Key Points
Question
Does restricting dietary free sugars reduce hepatic steatosis in children with nonalcoholic fatty liver disease?
Findings
In this randomized clinical trial that included 40 adolescent boys aged 11 to 16 years with nonalcoholic fatty liver disease followed up for 8 weeks, provision of a diet low in free sugars compared with usual diet resulted in a greater reduction in hepatic steatosis from 25% to 17% in the low free sugar diet group and from 21% to 20% in the usual diet group, a statistically significant difference of −6.23% when adjusted for baseline.
Meaning
These preliminary findings suggest potential benefit of a diet low in free sugars for children with nonalcoholic fatty liver disease, but further research is needed to assess long-term and clinical outcomes.
Abstract
Importance
Pediatric guidelines for the management of nonalcoholic fatty liver disease (NAFLD) recommend a healthy diet as treatment. Reduction of sugary foods and beverages is a plausible but unproven treatment.
Objective
To determine the effects of a diet low in free sugars (those sugars added to foods and beverages and occurring naturally in fruit juices) in adolescent boys with NAFLD.
Design, Setting, and Participants
An open-label, 8-week randomized clinical trial of adolescent boys aged 11 to 16 years with histologically diagnosed NAFLD and evidence of active disease (hepatic steatosis >10% and alanine aminotransferase level ≥45 U/L) randomized 1:1 to an intervention diet group or usual diet group at 2 US academic clinical research centers from August 2015 to July 2017; final date of follow-up was September 2017.
Interventions
The intervention diet consisted of individualized menu planning and provision of study meals for the entire household to restrict free sugar intake to less than 3% of daily calories for 8 weeks. Twice-weekly telephone calls assessed diet adherence. Usual diet participants consumed their regular diet.
Main Outcomes and Measures
The primary outcome was change in hepatic steatosis estimated by magnetic resonance imaging proton density fat fraction measurement between baseline and 8 weeks. The minimal clinically important difference was assumed to be 4%. There were 12 secondary outcomes, including change in alanine aminotransferase level and diet adherence.
Results
Forty adolescent boys were randomly assigned to either the intervention diet group or the usual diet group (20 per group; mean [SD] age, 13.0 [1.9] years; most were Hispanic [95%]) and all completed the trial. The mean decrease in hepatic steatosis from baseline to week 8 was significantly greater for the intervention diet group (25% to 17%) vs the usual diet group (21% to 20%) and the adjusted week 8 mean difference was −6.23% (95% CI, −9.45% to −3.02%; P < .001). Of the 12 prespecified secondary outcomes, 7 were null and 5 were statistically significant including alanine aminotransferase level and diet adherence. The geometric mean decrease in alanine aminotransferase level from baseline to 8 weeks was significantly greater for the intervention diet group (103 U/L to 61 U/L) vs the usual diet group (82 U/L to 75 U/L) and the adjusted ratio of the geometric means at week 8 was 0.65 U/L (95% CI, 0.53 to 0.81 U/L; P < .001). Adherence to the diet was high in the intervention diet group (18 of 20 reported intake of <3% of calories from free sugar during the intervention). There were no adverse events related to participation in the study.
Conclusions and Relevance
In this study of adolescent boys with NAFLD, 8 weeks of provision of a diet low in free sugar content compared with usual diet resulted in significant improvement in hepatic steatosis. However, these findings should be considered preliminary and further research is required to assess long-term and clinical outcomes.
Trial Registration
ClinicalTrials.gov Identifier: NCT02513121
In this randomized clinical trial, a diet low in free sugars reduced hepatic steatosis and liver enzyme levels in Hispanic adolescent boys with nonalcoholic fatty liver disease compared with their usual diet combined with a weekly food stipend.
Introduction
From 1988 to 2010, the prevalence of nonalcoholic fatty liver disease (NAFLD) increased among children in the United States, becoming the most common liver disease in children.1,2 Pediatric NAFLD is more common in boys than girls, and in Hispanic children compared with other races and ethnicities.2,3 It is important to identify and treat NAFLD in children because it is associated with increased risk of type 2 diabetes, end-stage liver disease, liver cancer, and cardiovascular disease.4
There are no approved pharmacological therapies for the treatment of NAFLD. Pediatric guidelines recommend “lifestyle modification to improve diet,”5 but do not support one specific diet over another because of the limited available evidence. Among the various dietary options, limiting sugar intake is easily targetable in part because sugar is not a required nutrient. Basic science and epidemiological studies support a role for dietary sugar in the development and progression of NAFLD.6 Moreover, sugar added to foods and beverages as part of processing or preparation (added sugars) accounted for 13% to 17% of dietary calories, well over the recommended limit,7 among 6412 US children and adolescents in the 2009-2012 National Health and Nutrition Examination Survey.
Because of growing evidence implicating dietary sugars in NAFLD, well-controlled studies in children with NAFLD are needed to inform clinical practice and public policy. Previous studies in children with NAFLD have typically relied on nutritional education to induce dietary change; however, this approach may not lead to sufficient reduction of sugar due to its pervasive presence in foods and beverages. To maximize control over the diet while maintaining usual activities, a randomized clinical trial using a feeding study design was performed to test the hypothesis that free sugar restriction would reduce hepatic fat content in children with NAFLD.
Methods
Participants and Study Design
Children with NAFLD were enrolled at 2 US academic clinical research centers (Emory University and University of California, San Diego) from August 2015 to July 2017 and the final date of follow-up was September 2017. Written informed consent from the parent or guardian and assent from the adolescent boys were obtained. Ethics approval was obtained from the institutional review boards of the University of California, San Diego, and Emory University. The trial protocol was uploaded to the Open Science Framework prior to initiation of the study and also appears in Supplement 1.
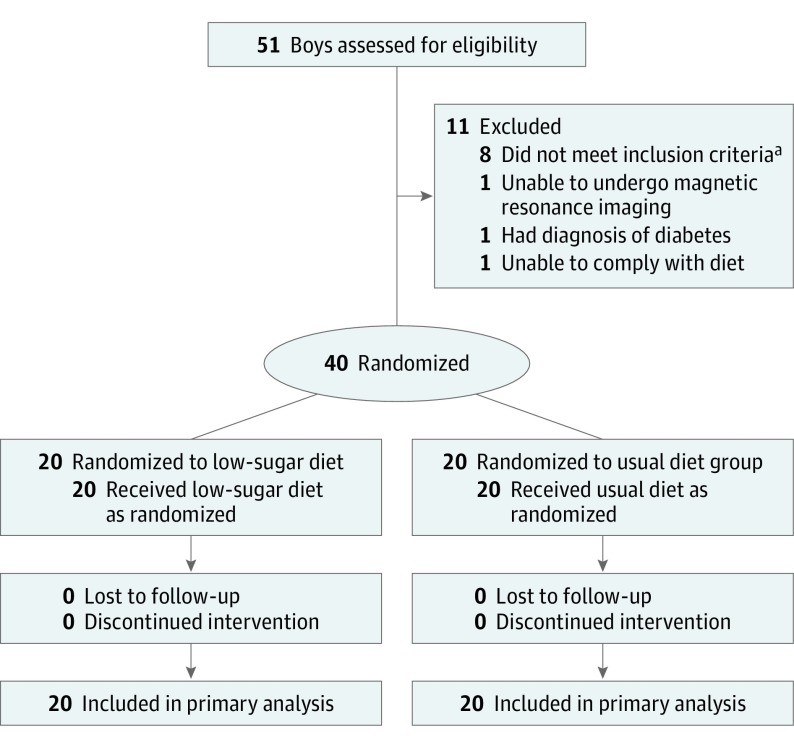
The study design was a randomized, parallel assignment clinical trial without blinding (open label) due to the impracticality of blinding diets. Participants eligible for the study were adolescent boys aged 11 to 16 years with a clinical-pathological diagnosis of NAFLD and current evidence of active disease, which was determined by the ongoing presence of hepatic steatosis estimated by a magnetic resonance imaging (MRI) proton density fat fraction (PDFF) measurement of 10% or greater and a serum alanine aminotransferase (ALT) level of 45 U/L or greater (Figure 1).
Figure 1. Consort Flow Diagram of Dietary Treatment Study Participants.
aBased on either the alanine aminotransferase level or the magnetic resonance imaging proton density fat fraction percentage measurement.
This study was performed in only adolescent boys to maximize participant homogeneity because the majority of pediatric patients with NAFLD are boys and the role of pubertal hormones and menarche are incompletely understood.8 Because all participants had a diagnosis of NAFLD, they had previously received counseling on diet and exercise as part of their clinical care. To determine whether ongoing excess free sugar consumption was present at the time of study enrollment, adolescent boys were eligible only if they currently consumed 3 servings or greater (8 ounces) of juice or sugar-sweetened beverages per week. The complete list of inclusion and exclusion criteria appears in the eMethods in Supplement 2.
Adolescent boys who met all inclusion criteria were randomly assigned to either the intervention (low in free sugar) diet group or the usual diet group in a 1:1 ratio. Randomization assignments were computer generated by the study statistician prior to the start of the study using random permuted block sizes of 2 or 4, stratifying by site, placing them in sealed envelopes, and supplying them to each site.
Details on the Diets
According to current guidelines from the World Health Organization,9 daily free sugar intake should be limited to less than 10% for all people and to less than 5% in specific circumstances. To ensure that daily free sugar intake was well below these targets, the goal for this study was set at a free sugar intake of less than 3% of daily calories for the intervention diet group.
Prior to initiation of the intervention diet, study staff inventoried all food items in the households of the participants in the intervention group. Sugar sweeteners and free sugar–containing products were removed and substituted with low or no-added-sugar food items. Use of artificial sweeteners was prohibited during the intervention because of the ongoing controversy regarding their effect on body weight and insulin sensitivity.10,11,12
Participants were instructed to avoid sugar-containing foods and drinks including fruit juice. Weekly meal plans were created collaboratively with the assistance of a registered dietitian and tailored to family preferences, habitual diet of the child (based on 24-hour food recall and a 7-day food diary), and within the requirement to keep free sugar intake to less than 3% of daily caloric intake without restricting total calorie intake and macronutrients. The provided study diet was matched to the reported baseline diet except for sugar content. Food sufficient for the entire family was delivered to each participant’s home twice per week. Participants and their families were instructed not to purchase any food and to consume only the food provided by the study staff. Food storage containers were supplied to facilitate transportation of the food from home to school.
Study-compliant meals were prepared at the Altman Clinical and Translational Research Institute metabolic kitchen by a certified research dietitian and by the Atlanta Clinical and Translational Science Institute metabolic kitchen by a certified registered dietician or purchased when low-sugar versions were readily available and preferred by families. When parents preferred to prepare their own meals, the ingredients for the meals were provided to ensure they met the free-sugar content requirement. To increase diet adherence, study staff performed twice-weekly telephone calls to assess food satisfaction, cravings, and assist with special family events or holiday alternatives. Participants in the usual diet group were instructed to continue their habitual diet and were provided with a weekly food stipend to be used at the retailer of their choice.
Clinical and Follow-up Evaluations
Study visits were conducted at baseline and at 4 and 8 weeks after initiation of the intervention. Race and ethnicity was collected because response to treatment may vary by race and ethnicity and was self-selected by a parent from a list of categories defined by the National Institutes of Health for ethnicity (Hispanic or non-Hispanic) and race (American Indian or Alaska Native, black, Native Hawaiian or Pacific Islander, white, Asian, other, or refused to answer). Each visit consisted of a medical history, vital signs, anthropometric assessments, and fasting blood collection for complete blood cell count, comprehensive metabolic panel, lipid panel, liver panel, prothrombin time, and international normalized ratio. Anthropometric assessments were performed twice at each visit and averaged.
The Nutrition Data System for Research (version 2015, University of Minnesota) was used for the collection and analysis of 24-hour dietary recalls along with the analysis of food records, menus, and recipes. Evaluation of each participant’s diet was performed during the screening phase and between study weeks 3 to 8. Each evaluation consisted of 3 separate 24-hour food recalls on 2 weekdays and on 1 weekend. Collected data included specific food items and estimated portion sizes for all meals consumed during the prior 24-hour period. In addition, a validated beverage consumption questionnaire (15-item Brief Questionnaire to Assess Habitual Beverage Intake) was used to assess sweetened beverage consumption at baseline.13 Participants were asked not to make any major changes to their physical activity routines during the study.
Primary Outcome
The primary outcome was change in percentage of hepatic steatosis measured by MRI-PDFF in the intervention diet group compared with change in the usual diet group over 8 weeks. Study participants were required to fast for longer than 4 hours prior to each MRI examination. The adolescent boys underwent MRI screening examinations at baseline and at the week 4 and 8 visits using an advanced magnitude-based, spoiled-gradient-echo MRI-PDFF estimation technique previously validated to measure hepatic steatosis in children.14,15 For each participant, data analysts co-localized the region-of-interest placement for the 4- and 8-week examinations to locations on the baseline examination. The liver imaging group at the University of California, San Diego, performed all analyses and was blinded to treatment group. Further details about the MRI screening examinations appear in the eMethods in Supplement 2.
Secondary Outcomes
Secondary outcomes included the homeostasis model assessment for insulin resistance; levels of ALT, aspartate aminotransferase, γ-glutamyl transpeptidase, fasting glucose, insulin, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides; sweetness perception testing (all outcomes compared for baseline vs 8 weeks and for change in the intervention diet group vs the usual diet group); diet adherence; and adverse events. All laboratory measurements were performed by the clinical laboratory at each site.
The sweetness perception testing was performed at baseline and at week 8. Participants rated the sweetness intensity of model beverages using a visual analog scale (range, 0-11) and the pleasantness of model beverages using a standard category scale (range, −4 [dislike extremely] to 0 [neither like nor dislike] to 4 [like extremely]) at 8 different sucrose concentrations ranging from barely sweet (100 mM) to very sweet (1000 mM). Diet adherence for the intervention group was defined as less than 3% consumption of free sugars during the intervention diet. Adverse events were monitored and summarized (details appear in the eMethods in Supplement 2).
Post hoc Comparisons
Data from the week 4 time point were collected and reported for hepatic steatosis and for some of the secondary outcomes. Anthropometric assessments and blood pressure were compared between groups in a post hoc analysis. A post hoc assessment of the correlations among weight, baseline free sugar consumption, and change in hepatic steatosis was performed.
Statistical Analysis
A sample size of 20 per group (total of 40 participants) was needed to provide 90% power to detect a true difference of means of 4% when the assumed standard deviation is 3% for hepatic steatosis measured by MRI-PDFF with a significance level of P = .05. A difference of 4% was assumed to be the minimal clinically significant change based on the clinical experience of the authors. Subsequently, a 4% change in steatosis has been shown to be associated with a histological response in adults with NAFLD.16 Power was calculated assuming that up to 20% of patients in each group would be lost to follow-up. Descriptive statistics including means and standard deviations, medians and interquartile ranges, and counts and percentages were calculated for the demographic characteristics, laboratory measures, and nutritional values at baseline.
To estimate the intervention effect for all primary, secondary, and post hoc analyses, mixed-effects models using baseline, week 4, and week 8 measurements conditioned on baseline values were used. This conditional joint response model, which is an extension to the traditional analysis of covariance model, is more tolerant to missing data and is less biased than the carrying forward of baseline measurements.17 This modeling approach was chosen to adjust for possible differences between groups at baseline.
Models were constructed using the PROC MIXED procedure in SAS.18 Standard errors were estimated using an unstructured covariance matrix. The Kenward-Roger method was used to estimate the degrees of freedom for the fixed effects. The results generated from these models are presented as differences in group means at week 8, are adjusted for baseline, and appear with 95% confidence intervals.19 Group-specific means and 95% confidence intervals are summarized for baseline and week 8.
Prior to modeling, outcomes were assessed for normality using histograms and probability density plots. Data were transformed prior to modeling to meet the assumption of normality. Residual plots by group were inspected to assess heteroscedasticity. In cases in which assumptions of normality were not met, log-transformed variables were modeled and an analysis was conducted on a log scale. Model estimates were exponentiated to present geometric means and mean ratios in place of mean differences. All models controlled for center as a fixed effect.
In a post hoc sensitivity analysis, the primary outcome was reanalyzed using treating center as a random effect. The usual diet group was used as the reference group. As a result, ratios less than 1 indicated a lower mean in the intervention diet group, whereas a ratio greater than 1 indicated a higher mean in the intervention diet group relative to the usual diet group. A post hoc analysis using a Pearson correlation coefficient with an associated 95% confidence interval was performed to correlate the change in weight with the change in hepatic fat content.
For the sweetness perception testing, the mean model rating estimates of sweetness perception and pleasantness at 5 different sucrose concentrations were compared between study groups and visits using penalized B-splines because of the nonlinear relationships among sucrose concentrations and perceived sweetness and pleasantness. Patient-specific random intercepts were used to account for patient variation. The Tukey method was used for adjustment of multiple comparisons. The sucrose concentration associated with the highest pleasantness rating was determined using a repeated-measures analysis of variance with a group × visit interaction. Square-root transformations of the most preferred sucrose concentration was used to meet model assumptions.
One participant’s week 8 MRI-PDFF data were missing due to a clerical error. In a post hoc sensitivity analysis, this participant’s week 4 MRI-PDFF data were carried forward to week 8. No imputation was performed for any secondary or post hoc outcomes because data were only incomplete for insulin and homeostasis model assessment for insulin resistance.
All statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc). Statistical significance was indicated by P < .05 and all significance testing was 2-sided. Additional details regarding the statistical analyses appear in Supplement 3.
Results
Study Participants
Fifty-one adolescent boys with NAFLD were screened to obtain the target of 40 participants (20 per group) who were randomized (Figure 1). The baseline demographic, clinical, diet, and laboratory characteristics of the 2 groups appear in Table 1. The mean (SD) age was 13.0 (1.9) years, most participants were Hispanic (95%), and baseline free sugar intake was 10% of total kilocalories in the intervention diet group and 11% in the usual diet group.
Table 1. Baseline Characteristics of Adolescent Boys With Nonalcoholic Fatty Liver Disease Randomized to Intervention (Low Free Sugar) Diet or Usual Diet.
| Intervention Diet (n = 20) |
Usual Diet (n = 20) |
|
|---|---|---|
| Age at screening, mean (SD), y | 12.8 (1.8) | 13.4 (1.9) |
| Income of household, No. (%) | ||
| <$15 000 | 4 (20) | 4 (20) |
| $15 000-$29 999 | 3 (15) | 9 (45) |
| $30 000-$49 999 | 7 (35) | 4 (20) |
| ≥$50 000 | 2 (10) | 2 (10) |
| Declined to answer | 4 (20) | 1 (5) |
| Race/ethnicity, No. (%) | ||
| Hispanic | 18 (90) | 20 (100) |
| Non-Hispanic white | 2 (10) | 0 |
| Dietary intake, median (IQR) | ||
| Total energy intake, kcal | 1420 (1220-1778) | 1721 (1440-1953) |
| Carbohydrates, % of total energy | 49.4 (46.4-54.3) | 50.5 (43.4-55.2) |
| Sugars | 21.6 (14.0-24.2) | 19.5 (14.9-28.9) |
| Added sugars | 9.8 (4.9-14.0) | 11.2 (7.7-19.2) |
| Free sugars | 10.0 (5.2-13.9) | 11.2 (7.0-20.3) |
| Protein, % of total energy | 43.6 (39.5-49.6) | 37.3 (32.8-44.7) |
| Fat, % of total energy | 13.6 (12.7-15.3) | 15.1 (12.7-16.3) |
| Anthropometric assessments, mean (SD) | ||
| Height, cm | 160.7 (9.9) | 164.1 (12.8) |
| Weight, kg | 88.1 (21.5) | 88.7 (26.3) |
| Body mass indexa | 33.7 (5.6) | 32.3 (6.3) |
| z score | 2.38 (0.28) | 2.22 (0.48) |
| Waist circumference, cm | 108.9 (12.3) | 107.7 (16.0) |
| Blood pressure, mean (SD), mm Hg | ||
| Systolic | 116.2 (9.3) | 117.6 (12.5) |
| Diastolic | 66.1 (6.7) | 65.3 (10.9) |
| Lipid levels, mean (SD), mg/dL | ||
| Total cholesterol | 162.1 (42.5) | 157.3 (31.3) |
| Low-density lipoprotein cholesterol | 100.5 (34.9) | 95.2 (24.1) |
| High-density lipoprotein cholesterol | 40.1 (7.1) | 40.5 (7.2) |
| Triglycerides | 144.2 (83.0) | 148.0 (48.7) |
| Insulin and glucose levels | ||
| Fasting glucose, mean (SD), mg/dL | 91.1 (9.8) | 90.5 (13.9) |
| Insulin, median (IQR), μIU/mLb | 34.0 (26.1-57.8) | 43.8 (32.1-59.2) |
| Homeostasis model assessment for insulin resistance, median (IQR)b | 7.9 (5.2-13.6) | 9.2 (6.8-12.6) |
| Liver enzyme levels, median (IQR) | ||
| Alanine aminotransferase, U/L | 82.0 (57.0-144.0) | 72.5 (57.0-113.5) |
| Aspartate aminotransferase, U/L | 44.0 (32.0-79.0) | 39.0 (34.5-63.5) |
| γ-Glutamyl transpeptidase, mg/dL | 52.5 (24.0-62.5) | 41.5 (28.0-61.5) |
| Hepatic steatosis | ||
| Nonalcoholic fatty liver disease activity score, median (IQR)c | 4 (3-5) | 4 (4-5) |
| Nonalcoholic steatohepatitis diagnosis, No. (%) | ||
| Nonalcoholic fatty liverd | 7 (35) | 6 (30) |
| Borderline nonalcoholic steatohepatitise | 9 (45) | 7 (35) |
| Nonalcoholic steatohepatitisf | 4 (20) | 7 (35) |
| Proton density fat fraction, mean (SD), %g | 25 (11) | 21 (8) |
Abbreviation: IQR, interquartile range.
SI conversion factors: To convert alanine aminotransferase, aspartate aminotransferase, and γ-glutamyl transpeptidase to μkat/L, multiply by 0.0167; glucose to mmol/L, multiply by 0.0555; high-density lipoprotein, low-density lipoprotein, and total cholesterol to mmol/L, multiply by 0.0259; insulin to pmol/L, multiply by 6.945; triglycerides to mmol/L, multiply by 0.0113.
Calculated as weight in kilograms divided by height in meters squared.
One participant from the intervention group was missing the baseline insulin laboratory measurement.
Based on NASH Clinical Research Network scoring system.20 Score range, 0-8; steatosis, 0-3; hepatocyte ballooning, 0-2; and lobular inflammation, 0-3.
Defined as steatosis with minimal or no inflammation.
Defined as steatosis and zone 1 or 3 liver injury pattern not sufficient to meet diagnosis of nonalcoholic steatohepatitis.
Defined as steatosis, inflammation, and hepatocyte ballooning injury.
Measured using magnetic resonance imaging.
All enrolled participants attended all planned study visits and completed participation in the study. In the intervention diet group, free sugars were estimated to contribute a mean of 1% of daily calories consumed at week 8 compared with 10% of daily calories consumed in the usual diet group (adjusted mean difference, −7.8% [95% CI, −10.4% to −5.1%], P < .001; Table 2). Eighteen of the 20 (90%) participants in the intervention diet group reported consuming estimated amounts of less than 3% for free sugars during the intervention.
Table 2. Baseline and 8-Week Model-Based Estimates of Nutrient Intake.
| Nutrient | Time Point | Least-Squares Mean (SD), % Total kcala | Adjusted Week 8 Mean Difference (95% CI), %b |
|
|---|---|---|---|---|
| Intervention Diet | Usual Diet | |||
| Carbohydrates | Baseline | 49 (5) | 49 (8) | |
| Week 8 | 43 (9) | 49 (8) | −5.9 (−10.5 to −1.2) | |
| Sugars | Baseline | 21 (7) | 22 (8) | |
| Week 8 | 15 (7) | 19 (7) | −3.9 (−8.0 to 0.1) | |
| Added | Baseline | 10 (6) | 13 (8) | |
| Week 8 | 1 (1) | 9 (6) | −7.0 (−9.7 to 4.3) | |
| Free | Baseline | 11 (6) | 14 (8) | |
| Week 8 | 1 (1) | 10 (7) | −7.8 (−10.4 to −5.1) | |
| Fat | Baseline | 14 (2) | 15 (2) | |
| Week 8 | 16 (3) | 15 (2) | 1.2 (−0.6 to 2.9) | |
| Protein | Baseline | 45 (7) | 39 (8) | |
| Week 8 | 52 (8) | 42 (8) | 8.3 (2.9 to 13.7) | |
| Total energy intake, kcalc | Baseline | 1374d | 1741d | |
| Week 8 | 1665d | 1603d | 1.1 (1.0 to 1.3)e | |
Data are from the mixed model and adjusted for study site.
Data were estimated from mixed models conditioned on baseline measurement and adjusted for baseline and study site unless otherwise noted.
Data followed a right-skewed distribution and were log transformed prior to the analysis.
Data are expressed as geometric means and were back transformed via exponentiation.
Data are expressed as a ratio of week 8 measures and adjusted for baseline and study site.
Primary Outcome
At baseline, MRI-PDFF measurements were higher in the intervention diet group compared with the usual diet group (mean of 25% vs 21%, respectively). The mean decrease in hepatic steatosis from baseline to week 8 was significantly greater for the intervention diet group (25% to 17%) compared with the usual diet group (21% to 20%) and the adjusted week 8 mean difference was −6.23% (95% CI, −9.45% to −3.02%) (P < .001; Table 3).
Table 3. Baseline and 8-Week Model-Based Comparisons for Primary and Secondary End Points.
| Outcome | Time Point | Least-Squares Mean (SD)a | Adjusted Week 8 Mean Difference (95% CI)b |
P Valuec | |
|---|---|---|---|---|---|
| Intervention Diet | Usual Diet | ||||
| Primary End Point | |||||
| MRI-PDFF measurement of hepatic steatosis, % | Baseline | 25 (11) | 21 (8) | ||
| Week 8 | 17 (10) | 20 (9) | −6.23 (−9.45 to −3.02) | <.001 | |
| Adjusted measurement, %d | Baseline | 25 (11) | 21 (8) | ||
| Week 8 | 18 (10) | 20 (9) | −5.71 (−8.89 to −2.54) | .001 | |
| Secondary End Points | |||||
| Glucose, mg/dL | Baseline | 91 (10) | 90 (14) | ||
| Week 8 | 87 (9) | 91 (8) | −4.90 (−9.87 to 0.07) | .05 | |
| Total cholesterol, mg/dL | Baseline | 162 (42) | 157 (31) | ||
| Week 8 | 147 (36) | 158 (29) | −15.16 (−25.67 to −4.65) | .006 | |
| Low-density lipoprotein cholesterol, mg/dL | Baseline | 101 (39) | 95 (24) | ||
| Week 8 | 90 (30) | 96 (24) | −9.58 (−19.30 to 0.15) | .05 | |
| High-density lipoprotein cholesterol, mg/dL | Baseline | 40 (7) | 41 (7) | ||
| Week 8 | 38 (6) | 41 (8) | −2.05 (−4.59 to 0.48) | .11 | |
Abbreviations: MRI, magnetic resonance imaging; PDFF, proton density fat fraction.
SI conversion factors: To convert glucose to mmol/L, multiply by 0.0555; high-density lipoprotein, low-density lipoprotein, and total cholesterol to mmol/L, multiply by 0.0259.
Data are from the mixed model and adjusted for study site.
Data were estimated from mixed models conditioned on baseline measurement and adjusted for baseline and study site.
Calculated using mixed model conditioned on baseline measurement and adjusted for study site.
Adjusted for individual weight change.
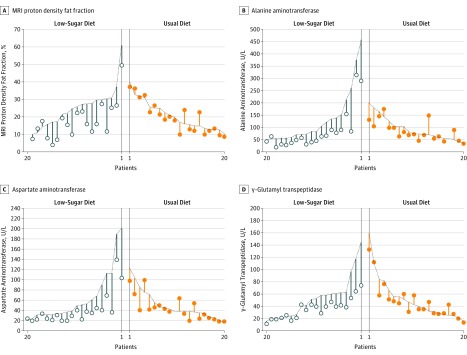
Individual-level data for change in MRI-PDFF measurements for the intervention diet group compared with the usual diet group appear in Figure 2. Upon further examination, there appeared to be outliers in the data causing the distribution of MRI-PDFF measurements to follow a right-skewed distribution. After log-transforming the MRI-PDFF measurements to reduce the effect of outliers, the week 8 adjusted geometric means were still lower in the intervention diet group compared with the usual diet group (15% vs 18%, respectively) and the adjusted ratio of the geometric means at week 8 was 0.69 (95% CI, 0.56 to 0.87, P = .003; Table 4).
Figure 2. Individual-Level Measurements for the Primary and Secondary Outcomes.
The primary outcome is magnetic resonance imaging (MRI) proton density fat fraction measurement of hepatic steatosis (panel A) and levels of alanine aminotransferase, aspartate aminotransferase, and γ-glutamyl transpeptidase (panels B-D) are secondary outcomes. Each vertical line represents 1 child sorted by treatment and baseline value. The baseline values are on the dotted line and the 8-week values are represented by the circles.
Table 4. Log-Transformed Baseline and 8-Week Model-Based Comparisons for Primary and Secondary End Points.
| Outcome | Time Point | Geometric Meana | Adjusted Week 8 Ratio of Geometric Means (95% CI)b |
P Valuec | |
|---|---|---|---|---|---|
| Intervention Diet | Usual Diet | ||||
| Primary End Point | |||||
| MRI-PDF measurement of hepatic steatosis, % | Baseline | 23 | 19 | ||
| Week 8 | 15 | 18 | 0.69 (0.56-0.87) | .003 | |
| Adjusted measurement, %d | Baseline | 23 | 19 | ||
| Week 8 | 15 | 18 | 0.72 (0.58-0.90) | .004 | |
| Secondary Endpoints | |||||
| Alanine aminotransferase, U/L | Baseline | 103 | 82 | ||
| Week 8 | 61 | 75 | 0.65 (0.53-0.81) | <.001 | |
| Aspartate aminotransferase, U/L | Baseline | 52 | 44 | ||
| Week 8 | 35 | 39 | 0.78 (0.65-0.92) | .005 | |
| γ-Glutamyl transpeptidase, U/L | Baseline | 44 | 44 | ||
| Week 8 | 33 | 43 | 0.77 (0.68-0.87) | <.001 | |
| Insulin, μIU/mLe | Baseline | 38 | 42 | ||
| Week 8 | 38 | 44 | 0.91 (0.72-1.14) | .37 | |
| Homeostasis model assessment for insulin resistancee | Baseline | 8.6 | 9.2 | ||
| Week 8 | 7.7 | 10.2 | 0.79 (0.60-1.03) | .07 | |
| Triglycerides, mg/dL | Baseline | 122 | 139 | ||
| Week 8 | 104 | 134 | 0.87 (0.72-1.04) | .13 | |
Abbreviations: MRI, magnetic resonance imaging; PDFF, proton density fat fraction.
SI conversion factors: To convert alanine aminotransferase, aspartate aminotransferase, and γ-glutamyl transpeptidase to μkat/L, multiply by 0.0167; insulin to pmol/L, multiply by 6.945; triglycerides to mmol/L, multiply by 0.0113.
Data are from the mixed model and adjusted for study site.
Data were estimated from mixed models conditioned on baseline measurement.
Calculated using mixed model conditioned on baseline measurement and adjusted for study site.
Adjusted for individual weight change.
Analyzed using only 19 participants with data at both baseline and week 8.
Secondary Outcomes
At week 8, mean levels of ALT, aspartate aminotransferase, and γ-glutamyl transpeptidase were significantly lower in the intervention diet group compared with the usual diet group after adjusting for center and conditioning on baseline levels. The geometric mean ALT level changed from 103 to 61 U/L in the intervention diet group and from 82 to 75 U/L in the usual diet group (adjusted ratio of the geometric means, 0.65 U/L [95% CI, 0.53 to 0.81 U/L], P < .001; Table 4).
At week 8, the mean total cholesterol level changed from 162 to 147 mg/dL in the intervention diet group and from 157 to 158 mg/dL in the usual diet group and there was a significant adjusted mean between-group difference of −15.16 mg/dL (95% CI, −25.67 to −4.65 mg/dL; P = .006). There were no significant differences in glucose, insulin, homeostasis model assessment for insulin resistance, triglycerides, low-density lipoprotein cholesterol, or high-density lipoprotein cholesterol.
No significant differences were observed for sweetness perception or pleasantness in each of the 5 sucrose concentrations (eTable 1 in Supplement 2). The most preferred sucrose concentration in the intervention diet group ranged from 416 mM (95% CI, 310 to 538 mM) at baseline to 509 mM (95% CI, 399 to 633 mM) at week 8. The intervention diet had no measurable effect on pleasantness perception; neither the main effects of group (P = .66), visit (P = .16), nor the group × visit interaction (P = .53) were significant.
Post hoc Comparisons
Mean weight decreased from 88.1 kg to 86.7 kg in the intervention diet group compared with an increase in weight in the usual diet group from 88.7 kg to 89.3 kg (between-group difference in means adjusted for baseline, −2.00 kg [95% CI, −3.30 to −0.79 kg]; P = .002). There were also significant differences in body mass index (calculated as weight in kilograms divided by height in meters squared), z score for body mass index, and systolic blood pressure (eTable 2 in Supplement 2).
There was a moderate correlation between change in weight and change in MRI-PDFF measurement for hepatic steatosis (r = 0.42; 95% CI, 0.12 to 0.65). When including weight change in the mixed model of MRI-PDFF measurement for hepatic steatosis conditional on baseline values, a significant between-group difference was maintained for week 8 MRI-PDFF measurements (adjusted week 8 ratio of the geometric means, 0.72 [95% CI, 0.58 to 0.90]; P = .004). After adding both weight change and baseline free sugar percentage to the model, the between-group difference at week 8 was unchanged (ratio of the geometric means, 0.72 [95% CI, 0.58 to 0.90]; P = .004).
In post hoc sensitivity analyses, the primary outcome was reanalyzed and there was minimal change in the results when treatment center was used as a random effect (eTable 3 in Supplement 2) and with the missing week 8 data point imputed (eTable 4 in Supplement 2). Week 4 results appear in the eFigure in Supplement 2.
Adverse Events
There were 3 adverse events reported during the study but none were related to participation (eResults in Supplement 2).
Discussion
This study demonstrated that a significant improvement in hepatic steatosis could be achieved in adolescent boys with NAFLD by restricting dietary sugars over 8 weeks. Current guidelines hold dietary intervention as an important component of treatment for NAFLD. However, the specific dietary recommendations vary. The most common recommendation is a reduced calorie diet, with many guidelines focused on restriction of carbohydrate intake.4,5,21,22 Nonetheless, the specific instructions as to what type of carbohydrate restriction should be implemented typically are vague and remain controversial.
Reduction of free sugars involves decreasing a number of dietary sugars including glucose, fructose, and sucrose commonly consumed in sweetened foods and beverages and in naturally sweet fruit juices. Biologically, fructose and fructose-containing sugars such as sucrose and high-fructose corn syrup appear to be of greater importance in elevating risk of liver fat accumulation than glucose, but both are sources of excess calories.23,24 Fructose increases hepatic de novo lipogenesis in a dose-dependent fashion25 and de novo lipogenesis has been shown to be abnormally unregulated in patients with NAFLD.26
Improvements in diet and increased exercise are the first-line therapy for NAFLD.5 However, despite the time spent by physicians counseling patients and families, implementation, long-term adherence, and sustainability of a healthy diet remain challenges for patients, researchers, and physicians. This clinical trial has shown that children and families can follow a diet low in free sugars for up to 8 weeks when the research team plans, purchases, and provides all meals. This is not practical to generalize widely; however, it shows that a low-sugar diet reduces biomarkers of NAFLD activity at least in the short term. Further studies will be needed to assess longer-term clinical benefit such as preventing progression or lowering the incidence of complications and to solve the challenges of implementing a low free sugar diet for patients with NAFLD in clinical practice.
This study had several strengths. First, all participants had a diagnosis of NAFLD based on history, physical examination, laboratory findings, and liver histology. Second, the provision of the food for the diet rather than just diet advice or education optimized the ability of participants to follow the diet and decreased the potential for other confounding dietary changes. Third, to assess hepatic steatosis, the primary outcome was change in hepatic MRI-PDFF, which is a precise measurement method.27 Changes in MRI-PDFF have been shown to correlate with changes in histological steatosis grade.14,16
Moreover, among adults, a 30% decrease in MRI-PDFF relative to baseline has been associated with greater odds of improvement in the NAFLD activity score.28 Liver transaminases also were obtained as a secondary outcome. Although reliance on liver enzyme levels as a surrogate for improvement in NAFLD has been controversial, recent data support that change in ALT level is a meaningful marker of change in liver histology.29 In 1 trial, histological improvement was significantly associated with a decrease in liver transaminases; for every 10% decrease in ALT level during the treatment period, there was a 1.24 times greater odds of histological improvement.30 In another trial, there was a 1.37 times greater odds of resolution of nonalcoholic steatohepatitis with every 10 U/L decrease in ALT level.31 The decrease in ALT level by 42 U/L (decrease of 40%) in the intervention diet group in the current trial is suggestive of improvements in the histology of NAFLD.
Limitations
The study has several limitations. First, the study was done in adolescent boys only; therefore, the efficacy of the same intervention in girls is not known. Second, because of the high prevalence of NAFLD in Hispanic children, most of the children in the study were Hispanic, which limits generalizability. Third, participants in the intervention diet group self-reported lower total calories and dietary sugar intake at baseline compared with participants in the usual diet group despite having similar body mass index and body weight. Fourth, the intervention showed significant change, but did not achieve reduction of hepatic steatosis or ALT level into the normal range. Liver biopsies would be needed to determine changes in liver inflammation and fibrosis but they were not included in this short, early-stage study. The absence of long-term or clinical outcomes such as liver biopsy and the intensive intervention raise the question of the cost-effectiveness of this approach.
Fifth, the artificiality of food delivery compared with implementation outside a study setting makes the likelihood of adherence to the intervention diet and its practicality lower if implemented. Studies testing other methods to reduce sugar in the diet of children with NAFLD and increased adherence to a low sugar diet should be considered. Sixth, the study was unable to blind participants to the intervention diet. Seventh, provision of food to one group and not the other may have imbalanced the amount of attention between the groups.
Conclusions
In this study of adolescent boys with NAFLD, 8 weeks of provision of a diet low in free sugar content compared with usual diet resulted in significant improvement in hepatic steatosis. However, these findings should be considered preliminary and further research is required to assess long-term and clinical outcomes.
Trial protocol
eMethods
eResults
eTable 1. Estimated Mean Sweetness and Pleasantness Scores
eTable 2. Model Based Baseline and Comparison of Week 8 Post Hoc Outcomes
eTable 3. Post Hoc Analysis of Effect of Center in the Model
eTable 4. Post Hoc Sensitivity Analysis for Missing Data
eFigure. Means of Hepatic Steatosis, ALT, AST and GGT at 0, 4 and 8 Weeks for Intervention and Control Groups
Statistical analysis plan
Data sharing plan
References
- 1.Anderson EL, Howe LD, Jones HE, et al. The prevalence of non-alcoholic fatty liver disease in children and adolescents. PLoS One. 2015;10(10):e0140908. doi: 10.1371/journal.pone.0140908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988-1994 to 2007-2010. J Pediatr. 2013;162(3):496-500.e1. doi: 10.1016/j.jpeds.2012.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115(5):e561-e565. doi: 10.1542/peds.2004-1832 [DOI] [PubMed] [Google Scholar]
- 4.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease. Gastroenterology. 2012;142(7):1592-1609. doi: 10.1053/j.gastro.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 5.Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children. J Pediatr Gastroenterol Nutr. 2017;64(2):319-334. doi: 10.1097/MPG.0000000000001482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vos MB, Lavine JE. Dietary fructose in nonalcoholic fatty liver disease. Hepatology. 2013;57(6):2525-2531. doi: 10.1002/hep.26299 [DOI] [PubMed] [Google Scholar]
- 7.Vos MB, Kaar JL, Welsh JA, et al. Added sugars and cardiovascular disease risk in children: a scientific statement from the American Heart Association. Circulation. 2017;135(19):e1017-e1034. doi: 10.1161/CIR.0000000000000439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller NT, Pereira MA, Demerath EW, et al. Earlier menarche is associated with fatty liver and abdominal ectopic fat in midlife, independent of young adult BMI: The CARDIA study. Obesity (Silver Spring). 2015;23(2):468-474. doi: 10.1002/oby.20950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization Guidelines: sugars intake for adults and children. https://www.who.int/nutrition/publications/guidelines/sugars_intake/en/. Accessed December 13, 2018. [PubMed]
- 10.Brown RJ, de Banate MA, Rother KI. Artificial sweeteners. Int J Pediatr Obes. 2010;5(4):305-312. doi: 10.3109/17477160903497027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackenzie T, Brooks B, O’Connor G. Beverage intake, diabetes, and glucose control of adults in America. Ann Epidemiol. 2006;16(9):688-691. doi: 10.1016/j.annepidem.2005.11.009 [DOI] [PubMed] [Google Scholar]
- 12.McNaughton SA, Mishra GD, Brunner EJ. Dietary patterns, insulin resistance, and incidence of type 2 diabetes in the Whitehall II Study. Diabetes Care. 2008;31(7):1343-1348. doi: 10.2337/dc07-1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hedrick VE, Myers EA, Zoellner JM, Duffey KJ, Davy BM. Validation of a rapid method to assess habitual beverage intake patterns. Nutrients. 2018;10(1):E83. doi: 10.3390/nu10010083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Middleton MS, Van Natta ML, Heba ER, et al. Diagnostic accuracy of magnetic resonance imaging hepatic proton density fat fraction in pediatric nonalcoholic fatty liver disease. Hepatology. 2018;67(3):858-872. doi: 10.1002/hep.29596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokoo T, Shiehmorteza M, Hamilton G, et al. Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology. 2011;258(3):749-759. doi: 10.1148/radiol.10100659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel J, Bettencourt R, Cui J, et al. Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Therap Adv Gastroenterol. 2016;9(5):692-701. doi: 10.1177/1756283X16656735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carpenter J, Kenward M. Data in Randomized Controlled Trials: A Practical Guide. London, England: London School of Hygiene and Tropical Medicine; 2007. [Google Scholar]
- 18.SAS/STAT® 9.2 User’s Guide. Cary, NC: SAS Institute Inc; 2008. [Google Scholar]
- 19.London School of Hygiene and Tropical Medicine Missing data in randomised controlled trials: a practical guide. http://www.missingdata.org.uk. Accessed Accessed December 1, 2015.
- 20.Brunt EM, Kleiner DE, Wilson LA, et al. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD. Hepatology. 2011;53(3):810-820. doi: 10.1002/hep.24127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arab JP, Candia R, Zapata R, et al. Management of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20(34):12182-12201. doi: 10.3748/wjg.v20.i34.12182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loria P, Adinolfi LE, Bellentani S, et al. Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42(4):272-282. doi: 10.1016/j.dld.2010.01.021 [DOI] [PubMed] [Google Scholar]
- 23.Chiu S, Mulligan K, Schwarz JM. Dietary carbohydrates and fatty liver disease: de novo lipogenesis. Curr Opin Clin Nutr Metab Care. 2018;21(4):277-282. doi: 10.1097/MCO.0000000000000469 [DOI] [PubMed] [Google Scholar]
- 24.Jin R, Welsh JA, Le NA, et al. Dietary fructose reduction improves markers of cardiovascular disease risk in Hispanic-American adolescents with NAFLD. Nutrients. 2014;6(8):3187-3201. doi: 10.3390/nu6083187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beysen C, Ruddy M, Stoch A, et al. Dose-dependent quantitative effects of acute fructose administration on hepatic de novo lipogenesis in healthy humans. Am J Physiol Endocrinol Metab. 2018;315(1):E126-E132. doi: 10.1152/ajpendo.00470.2017 [DOI] [PubMed] [Google Scholar]
- 26.Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146(3):726-735. doi: 10.1053/j.gastro.2013.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwimmer JB, Middleton MS, Behling C, et al. Magnetic resonance imaging and liver histology as biomarkers of hepatic steatosis in children with nonalcoholic fatty liver disease. Hepatology. 2015;61(6):1887-1895. doi: 10.1002/hep.27666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Middleton MS, Heba ER, Hooker CA, et al. Agreement between magnetic resonance imaging proton density fat fraction measurements and pathologist-assigned steatosis grades of liver biopsies from adults with nonalcoholic steatohepatitis. Gastroenterology. 2017;153(3):753-761. doi: 10.1053/j.gastro.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arsik I, Frediani JK, Frezza D, et al. Alanine aminotransferase as a monitoring biomarker in children with nonalcoholic fatty liver disease. Children (Basel). 2018;5(6):E64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwimmer JB, Lavine JE, Wilson LA, et al. In children with nonalcoholic fatty liver disease, cysteamine bitartrate delayed release improves liver enzymes but does not reduce disease activity scores. Gastroenterology. 2016;151(6):1141-1154.e9, e1149. doi: 10.1053/j.gastro.2016.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vuppalanchi R, Jain AK, Deppe R, et al. Relationship between changes in serum levels of keratin 18 and changes in liver histology in children and adults with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12(12):2121-30.e1, 2. doi: 10.1016/j.cgh.2014.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eMethods
eResults
eTable 1. Estimated Mean Sweetness and Pleasantness Scores
eTable 2. Model Based Baseline and Comparison of Week 8 Post Hoc Outcomes
eTable 3. Post Hoc Analysis of Effect of Center in the Model
eTable 4. Post Hoc Sensitivity Analysis for Missing Data
eFigure. Means of Hepatic Steatosis, ALT, AST and GGT at 0, 4 and 8 Weeks for Intervention and Control Groups
Statistical analysis plan
Data sharing plan