based Multi-Ethnic Study of Atherosclerosis, CMV antibody titer was strongly associated with a bias toward T helper type 1 response and increased coronary artery calcification [27]. The effect appears even stronger in patients with HIV [28]. Animal models have implicated a Toll-like receptor–mediated CMV–platelet interaction leading to pro-inflammatory and pro-angiogenic responses, ultimately producing atherosclerosis [29]. While the effect has been well demonstrated in coronary and carotid arteries, studies of cerebral arteries are lacking and more research is needed. A fascinating development is the recent discovery that statins have among their pleiotropic effects anti-viral properties, with an effect on CMV comparable to ganciclovir [30•]. This raises important new questions regarding the mechanisms by which statins reduce the risk of stroke, particularly in the case of intracranial atherosclerosis, which has proven difficult to treat effectively. If the beneficial effect of statins is partially mediated by viral suppression of CMV, might other CMV treatments offer similar benefits?
Increasingly, other common herpesviruses, such as varicella zoster virus (VZV), have been implicated in the pathogenesis of cerebral arteriopathy. VZV causes varicella (chickenpox) in humans only; following initial infection, the virus becomes dormant in neural ganglia, only to reactivate at times of decreased cell-mediated immunity, causing zoster (shingles). Zoster is common and usually self-limited but can be deadly, particularly to the elderly and immunosuppressed.
The first cases of autopsy-proven central nervous system angiitis associated with VZV were described in the 1970s, often in young patients with Hodgkin disease and recent episodes of zoster ophthalmicus [31]. In 1996, Gilden and colleagues identified VZV DNA and VZV-specific antigen in the cerebral arteries of a man who died of unspecified CNS vasculitis, with no prior history of zoster rash [32]. This sparked an ongoing exploration into the role of VZV in vasculopathy and stroke. It appears that VZV can invade the vessel walls of both small and large cerebral arteries in a patchy fashion, causing a broad spectrum of neurovascular disorders, from large vessel vasculopathy associated with ischemic stroke, to arterial dissections and aneurysmal subarachnoid hemorrhage [33]. Purported mechanisms include invasion by virions into the adventitia in early disease and the media in late disease, leading to chronic inflammation and eventual intimal thickening and arterial remodeling [34, 35•]. The presence of anti-VZV IgG antibodies in the cerebrospinal fluid has emerged as a more sensitive indicator of VZV vasculopathy than the more commonly used VZV PCR [36].
One recent study identified VZV antigen in the cerebral arteries of 14 of 18 subjects at high risk of VZV reactivation due to lowered cell-mediated immunity [37]. Importantly, pathological study of 63 normal cerebral arteries from 45 subjects revealed no evidence of VZV antigen or DNA [38], fulfilling the first of Koch’s postulates. Most recently, VZV antigen was found in 61/82 (74 %) of temporal arteries from patients with biopsy proven giant cell arteritis (GCA), compared to 1/13 (8 %) of normal temporal arteries [39••]. These results support the hypothesis that VZV may be a causative organism in the pathogenesis of GCA, a finding with broad clinical implications for treatment, if confirmed by others. A link between recent zoster infection and increased stroke risk has also been identified in multiple epidemiological studies [40–42].
Herpes simplex virus (HSV) is closely related to VZV, and new work suggests a potential role for this herpesvirus in stroke pathogenesis. Recent case reports suggest that HSV (both type 1 and type 2) can directly cause both ischemic and hemorrhagic stroke in children and adults, via mechanisms similar to that of VZV [43–46]. Recent results of the international Vascular Effects of Infection in Pediatric Stroke (VIPS) study indicate that serological evidence of recent infection with several herpesviruses (Epstein–Barr virus, VZV, CMV, HSV1, or HSV2) doubles the odds of stroke in children aged 29 days through 18 years [47]. HSV, found in one quarter of cases, was the most common herpesvirus to be detected; HSV subtype could not be determined in the majority of these, but in those that could be identified, all were HSV1. These results provide novel evidence that acute infection with HSV may trigger stroke in children. More research is needed to see if the same may hold true for adults. If confirmed, these results may lead to new drug or vaccine targets both for primary and secondary prevention.
Human Immunodeficiency Virus
AIDS patients were recognized to be at increased risk of stroke in the 1980s [48]. Autopsy studies revealed multiple cases of AIDS patients with stroke due to cerebral vasculitis from lymphoma or opportunistic pathogens such as Candida albicans and herpesviruses [49]. Other patients died of hemorrhagic stroke due to immune-related thrombocytopenia or coagulopathy or developed cardioembolic stroke due to AIDS-related cardiomyopathy or endocarditis [50]. With the arrival of highly active anti-retroviral therapy (HAART), HIV disease transformed from an inevitably fatal condition to a chronic, manageable disease; yet patients continued to suffer strokes at alarming rates. In fact, the number of stroke hospitalizations for patients with HIV rose by 60 % from 1997 to 2006 in a large population-based study, despite an overall decrease in stroke hospitalizations during this time period [51]. Proposed reasons for this increase have included improved survival and aging of the HIV-positive population, high prevalence of comorbidities such as smoking and substance abuse, accelerated atherosclerosis due to HAART-induced dyslipidemia, cerebral vasculitis from comorbid infections such as syphilis or tuberculosis, and HIV-associated vasculopathy [50].
New research suggests that traditional cardiovascular risk factors may be more prevalent in patients with HIV disease [52, 53]. Despite this, the Framingham Risk Score for Stroke appears to underestimate the risk of stroke in HIV-positive men; [54] furthermore, statins appear not to have the same benefits in patients with HIV that we see in other patients with cardiovascular risk factors [55]. Even after controlling for other risk factors, HIV increases the risk of both ischemic [56•] and hemorrhagic [57•] stroke, in women and men [58], and outcomes in stroke patients with HIV tend to be poor [59, 60]. Interestingly, the increased risk appears to correlate with lower CD4 counts and higher viral load [55, 59, 61, 62], suggesting that the virus itself as the causal agent rather than HAART-related dyslipidemia. The effect of HIV on risk of hemorrhagic stroke appears more pronounced in younger patients and in women [57•].
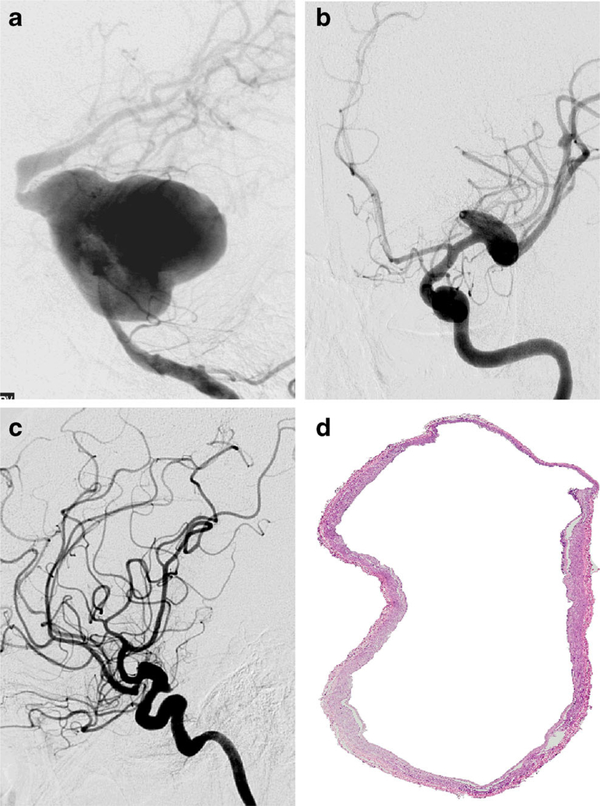
Why is stroke risk increased in HIV patients with higher viral load? Accumulating evidence suggests that direct vasculopathic effects of the virus contribute. HIV is known to be associated with fusiform aneurysmal cerebral vasculopathy (Fig. 1) [63]. Pathological studies of both large and penetrating cerebral arteries of patients with HIV reveal thinning of the media, often accompanied by dilatation [64, 65•, 66]. Infarcts in HIV-positive patients appear to be associated with extremes of arterial remodeling: either accelerated atherosclerosis with severely stenotic vessels, or dilated, dolichoectatic vessels predisposing both to thrombotic and hemorrhagic strokes [67••]. Our data suggest that the dolichoectatic phenotype is associated with increased inflammation in the adventitia [J. Gutierrez, personal communication]. More research is needed to better define the mechanisms by which HIV appears to cause vasculopathy. Meanwhile, we recommend that HIV screening be considered in patients with unknown HIV status admitted with stroke, particularly if other risk factors are not present.
Fig. 1.
HIV vasculopathy. HIV vasculopathy is a form of secondary dolichoectasia frequently found among patients with HIV. This arteriopathy may simultaneously affect different arterial segments, as shown in (a) where extreme dilatation of the basilar artery is observed, with other areas of dilatation noted in the right ICA (b) and left ICA (c) in a 36-year-old woman with HIV and hydrocephalus. Pathologically, cerebral brain arteries of patients with HIV vasculopathy have thinning of the media and the wall with a dilated lumen (d), image obtained from the Brain Arterial Remodeling Study). Figure courtesy of Dr. Jose Gutierrez, Columbia University Medical Center
Parvovirus B19
Acute infection with a variety of viruses may trigger stroke (see “Infection as Stroke Trigger” section), but parvovirus B19 (PVB19) deserves special mention. A single-stranded DNA virus, PVB19, commonly causes erythema infectiosum (“fifth disease” or “slapped-cheek disease”) in children. Numerous case reports document acute parvovirus infection or reactivation associated with ischemic stroke, particularly in children [68]. Many patients had evidence of active PVB19 replication in their cerebrospinal fluid; some authors postulate a direct viral effect on the endothelium [69]. Maternal PVB19 infection during pregnancy has been associated with intrauterine fetal death; on post-mortem, perivascular calcifications were found in the fetal cerebral arteries, and PVB19 DNA and capsid antigen were demonstrated in cerebral endothelial cells and macrophages [70]. Recent results from the VIPS study also found DNA evidence of PVB19 in 6 % of children with stroke, and 0 % of controls [71]. PVB19 may join the ranks of viruses known to cause cerebral arteriopathy, but more research is needed.
Infection as Stroke Trigger
Why does a patient with chronic vascular risk factors have a stroke today, rather than last week or next month? Systemic infection has been proposed as a trigger of acute stroke based on accumulating evidence from multiple studies [72]. A large prospective study in Britain found a substantial increase in the risk of stroke and myocardial infarction in the days following acute upper respiratory infection. The effect diminished over time after the infection and was seen to a lesser degree after urinary tract infection. Recent vaccination, in contrast, did not increase cardiovascular event rate [73]. In the Cardiovascular Health Study, a multicenter prospective cohort study of vascular risk factors in an elderly population, we found that recent hospitalization for an infection (mostly respiratory and urinary infections) was associated with an increased risk of stroke [74]. Proposed mechanisms include increased platelet activation and aggregation, impaired endothelial function, infection-provoked cardiac arrhythmias, and dehydration-induced thrombosis. However, despite the strong association and biological plausibility, this analysis did not prove causality; hospitalization itself, rather than infection per se, may have increased the risk of stroke.
If acute systemic infections can precipitate stroke, could vaccination prevent it? The randomized FLUVACS trial demonstrated the benefits of influenza vaccination in prevention of cardiovascular death [75]. Influenza vaccination has been shown to be associated with reduced stroke risk, particularly in older patients [76]. These and other studies prompted the American Heart Association to recommend influenza vaccination as a secondary prevention strategy in patients with coronary or atherosclerotic vascular disease [77]. The benefits of influenza vaccination for stroke prevention appear to increase with successive yearly vaccinations [78]. However, some evidence suggests that there may be little or no benefit to influenza vaccination, and the topic remains controversial [79•, 80]
What about other vaccines? The CAPAMIS study, a large, prospective cohort study, evaluated the effectiveness of the 23-valent pneumococcal vaccine in prevention of myocardial infarction and stroke. At 1-year interim follow-up, patients who had received the vaccine appeared to have a 35 % reduction in risk of stroke; there was no evidence for reduction in risk of MI [81]. However, at conclusion of the study after 3 years, no benefit was seen [82•]. Another, larger cohort study in men also found no stroke prevention benefit to the pneumococcal vaccine [83]. This could be due to limited efficacy of the vaccine in preventing pneumococcal disease; evidence from meta-analyses has shown conflicting results regarding the vaccine’s efficacy, particularly in adults with chronic disease [84, 85]. Alternatively, vascular events may be triggered by pneumococcal serotypes not included in the 23-valent vaccine.
Infectious Burden
In addition to the agents discussed in the first section, many other specific pathogens have been suggested to play a role in stroke. Chlamydia pneumoniae, Helicobacter pylori, hepatitis A, and periodontal infections have all been explored as causative agents [86–88]. However, the evidence was mixed and multiple studies found no association [89–91]. More recently, the concept of “infectious burden” as a stroke risk factor has emerged. Exposure to increasing numbers of pathogens appears to exert a cumulative effect, leading to increased progression of coronary artery disease and carotid atherosclerosis [92, 93]. We explored this further in the Northern Manhattan Study, a large, prospective cohort study in a randomly selected, multi-ethnic urban stroke-free population [94]. We hypothesized that a weighted measure of multiple serological results might be more strongly associated with incident stroke than infection with any one pathogen. We assessed patients for serological evidence at stroke-free baseline of five common infections that have been linked to atherosclerotic risk in prior studies: C. pneumoniae, H. pylori, CMV, HSV1, and HSV2. While no individual infection showed a statistically significant association with increased stroke risk, all had a trend toward increased risk. We created a weighted index for infectious burden (IB) based on the strength of each individual infection’s association. We found that higher scores on this IB index were associated with increased risk of stroke (adjusted HR per standard deviation in the IB index 1.39, 95 % confidence interval (CI) 1.02–1.9), even after adjusting for demographic factors and vascular comorbidities. Adjusting for leukocyte count and levels of C-reactive protein had no effect on the association. Mean IB index was higher in Hispanics, non-Hispanic blacks, and women. Interestingly, elevated IB index was also associated with non-vascular death, carotid plaque thickness, and cognitive impairment [95•], particularly in the domains of executive function and memory [96]. An inverse correlation between IB and score on the Mini-Mental Status Exam was also observed in a recent study of patients in China with Alzheimer disease, although the authors did not employ a weighted index [97].
These results have important clinical implications. First, the absence of association with inflammatory markers suggests that the pathogens themselves may mediate increased stroke risk, rather than simply chronic inflammation. Second, IB index was higher in non-whites and women, groups that have been historically understudied in stroke research. Might this be a risk factor with particular importance for these populations? Third, infectious burden is a potentially modifiable and preventable risk factor. Prior studies have found no benefit to antibiotic treatment aimed at eradication of C. pneumoniae with regard to coronary disease [98, 99], but this has not been studied in stroke prevention. Serological evidence of bacterial infections (such as C. pneumonia and H. pylori) does not always indicate active infection, making the benefits of treatment uncertain; herpesvirus infections, in contrast, remain latent for life, offering a potential treatment target. Prospective, randomized trials are needed to ascertain whether prevention or treatment of common infections could be an effective stroke prevention strategy.
Post-stroke Infection
Infection after stroke is common, associated with poor outcomes and potentially deadly. A systematic review and meta-analysis in 2011 found that 30 % of acute stroke hospitalizations are complicated by post-stroke infection, most commonly pneumonia (10 %) and urinary tract infection (10 %). In intensive care units, the infection rate was 45 % (pneumonia 28 %, urinary tract infection 20 %). Of patients with post-stroke infections, almost half died, compared to less than one fifth of patients without infections [100]. Infections are more common in severe strokes, which have higher morbidity and mortality; however, in a recent study of 800 patients with intracerebral hemorrhage (ICH), infection was not associated with ICH volume or Glasgow Coma Scale score yet appeared to be an independent predictor of mortality and poor functional outcome [101•]
Stroke appears to induce endogenous immunosuppression through suppression of cytokine induction, making patients more vulnerable to infection [102]. It has been proposed that this might be an adaptive mechanism aimed at minimizing post-ischemic immune activation against brain antigens [103]. In the setting of infection, however, this adaptive immunosuppression may be reversed, worsening immunological activation directed against the brain.
The frequency of infection after stroke and its apparent adverse effects on outcomes underscore the paramount importance of infection prevention in post-stroke care. Many nursing protocols incorporated into stroke unit care have precisely this aim: dysphagia screening, avoidance of indwelling catheters, and early mobilization. Could there be a role for prophylactic antibiotics in the immediate poststroke period? The recent multicenter randomized Preventive Antibiotics in Stroke Study (PASS) evaluated this strategy by assigning 2550 stroke patients within 24 hours of symptom onset either to standard post-stroke care, or standard care plus prophylactic intravenous ceftriaxone for 4 days. The primary endpoint was functional outcome at 6 months. Post-stroke ceftriaxone did not affect the distribution of functional outcomes on the modified Rankin scale (adjusted common odds ratio 0.95, 95 % CI 0.82–1.09, p = 0.46) [104•]. Other investigators tested multiple classes of antibiotics as post-stroke prophylaxis in rats, to assess for possible differential effects of different medications. They found that no individual antibiotic class was associated with a functional benefit, but rats given fluoroquinolones had significantly worse functional outcomes [105]. It is therefore possible that toxic effects of treatment as well as the infections themselves mediate the poor outcomes seen in infected stroke patients. At this time, the bulk of evidence argues against prophylactic antibiotics after stroke.
Conclusion
Studies in the past 5 years have shed increasing light on the complex interactions between infection and stroke. Abundant research, both basic and clinical, is needed to confirm and expand on these findings. Investigation into mechanisms of viral vascular toxicity may provide insight into other neurological illnesses. Specific infectious pathogens such as herpesviruses may offer new therapeutic targets for stroke prevention. Epidemiological studies are needed to assess whether infection poses the same risk of stroke in adults that it appears to in children. Vaccines may offer new prevention strategies with substantial benefit to the public health. Quality initiatives and outcomes research may lead to reductions in post-stroke infection with sweeping consequences for patients and health care costs. As our understanding of the relationship between stroke and infection deepens, we hope to identify new strategies to reduce the burden of suffering that stroke continues to cause.
Acknowledgments
The authors thank Dr. José Gutierrez for his assistance with the figure on HIV vasculopathy.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Eliza C. Miller declares no conflict of interest.
Mitchell S.V. Elkind has received personal fees from UpToDate, Boehringer-Ingelheim, Inc., BMS-Pfizer, Daiichi-Sankyo, Janssen Pharmaceuticals, and BioTelemetry/Cardionet. He also has received grants from Diadexus, Inc. and BMS-Pfizer.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Karenberg A, Hort I. Medieval descriptions and doctrines of stroke: preliminary analysis of select sources. Part I: the struggle for terms and theories—late antiquity and early middle ages. J Hist Neurosci. 1998;7:162–73. [DOI] [PubMed] [Google Scholar]
- 2.Marie P Hémiplégie cérébrale infantile et maladies infectieuses. Prog Med Paris. 1885;13:167–9. [Google Scholar]
- 3.Freud S Die infantile Cerebrallähmung. Nothnagel H, editor. Specielle Pathologie 9, Teil 3 (I. Hälfte) Vienna, Austria: Holder; 1897. pp. 1–327. [Google Scholar]
- 4.Osler W The principles and practice of medicine, designed for the use of practitioners and students of medicine. 7th ed. New York: D. Appleton and company; 1910. [Google Scholar]
- 5.Frothingham C The relation between acute infectious diseases and arterial lesions. Arch Intern Med. 1911;8:153–62. [Google Scholar]
- 6.Ophüls W. Arteriosclerosis and cardiovascular disease: their relation to infectious diseases. JAMA. 1921;76:700–1. [Google Scholar]
- 7.Fabricant CG, Fabricant J, Litrenta MM, Minick CR. Virus-induced atherosclerosis. J Exp Med. 1978;148:335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hindfelt B, Nilsson O. Brain infarction in young adults (with particular reference to pathogenesis). Acta Neurol Scand. 1977;55:145–57. [DOI] [PubMed] [Google Scholar]
- 9.Grindal AB, Cohen RJ, Saul RF, Taylor JR. Cerebral infarction in young adults. Stroke. 1978;9:39–42. [DOI] [PubMed] [Google Scholar]
- 10.Chopra JS, Prabhakar S. Clinical features and risk factors in stroke in young. Acta Neurol Scand. 1979;60:289–300. [DOI] [PubMed] [Google Scholar]
- 11.Syrjanen J, Valtonen VV, Iivanainen M, Kaste M, Huttunen JK. Preceding infection as an important risk factor for ischaemic brain infarction in young and middle aged patients. Br Med J (Clin Res Ed). 1988;296:1156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finsterer J, Auer H. Parasitoses of the human central nervous system. J Helminthol. 2013;87:257–70. [DOI] [PubMed] [Google Scholar]
- 13.Nieto FJ, Adam E, Sorlie P, Farzadegan H, Melnick JL, Comstock GW, et al. Cohort study of cytomegalovirus infection as a risk factor for carotid intimal-medial thickening, a measure of subclinical atherosclerosis. Circulation. 1996;94:922–7. [DOI] [PubMed] [Google Scholar]
- 14.Zhu J, Quyyumi AA, Norman JE, Csako G, Epstein SE. Cytomegalovirus in the pathogenesis of atherosclerosis: the role of inflammation as reflected by elevated C-reactive protein levels. J Am Coll Cardiol. 1999;34:1738–43. [DOI] [PubMed] [Google Scholar]
- 15.Sorlie PD, Nieto FJ, Adam E, Folsom AR, Shahar E, Massing M. A prospective study of cytomegalovirus, herpes simplex virus 1, and coronary heart disease: the atherosclerosis risk in communities (ARIC) study. Arch Intern Med. 2000;160:2027–32. [DOI] [PubMed] [Google Scholar]
- 16.Grahame-Clarke C, Chan NN, Andrew D, Ridgway GL, Betteridge DJ, Emery V, et al. Human cytomegalovirus seropositivity is associated with impaired vascular function. Circulation. 2003;108:678–83. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, Pei W, Welte T, Wu Y, Ye S, Yang Y. Cytomegalovirus infection is associated with elevated interleukin-10 in coronary artery disease. Atherosclerosis. 2005;179:133–7. [DOI] [PubMed] [Google Scholar]
- 18.Hsue PY, Hunt PW, Sinclair E, Bredt B, Franklin A, Killian M, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS. 2006;20:2275–83. [DOI] [PubMed] [Google Scholar]
- 19.Nerheim PL, Meier JL, Vasef MA, Li W-G, Hu L, Rice JB, et al. Enhanced cytomegalovirus infection in atherosclerotic human blood vessels. Am J Pathol. 2004;164:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridker PM, Hennekens CH, Stampfer MJ, Wang F. Prospective study of herpes simplex virus, cytomegalovirus, and the risk of future myocardial infarction and stroke. Circulation. 1998;98: 2796–9. [DOI] [PubMed] [Google Scholar]
- 21.Fagerberg B, Gnarpe J, Gnarpe H, Agewall S, Wikstrand J. Chlamydia pneumoniae but not cytomegalovirus antibodies are associated with future risk of stroke and cardiovascular disease: a prospective study in middle-aged to elderly men with treated hypertension. Stroke. 1999;30:299–305. [DOI] [PubMed] [Google Scholar]
- 22.Borgia MC, Mandolini C, Barresi C, Battisti G, Carletti F, Capobianchi MR. Further evidence against the implication of active cytomegalovirus infection in vascular atherosclerotic diseases. Atherosclerosis. 2001;157:457–62. [DOI] [PubMed] [Google Scholar]
- 23.Betjes MGH, Litjens NHR, Zietse R. Seropositivity for cytomegalovirus in patients with end-stage renal disease is strongly associated with atherosclerotic disease. Nephrol Dial Transplant. 2007;22:3298–303. [DOI] [PubMed] [Google Scholar]
- 24.Ozdemir FN, Akgul A, Altunoglu A, Bilgic A, Arat Z, Haberal M. The association between cytomegalovirus infection and atherosclerotic events in renal transplant recipients. Transplant Proc. 2007;39:990–2. [DOI] [PubMed] [Google Scholar]
- 25.Wolf SC, Brehm BR, Mayer O, Jurgens S, Schultze G, Risler T. Infectious risk factors for atherosclerotic vascular disease in hemodialysis patients–Chlamydia pneumoniae but not Helicobacter pylori or cytomegalovirus is associated with increased C-reactive protein. Ren Fail. 2004;26:279–87. [DOI] [PubMed] [Google Scholar]
- 26.Ji Y-N, An L, Zhan P, Chen X-H. Cytomegalovirus infection and coronary heart disease risk: a meta-analysis. Mol Biol Rep. 2012;39:6537–46.• This systematic review and meta-analysis examined the association of CMV and coronary artery atherosclerosis, looking at 55 case–control studies (6 prospective, 49 retrospective) involving 9000 cases and 8608 controls. CMV was a clear risk factor for coronary disease; the association was even stronger (OR 8.21) in studies where PCR was used as the detection method.
- 27.Tracy RP, Doyle MF, Olson NC, Huber SA, Jenny NS, Sallam R, et al. T-helper type 1 bias in healthy people is associated with cytomegalovirus serology and atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2013;2:e000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sacre K, Hunt PW, Hsue PY, Maidji E,Martin JN, Deeks SG, et al. A role for cytomegalovirus-specific CD4+CX3CR1+ T cells and cytomegalovirus-induced T-cell immunopathology in HIV-associated atherosclerosis. AIDS. 2012;26:805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Assinger A, Kral JB, Yaiw KC, Schrottmaier WC, Kurzejamska E, Wang Y, et al. Human cytomegalovirus-platelet interaction triggers toll-like receptor 2-dependent proinflammatory and proangiogenic responses. Arterioscler Thromb Vasc Biol. 2014;34:801–9. [DOI] [PubMed] [Google Scholar]
- 30.Ponroy N, Taveira A, Mueller NJ, Millard A-L. Statins demonstrate a broad anti-cytomegalovirus activity in vitro in ganciclovirsusceptible and resistant strains. J Med Virol. 2015;87:141–53.• This bench study examined the effect of statins on CMV-infected human aortic endothelial cells and fibroblasts in vitro. Atorva-, fluva-, and pravastatin, but not simvastatin, reduced CMV titers in both cell types with an efficacy comparable to ganciclovir. The effect was retained in ganciclovir-resistant strains.
- 31.Rosenblum WI, Hadfield MG, Young HF. Granulomatous angiitis with preceding varicella zoster. Ann Neurol. 1978;3:374–5. [DOI] [PubMed] [Google Scholar]
- 32.Gilden DH, Kleinschmidt-DeMasters BK, Wellish M, Hedley-Whyte ET, Rentier B, Mahalingam R. Varicella zoster virus, a cause of waxing and waning vasculitis: the New England Journal of Medicine case 5–1995 revisited. Neurology. 1996;47: 1441–6. [DOI] [PubMed] [Google Scholar]
- 33.Nagel MA, Gilden D. Update on varicella zoster virus vasculopathy. Curr Infect Dis Rep. 2014;16:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagel MA, Traktinskiy I, Azarkh Y, Kleinschmidt-DeMasters B, Hedley-Whyte T, Russman A, et al. Varicella zoster virus vasculopathy: analysis of virus-infected arteries. Neurology. 2011;77: 364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagel MA, Traktinskiy I, Stenmark KR, Frid MG, Choe A, Gilden D. Varicella-zoster virus vasculopathy: immune characteristics of virus-infected arteries. Neurology. 2013;80:62–8.• This study examined pathological changes in VZV-infected cerebral and temporal arteries at various stages of infection, and compared with control normal arteries. Inflammatory cells and viral antigen were found in abundance in the adventitia of VZV-infected arteries only, along with thickened intima, supporting a role for viral-induced inflammation in arterial wall remodeling.
- 36.Nagel MA, Forghani B, Mahalingam R, Wellish MC, Cohrs RJ, Russman AN, et al. The value of detecting anti-VZV IgG antibody in CSF to diagnose VZV vasculopathy. Neurology. 2007;68: 1069–73. [DOI] [PubMed] [Google Scholar]
- 37.Nagel MA, Khmeleva N, Choe A, Gutierrez J, Gilden D. Varicella zoster virus (VZV) in cerebral arteries of subjects at high risk for VZV reactivation. J Neurol Sci. 2014;339:32–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagel MA, Choe A, Khmeleva N, Overton L, Rempel A, Wyborny A, et al. Search for varicella zoster virus and herpes simplex virus-1 in normal human cerebral arteries. J Neurovirol. 2013;19:181–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilden D, White T, Khmeleva N, Heintzman A, Choe A, Boyer PJ, et al. Prevalence and distribution of VZV in temporal arteries of patients with giant cell arteritis. Neurology. 2015;84:1948–55.•• This pathological study looked for VZV antigen, virions and DNA in 82 temporal arteries from patients with biopsy-proven giant cell arteries, compared with 13 normal temporal arteries. VZV antigen was found in 74% of GCA-positive TAs, compared with 1/13 normal TAs (p<0.0001, RR 9.67, 95%CI 1.46–63.69). The VZV was found mostly in skip areas adjacent to regions with GCA pathology.
- 40.Langan SM, Minassian C, Smeeth L, Thomas SL. Risk of stroke following herpes zoster: a self-controlled case-series study. Clin Infect Dis. 2014;58:1497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang J-H, Ho J-D, Chen Y-H, Lin H-C. Increased risk of stroke after a herpes zoster attack: a population-based follow-up study. Stroke. 2009;40:3443–8. [DOI] [PubMed] [Google Scholar]
- 42.Lin H-C, Chien C-W, Ho J-D. Herpes zoster ophthalmicus and the risk of stroke: a population-based follow-up study. Neurology. 2010;74:792–7. [DOI] [PubMed] [Google Scholar]
- 43.Zepper P, Wunderlich S, Forschler A, Nadas K, Hemmer B, Sellner J. Pearls & Oy-sters: cerebral HSV-2 vasculitis presenting as hemorrhagic stroke followed by multifocal ischemia. Neurology. 2012;78:e12–5. [DOI] [PubMed] [Google Scholar]
- 44.Guerrero WR, Dababneh H, Hedna S, Johnson JA, Peters K, Waters MF. Vessel wall enhancement in herpes simplex virus central nervous system vasculitis. J Clin Neurosci. 2013;20:1318–9. [DOI] [PubMed] [Google Scholar]
- 45.Snider SB, Jacobs CS, Scripko PS, Klein JP, Lyons JL. Hemorrhagic and ischemic stroke secondary to herpes simplex virus type 2 meningitis and vasculopathy. J Neurovirol. 2014;20:419–22. [DOI] [PubMed] [Google Scholar]
- 46.Terlizzi V, Improta F, Di Fraia T, Sanguigno E, D’Amico A, Buono S, et al. Primary herpes virus infection and ischemic stroke in childhood: a new association? J Clin Neurosci. 2014;21:1656–8. [DOI] [PubMed] [Google Scholar]
- 47.Fullerton HJ, Elkind MSV, Glaser CA, Hills NK, Luna JM, Sear K, et al. Herpes viruses in childhood arterial ischemic stroke: interim results of the VIPS Study. Stroke. 2014;45:A38. [Google Scholar]
- 48.Mizusawa H, Hirano A, Llena JF, Shintaku M. Cerebrovascular lesions in acquired immune deficiency syndrome (AIDS). Acta Neuropathol. 1988;76:451–7. [DOI] [PubMed] [Google Scholar]
- 49.Kieburtz KD, Eskin TA, Ketonen L, Tuite MJ. Opportunistic cerebral vasculopathy and stroke in patients with the acquired immunodeficiency syndrome. Arch Neurol. 1993;50:430–2. [DOI] [PubMed] [Google Scholar]
- 50.Benjamin LA, Bryer A, Emsley HCA, Khoo S, Solomon T, Connor MD. HIV infection and stroke: current perspectives and future directions. The Lancet Neurology. 2012;11:878–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ovbiagele B, Nath A. Increasing incidence of ischemic stroke in patients with HIV infection. Neurology. 2011;76:444–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gutierrez J, Elkind MSV, Marshall RS. Cardiovascular profile and events of US adults 20–49 years with HIV: results from the NHANES 1999–2008. AIDS Care. 2013;25:1385–91. [DOI] [PubMed] [Google Scholar]
- 53.Vinikoor MJ, Napravnik S, Floris-Moore M, Wilson S, Huang DY, Eron JJ. Incidence and clinical features of cerebrovascular disease among HIV-infected adults in the Southeastern United States. AIDS Res Hum Retroviruses. 2013;29:1068–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mateen FJ, Post WS, Sacktor N, Abraham AG, Becker JT, Smith BR, et al. Long-term predictive value of the Framingham Risk Score for Stroke in HIV-positive vs HIV-negative men. Neurology. 2013;81:2094–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krsak M, Kent DM, Terrin N, Holcroft C, Skinner SC, Wanke C. Myocardial infarction, stroke, and mortality in cART-treated HIV patients on statins. AIDS Patient Care STDS. 2015;29:307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sico JJ, Chang C-CH, So-Armah K, Justice AC, Hylek E, Skanderson M, et al. HIV status and the risk of ischemic stroke among men. Neurology. 2015;84:1933–40.• This study examined the incidence of ischemic stroke in a cohort of 76,835 male veterans free of baseline cardiovascular disease. Over a median follow-up period of 5.9 years, HIV-infected veterans had a higher stroke rate than matched uninfected veterans (incidence rate ratio 1.25, p<0.01). The rate remained higher even after adjusting for comorbidities.
- 57.Chow FC, He W, Bacchetti P, Regan S, Feske SK, Meigs JB, et al. Elevated rates of intracerebral hemorrhage in individuals from a US clinical care HIV cohort. Neurology. 2014;83:1705–11.• This retrospective cohort study used billing data to compare rates of ICH in HIV-infected and uninfected patients. The incidence of ICH was higher in the HIV-infected patients (unadjusted incidence rate ratio of 1.85, 95%CI 1.37–2.47, p<0.001). In the multivariable model, the effect was more pronounced in young patients and in women.
- 58.Womack JA, Chang C-CH, So-Armah KA, Alcorn C, Baker JV, Brown ST, et al. HIV infection and cardiovascular disease in women. J Am Heart Assoc. 2014;3:e001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thakur KT, Lyons JL, Smith BR, Shinohara RT, Mateen FJ. Stroke in HIV-infected African Americans: a retrospective cohort study. J Neurovirol. 2015. [DOI] [PubMed] [Google Scholar]
- 60.Sweeney EM, Thakur KT, Lyons JL, Smith BR, Willey JZ, Cervantes-Arslanian AM, et al. Outcomes of intravenous tissue plasminogen activator for acute ischaemic stroke in HIV-infected adults. Eur J Neurol. 2014;21:1394–9. [DOI] [PubMed] [Google Scholar]
- 61.Chow FC, Bacchetti P, Kim AS, Price RW, Hsue PY. Effect of CD4+ cell count and viral suppression on risk of ischemic stroke in HIV infection. AIDS. 2014;28:2573–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marcus JL, Leyden WA, Chao CR, Chow FC, Horberg MA, Hurley LB, et al. HIV infection and incidence of ischemic stroke. AIDS. 2014;28:1911–9. [DOI] [PubMed] [Google Scholar]
- 63.Goldstein DA, Timpone J, Cupps TR. HIV-associated intracranial aneurysmal vasculopathy in adults. J Rheumatol. 2010;37:226–33. [DOI] [PubMed] [Google Scholar]
- 64.Gutierrez J, Glenn M, Isaacson RS, Marr AD, Mash D, Petito C. Thinning of the arterial media layer as a possible preclinical stage in HIV vasculopathy: a pilot study. Stroke. 2012;43:1156–8. [DOI] [PubMed] [Google Scholar]
- 65.Gutierrez J, Elkind MSV, Petito C, Chung DY, Dwork AJ, Marshall RS. The contribution of HIV infection to intracranial arterial remodeling: a pilot study. Neuropathology. 2013;33:256–63.• This pathological study examined a measure of arterial remodeling by comparing the ratio of arterial lumen diameter to wall thickness in 51 cerebral arteries from 5 HIV-infected and 13 uninfected brain donors. Arteries from HIV-infected donors had significantly higher ratios, suggesting an association of HIV infection with brain outward arterial remodeling.
- 66.Gutierrez J, Rosoklija G, Murray J, Chon C, Elkind MSV, Goldman J, et al. A quantitative perspective to the study of brain arterial remodeling of donors with and without HIV in the Brain Arterial Remodeling Study (BARS). Front Physiol. 2014;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gutierrez J, Goldman J, Dwork AJ, Elkind MSV, Marshall RS, Morgello S. Brain arterial remodeling contribution to nonembolic brain infarcts in patients with HIV. Neurology. 2015;85:1–8.•• Expanding on prior work (see ref 65), this study examined 1, 878 cerebral artery segments from 284 HIV-infected and uninfected brain donors. HIV infection was associated with two extremes of arterial remodeling: inward remodeling with accelerated cerebral atherosclerosis causing ischemic infarcts, and outward remodeling causing dolichoectasia and otherwise unexplained infarcts. The dolichoectatic subtype predominated in more immunosuppressed patients.
- 68.Douvoyiannis M, Litman N, Goldman DL. Neurologic manifestations associated with parvovirus B19 infection. Clin Infect Dis. 2009;48:1713–23. [DOI] [PubMed] [Google Scholar]
- 69.Mandrioli J, Portolani M, Cortelli P, Sola P. Middle cerebral artery thrombosis in course of parvovirus B19 infection in a young adult: a new risk factor for stroke? J Neurovirol. 2004;10:71–4. [DOI] [PubMed] [Google Scholar]
- 70.Isumi H, Nunoue T, Nishida A, Takashima S. Fetal brain infection with human parvovirus B19. Pediatr Neurol. 21:661–3. [DOI] [PubMed] [Google Scholar]
- 71.Luna JM, Fullerton HJ, Wintermark M, deVeber GA, Hills NK, Muhammad K, et al. Parvovirus B19 DNA prevalence is increased in pediatric stroke patients compared to controls: pilot findings from the Vascular Effects of Infection in Pediatric Stroke (VIPS) study. Stroke. 2014;45:A36. [Google Scholar]
- 72.Elkind MSV. Why now? Moving from stroke risk factors to stroke triggers. Curr Opin Neurol. 2007;20:51–7. [DOI] [PubMed] [Google Scholar]
- 73.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–8. [DOI] [PubMed] [Google Scholar]
- 74.Elkind MSV, Carty CL, O’Meara ES, Lumley T, Lefkowitz D, Kronmal RA, et al. Hospitalization for infection and risk of acute ischemic stroke: the Cardiovascular Health Study. Stroke. 2011;42:1851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gurfinkel EP, de la Fuente RL. Two-year follow-up of the FLU Vaccination Acute Coronary Syndromes (FLUVACS) Registry. Tex Heart Inst J. 2004;31:28–32. [PMC free article] [PubMed] [Google Scholar]
- 76.Grau AJ, Fischer B, Barth C, Ling P, Lichy C, Buggle F. Influenza vaccination is associated with a reduced risk of stroke. Stroke. 2005;36:1501–6. [DOI] [PubMed] [Google Scholar]
- 77.Davis MM, Taubert K, Benin AL, Brown DW, Mensah GA, Baddour LM, et al. Influenza vaccination as secondary prevention for cardiovascular disease: a science advisory from the American Heart Association/American College of Cardiology. J Am Coll Cardiol. 2006;48:1498–502. [DOI] [PubMed] [Google Scholar]
- 78.Lin H-C, Chiu H-F, Ho S-C, Yang C-Y. Association of influenza vaccination and reduced risk of stroke hospitalization among the elderly: a population-based case–control study. Int J Environ Res Public Health. 2014;11:3639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lavallee PC, Labreuche J, Fox KM, Lavados P, Mattle H, Steg PG, et al. Influenza vaccination and cardiovascular risk in patients with recent TIA and stroke. Neurology. 2014;82:1905–13.• This meta-analysis pooled data from 2 prospective cohort studies and one randomized trial (total 23,110 patients) to examine whether influenza vaccination was associated with reduced risk of major vascular events in patients with recent TIA or ischemic stroke. After adjusting for baseline characteristics, influenza vaccination was not associated with a reduction in vascular event rate. Subgroup analysis similarly showed no reduction in stroke or MI separately.
- 80.Rahman B, Heywood A, Moa A, MacIntyre CR. Influenza vaccination and cardiovascular risk in patients with recent TIA and stroke. Neurology. 2015;84:105. [DOI] [PubMed] [Google Scholar]
- 81.Vila-Corcoles A, Ochoa-Gondar O, Rodriguez-Blanco T, Gutierrez-Perez A, Vila-Rovira A, Gomez F, et al. Clinical effectiveness of pneumococcal vaccination against acute myocardial infarction and stroke in people over 60 years: the CAPAMIS study, one-year follow-up. BMC Public Health. 2012;12:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vila-Corcoles A, Ochoa-Gondar O, Rodriguez-Blanco T, de Diego C, Satue E. Ineffectiveness of pneumococcal vaccination in cardiovascular prevention: the CAPAMIS study. JAMA Intern Med. 2013;173:1918–20.• This prospective cohort study followed 27,204 patients over age 60 in primary care practices in Spain, to assess the effect of pneumococcal vaccination on hospitalization for stroke, MI and community acquired pneumonia. At 3 year follow-up, there was no protective effect of vaccination on any of these measures, in contrast to interim analysis that had shown such an effect.
- 83.Tseng HF, Slezak JM, Quinn VP, Sy LS, Van den Eeden SK, Jacobsen SJ. Pneumococcal vaccination and risk of acute myocardial infarction and stroke in men. JAMA. 2010;303:1699–706. [DOI] [PubMed] [Google Scholar]
- 84.Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1:CD000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bloemenkamp DGM, Mali WPTM, Tanis BC, Rosendaal FR, van den Bosch MAAJ, Kemmeren JM, et al. Chlamydia pneumoniae, Helicobacter pylori and cytomegalovirus infections and the risk of peripheral arterial disease in young women. Atherosclerosis. 2002;163:149–56. [DOI] [PubMed] [Google Scholar]
- 87.Haider AW, Wilson PWF, Larson MG, Evans JC, Michelson EL, Wolf PA, et al. The association of seropositivity to Helicobacter pylori, Chlamydia pneumoniae, and cytomegalovirus with risk of cardiovascular disease: a prospective study. J Am Coll Cardiol. 2002;40:1408–13. [DOI] [PubMed] [Google Scholar]
- 88.Smieja M, Gnarpe J, Lonn E, Gnarpe H, Olsson G, Yi Q, et al. Multiple infections and subsequent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation. 2003;107:251–7. [DOI] [PubMed] [Google Scholar]
- 89.Khairy P, Rinfret S, Tardif J-C, Marchand R, Shapiro S, Brophy J, et al. Absence of association between infectious agents and endothelial function in healthy young men. Circulation. 2003;107: 1966–71. [DOI] [PubMed] [Google Scholar]
- 90.Muller BT, Huber R, Henrich B, Adams O, Berns G, Siebler M, et al. Chlamydia pneumoniae, herpes simplex virus and cytomegalovirus in symptomatic and asymptomatic high-grade internal carotid artery stenosis. Does infection influence plaque stability? Vasa. 2005;34:163–9. [DOI] [PubMed] [Google Scholar]
- 91.Hagiwara N, Toyoda K, Inoue T, Shimada H, Ibayashi S, Iida M, et al. Lack of association between infectious burden and carotid atherosclerosis in Japanese patients. J Stroke Cerebrovasc Dis. 2007;16:145–52. [DOI] [PubMed] [Google Scholar]
- 92.Rupprecht HJ, Blankenberg S, Bickel C, Rippin G, Hafner G, Prellwitz W, et al. Impact of viral and bacterial infectious burden on long-term prognosis in patients with coronary artery disease. Circulation. 2001;104:25–31. [DOI] [PubMed] [Google Scholar]
- 93.Espinola-Klein C, Rupprecht HJ, Blankenberg S, Bickel C, Kopp H, Victor A, et al. Impact of infectious burden on progression of carotid atherosclerosis. Stroke. 2002;33:2581–6. [DOI] [PubMed] [Google Scholar]
- 94.Elkind MSV, Ramakrishnan P, Moon YP, Boden-Albala B, Liu KM, Spitalnik SL, et al. Infectious burden and risk of stroke: the northern Manhattan study. Arch. Neurol. [Internet]. 2009;67:33–8. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=19901154&retmode=ref&cmd=prlinks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Katan M, Moon YP, Paik MC, Sacco RL, Wright CB, Elkind MSV. Infectious burden and cognitive function: the Northern Manhattan Study. Neurology. 2013;80:1209–15.• A composite index of infectious burden was previously shown to be associated with stroke risk and atherosclerosis in this multi-ethnic urban cohort. In this study, the same measure was found to be associated with worse performance on cognitive assessments, even after adjusting for vascular risk factors. The measure was not associated with cognitive decline over time.
- 96.Wright CB, Gardener H, Dong C, Yoshita M, DeCarli C, Sacco RL, et al. Infectious burden and cognitive decline in the northern Manhattan study. J Am Geriatr Soc. 2015;63:1540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bu X-L, Yao X-Q, Jiao S-S, Zeng F, Liu Y-H, Xiang Y, et al. A study on the association between infectious burden and Alzheimer’s disease. Eur J Neurol. 2014. [DOI] [PubMed] [Google Scholar]
- 98.O’Connor CM, Dunne MW, Pfeffer MA, Muhlestein JB, Yao L, Gupta S, et al. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459–66. [DOI] [PubMed] [Google Scholar]
- 99.Grayston JT, Kronmal RA, Jackson LA, Parisi AF, Muhlestein JB, Cohen JD, et al. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637–45. [DOI] [PubMed] [Google Scholar]
- 100.Westendorp WF, Nederkoorn PJ, Vermeij J-D, Dijkgraaf MG, van de Beek D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 2011;11:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lord AS, Langefeld CD, Sekar P, Moomaw CJ, Badjatia N, Vashkevich A, et al. Infection after intracerebral hemorrhage: risk factors and association with outcomes in the ethnic/racial variations of intracerebral hemorrhage study. Stroke. 2014;45:3535–42.• This retrospective study of 800 patients with ICH found that post-ICH infections were more likely to occur in patients with larger and deeper hemorrhages, and in black patients. Patients with infections had higher discharge mortality (16% vs 8%, p=0.001) and worse 3-month functional outcomes.
- 102.Emsley HCA, Smith CJ, Gavin CM, Georgiou RF, Vail A, Barberan EM, et al. Clinical outcome following acute ischaemic stroke relates to both activation and autoregulatory inhibition of cytokine production. BMC Neurol. 2007;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chamorro A, Urra X, Planas AM. Infection after acute ischemic stroke: a manifestation of brain-induced immunodepression. Stroke. 2007;38:1097–103. [DOI] [PubMed] [Google Scholar]
- 104.Westendorp WF, Vermeij J-D, Zock E, Hooijenga IJ, Kruyt ND, Bosboom HJLW, et al. The Preventive Antibiotics in Stroke Study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet. 2015;385:1519–26.• This PROBE-design trial randomly assigned 2550 patients in the first 24 hours after acute stroke admitted to stroke units to IV ceftriaxone for 4 days vs usual care. In the intention-to-treat analysis, antibiotic treatment did not improve functional outcomes at three months.
- 105.Zierath D, Kunze A, Fecteau L, Becker K. Effect of antibiotic class on stroke outcome. Stroke. 2015;46:2287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]