Key Points
Question
What are the complication rates and downstream medical costs associated with invasive diagnostic procedures for lung abnormalities in the community setting?
Findings
In this cohort study of 344 510 patients in national databases, the estimated complication rate was 22.2% for individuals in the younger age group (55-64 years) and 23.8% for those in the Medicare group (65-77 years). The complication costs varied by patient age and complication type, ranging from $6320 to $56 845.
Meaning
Shared decision-making communications between physicians and patients on lung cancer screening should include a discussion on the risks of subsequent adverse events and downstream costs associated with invasive diagnostic procedures.
Abstract
Importance
The Centers for Medicare & Medicaid Services added lung cancer screening with low-dose computed tomography (LDCT) as a Medicare preventive service benefit in 2015 following findings from the National Lung Screening Trial (NLST) that showed a 16% reduction in lung cancer mortality associated with LDCT. A challenge in developing and promoting a national lung cancer screening program is the high false-positive rate of LDCT because abnormal findings from thoracic imaging often trigger subsequent invasive diagnostic procedures and could lead to postprocedural complications.
Objective
To determine the complication rates and downstream medical costs associated with invasive diagnostic procedures performed for identification of lung abnormalities in the community setting.
Design, Setting, and Participants
A retrospective cohort study of non–protocol-driven community practices captured in MarketScan Commercial Claims & Encounters and Medicare supplemental databases was conducted. A nationally representative sample of 344 510 patients aged 55 to 77 years who underwent invasive diagnostic procedures between 2008 and 2013 was included.
Main Outcomes and Measures
One-year complication rates were calculated for 4 groups of invasive diagnostic procedures. The complication rates and costs were further stratified by age group.
Results
Of the 344 510 individuals aged 55 to 77 years included in the study, 174 702 comprised the study group (109 363 [62.6%] women) and 169 808 served as the control group (106 007 [62.4%] women). The estimated complication rate was 22.2% (95% CI, 21.7%-22.7%) for individuals in the young age group and 23.8% (95% CI, 23.0%-24.6%) for those in the Medicare group; the rates were approximately twice as high as those reported in the NLST (9.8% and 8.5%, respectively). The mean incremental complication costs were $6320 (95% CI, $5863-$6777) for minor complications to $56 845 (95% CI, $47 953-$65 737) for major complications.
Conclusions and Relevance
The rates of complications after invasive diagnostic procedures were higher than the rates reported in clinical trials. Physicians and patients should be aware of the potential risks of subsequent adverse events and their high downstream costs in the shared decision-making process.
This cohort study examines the rates and costs associated with complications of invasive diagnostic procedures performed in patients undergoing screening for lung cancer.
Introduction
Lung cancer is the leading cause of cancer-related deaths in the United States. Despite advances in cancer treatment, the 5-year survival rate of advanced-stage lung cancer has remained low at only 16%.1 Lung cancer is diagnosed at advanced stages in approximately 70% of the patients, making the development of effective screening strategies for lung cancer a public health priority nationwide. Efforts to establish an effective screening strategy for lung cancer had not been successful until the landmark National Lung Screening Trial (NLST). Published in 2011, the NLST demonstrated the efficacy of low-dose computed tomography (LDCT), reporting a 20% reduction in lung cancer–related death compared with chest radiography.2 Analyses with more mature data report an approximately 16% reduction in lung cancer mortality.3 Following the release of the NLST results, many professional societies issued guidelines recommending LDCT for individuals at high risk for lung cancer.4,5,6,7,8,9,10
Success of the NLST trial was somewhat shadowed by the high false-positive rate associated with LDCT. At a 23.3% false-positive rate across 3 rounds of screening, screening experts are concerned that the 8.5% to 9.8% complication rate from invasive diagnostic procedures reported in the NLST for those who experienced false-positive results associated with LDCT could translate to substantial harms and financial burden when lung cancer screening programs are implemented in the United States.11 This concern is heightened because the participants in the NLST tended to be healthier than the screening-eligible population in the United States. A recent analysis of Surveillance, Epidemiology, and End Results (SEER)-Medicare data showed that the mortality benefit from screening demonstrated by the NLST would likely diminish among elderly patients with significant comorbid conditions.12 Another concern is that most procedures used to evaluate abnormal findings in the NLST may have been performed at the participating sites, although this process was not required in the trial protocol. Because of the better health status of the trial participants and the observation that many NLST sites were high-volume facilities with proficiency in patient care,13,14,15,16,17,18,19 we hypothesized that the rates of complications after invasive diagnostic procedures observed among the screening-eligible general population would likely be higher than those reported in the NLST.
Using ages 55 to 77 years, which is the age eligibility criterion specified in the Decision Memo for Screening for Lung Cancer with Low-Dose Computed Tomography issued by the Centers for Medicare & Medicaid Services20 as the targeted age range for the lung cancer screening, we tested our hypothesis by estimating the complication rate associated with common invasive diagnostic procedures in community settings. To understand the potential financial outcome of the higher complication rates outside the trial setting, we also estimated the associated downstream costs.
Methods
Data
We used the 2008-2013 Truven MarketScan claims databases to identify a study cohort representative of the community practice setting. MarketScan data are from one of the largest US-based proprietary claims databases, covering nearly 240 million unique patients since 1995 and widely used in medical and health services research.21,22,23,24 MarketScan databases capture medical information on the full continuum of care in all settings, including inpatient and outpatient services and outpatient prescription drugs. Patient-level details can be linked via unique identifiers for consistency across services. The MarketScan Commercial Claims and Encounters database provides claims data for current employees and their spouses and dependents (age, ≤64 years), and the Coordination of Benefits database provides claims for Medicare-eligible retirees (age, ≥65 years) carrying supplemental insurance offered by their prior employers. Our study was considered exempt from review by the institutional review board at The University of Texas MD Anderson Cancer Center because of the use of deidentified data.
Invasive Diagnostic Procedures and Postprocedural Complications
The NLST protocol categorized 23 invasive diagnostic procedures into 4 groups2: cytology or needle biopsy, bronchoscopy, thoracic surgery, and others (eTable 1 in the Supplement). The NLST reported 43 complications classified as minor, intermediate, or major (eTable 2 in the Supplement).2 The clinicians on our research team mapped each of the diagnostic procedures and complications reported in the NLST to International Classification of Diseases, Ninth Revision (ICD-9) diagnosis and procedure codes as well as Healthcare Common Procedure Coding System (HCPCS) codes so that we could identify them from claims data. In determining an individual’s diagnostic procedure group, those who underwent more than 1 procedure for lung abnormality were categorized on the basis of the most invasive procedure.
Ascertainment of Study Cohort
The study cohort consisted of individuals aged 55 to 77 years, consistent with the Centers for Medicare & Medicaid Services age eligibility criterion for lung cancer screening with LDCT.20 Smoking status was not included because such information is unavailable in the claims databases. We further limited the cohort to individuals who had undergone invasive diagnostic procedures but did not have a diagnosis code indicative of lung cancer 12 months before and after these procedures. Because the period of our data was before February 5, 2015, the date that the billing codes for lung cancer screening with LDCT (HCPCS code G0297) became effective, our case cohort was not individuals who underwent invasive diagnostic procedures following a positive finding from LDCT but rather those who received the types of invasive procedures reported in the NLST to assess lung abnormalities.
In addition, we restricted our analysis to individuals who had continuous insurance coverage during the 12 months before and 12 months after the date of the index invasive procedure. By using complete, 12-month claims data before the index date, we calculated Charlson comorbidity index scores with the modified algorithm by Klabunde et al.25 The score was grouped as 0 for no comorbidity, 1 for mild, and 2 or higher for moderate to severe. We then extracted the following information from the MarketScan databases: year of procedure, age, sex, and state of residence.
Complications Attributable to Invasive Diagnostic Procedures
An analytical challenge for our study was that complications listed in the NLST report were not necessarily a result of the invasive diagnostic procedures; the complications could have developed for other reasons. To derive complications that were likely associated with the invasive procedures, we used an incremental approach by constructing a matched control cohort that did not undergo these invasive procedures between January 1, 2008, and December 31, 2013, compared the complication rates between the study and control cohorts, and attributed the differences in complication rates to the invasive procedures. Using a matched-control cohort to compute the attributable risks or costs associated with specific causes has been widely applied in oncology health services research.26,27,28
We assigned January 1 in the year of the index procedure received by the matched individuals in the study cohort as the pseudo-index date for individuals in the control cohort. The control group was required to have 1 year of insurance coverage before the pseudo-index date to calculate the comorbidity score and 1 year after the pseudo-index date to identify complication events. For both the study and control cohorts from the same year of data, we constructed the control cohort by conducting 1:1 propensity score matching by age, sex, state, and comorbidity.29 The balance between the study and control cohorts was tested using standardized differences.30
After stratifying individuals in the study cohort by the type of invasive diagnostic procedure, we further classified the study cohort into 4 age groups (55-59, 60-64, 65-69, and 70-77 years) within each procedural type, creating a total of 16 subgroups. Within each subgroup, we identified complication events that occurred within the 1-year observation window and calculated the gross rate of complications of each complication category by dividing the total number of individuals with complications in the corresponding category by the total number of individuals in that subgroup. Because some complications can be the trigger of these invasive procedures, we searched claims within 1 month prior to the invasive procedure date and, if the same diagnosis code used to determine complication was found within this 1-month time window, we did not consider that downstream event to be a complication. For the control group, we calculated the gross rate of complications of each category with age stratification. For each subgroup, we calculated the rate of complications attributable to invasive procedures by subtracting the gross rate of the control cohort from that of the study cohort.
Cost Analysis
Among individuals who experienced postprocedural complications, we estimated the 1-year complication costs by aggregating insurance payments and out-of-pocket expenditures for inpatient, outpatient, and physician services rendered on the dates for which an ICD-9 diagnosis, procedure, or HCPCS code was indicated for complications. We also estimated the mean procedure costs for each invasive diagnostic procedure group. All costs were normalized to 2017 US dollars by using the Consumer Price Index medical care component.31
Statistical Analysis
In addition to reporting complication rates and costs by 16 subgroups stratified by age group and type of procedure, we collapsed age strata from 4 to 2 groups (younger [age 55-64 years] vs Medicare [age 65-77 years]) so that the complications rates estimated from our study can be directly compared with the age-stratified complication rates reported from the NLST.11 Differences between individuals grouped on the basis of the 4 types of invasive diagnostic procedures, demographics, and comorbidities were analyzed using Pearson χ2 tests. A P value ≤.05, based on a 2-tailed test, was considered statistically significant. All analyses were conducted using the statistical software SAS, version 9.4 (SAS Institute Inc).
Results
Patient Characteristics
We identified 174 702 individuals (109 363 [62.6%] women) who underwent 1 of the invasive diagnostic procedures documented in the NLST for the overall cohort, and 169 808 individuals (106 007 [62.4%] women) for the control group after matching. Selected demographic and clinical characteristics of study patients and the matched controls are presented in Table 1.
Table 1. Descriptive Statistics of the Population Samples.
| Characteristic | Younger Age Group (n = 222 740) | Medicare Age Group (n = 116 876) | ||||
|---|---|---|---|---|---|---|
| Study Cohort, No. (%) (n = 111 370) | Matched Cohort, No. (%) (n = 111 370) | Standardized Differencea | Study Cohort, No. (%) (n = 58 438) | Matched Cohort, No. (%) (n = 58 438) | Standardized Differencea | |
| Year | ||||||
| 2008 | 13 820 (12.4) | 13 727 (12.3) | 0.0030 | 1219 (2.1) | 1284 (2.2) | 0.0249 |
| 2009 | 18 844 (16.9) | 18 858 (16.9) | 10 365 (17.7) | 10 191 (17.4) | ||
| 2010 | 21 422 (19.2) | 21 402 (19.2) | 10 963 (18.8) | 10 816 (18.5) | ||
| 2011 | 22 725 (20.4) | 22 760 (20.4) | 11 525 (19.7) | 11 343 (19.4) | ||
| 2012 | 17 815 (16.0) | 17 883 (16.1) | 11 171 (19.1) | 11 046 (18.9) | ||
| 2013 | 16 744 (15.0) | 16 740 (15.0) | 13 195 (22.6) | 13 758 (23.5) | ||
| Age, y | ||||||
| 55-59 | 60 469 (54.3) | 60 469 (54.3) | 0.0001 | 0.0001 | ||
| 60-64 | 50 901 (45.7) | 50 901 (45.7) | ||||
| 65-69 | 20 217 (34.6) | 20 249 (34.7) | ||||
| 70-77 | 38 221 (65.4) | 38 189 (65.4) | ||||
| Sex | ||||||
| Male | 40 788 (36.6) | 40 767 (36.6) | 0.0004 | 23 012 (39.4) | 23 034 (39.4) | 0.0008 |
| Female | 70 582 (63.4) | 70 603 (63.4) | 35 426 (60.6) | 35 404 (60.6) | ||
| Census divisionb | ||||||
| Pacific | 15 434 (13.9) | 15 436 (13.9) | 0.0038 | 8868 (15.5) | 8775 (15.3) | 0.0120 |
| New England | 6412 (5.8) | 6383 (5.7) | 3315 (5.8) | 3313 (5.8) | ||
| Middle Atlantic | 12 804 (11.5) | 12 709 (11.4) | 7532 (13.2) | 7732 (13.5) | ||
| Northeast central | 23 255 (20.9) | 23 224 (20.9) | 15 692 (27.4) | 15 649 (27.4) | ||
| Northwest central | 5071 (4.6) | 5075 (4.6) | 2662 (4.7) | 2689 (4.7) | ||
| South Atlantic | 22 455 (20.2) | 22 510 (20.2) | 9033 (15.8) | 8930 (15.6) | ||
| Southeast central | 8237 (7.4) | 8268 (7.4) | 2694 (4.7) | 2697 (4.7) | ||
| Southwest central | 11 810 (10.6) | 11 881 (10.7) | 4192 (7.3) | 4182 (7.3) | ||
| Mountain | 5892 (5.3) | 5884 (5.3) | 3215 (5.6) | 3254 (5.7) | ||
| Comorbidityc | ||||||
| 0 | 82 364 (74.0) | 82 012 (73.6) | 0.0093 | 36 012 (61.6) | 35 872 (61.4) | 0.0051 |
| 1 | 21 308 (19.1) | 21 416 (19.2) | 14 210 (24.3) | 14 274 (24.4) | ||
| 2+ | 7698 (6.9) | 7942 (7.1) | 8216 (14.1) | 8292 (14.2) | ||
Standardized difference measures the balance in baseline covariates between 2 groups, and a value less than 0.10 suggests the study cohort and control cohort are well balanced.
We used state to match the study cohort and control cohort, and we collapsed the states into census division to improve the readability of the table.
Charlson comorbidity index scores were grouped as 0 for no comorbidity, 1 for mild, and 2 or higher for moderate to severe.
Of the patients in the study cohort who were identified as having undergone an invasive diagnostic procedure, 44 319 (26.1%) had a cytology test or biopsy, 43 437 (25.6%) had a bronchoscopy, 9161 (5.4%) underwent thoracic surgery, and 72 891 (42.9%) underwent other procedures. Further data are reported in Table 2.
Table 2. Descriptive Statistics of the Study Sample, by Invasive Diagnostic Procedure.
| Characteristic | Total, No. | Cytology/Needle Biopsy, No. (%) (n = 44 319) | Bronchoscopy, No. (%) (n = 43 437) | Thoracic Surgery, No. (%) (n = 9161) | Other, No. (%) (n = 72 891) | P Value |
|---|---|---|---|---|---|---|
| Year | ||||||
| 2008 | 15 011 | 4063 (27.1) | 3221 (21.5) | 878 (5.8) | 6849 (45.6) | <.001 |
| 2009 | 29 049 | 7541 (26.0) | 7660 (26.4) | 1682 (5.8) | 12 166 (41.9) | |
| 2010 | 32 218 | 8487 (26.3) | 8357 (25.9) | 1848 (5.7) | 13 526 (42.0) | |
| 2011 | 34 103 | 8836 (25.9) | 8692 (25.5) | 1762 (5.2) | 14 813 (43.4) | |
| 2012 | 28 929 | 7554 (26.1) | 7268 (25.1) | 1501 (5.2) | 12 606 (43.6) | |
| 2013 | 30 498 | 7838 (25.7) | 8239 (27.0) | 1490 (4.9) | 12 931 (42.4) | |
| Age, y | ||||||
| 55-59 | 60 469 | 18 214 (30.1) | 12 833 (21.2) | 3156 (5.2) | 26 266 (43.4) | <.001 |
| 60-64 | 50 901 | 13 296 (26.1) | 12 008 (23.6) | 2745 (5.4) | 22 852 (44.9) | |
| 65-69 | 20 249 | 4695 (23.2) | 5935 (29.3) | 1175 (5.8) | 8444 (41.7) | |
| 70-77 | 38 189 | 8114 (21.2) | 12 661 (33.2) | 2085 (5.5) | 15 329 (40.1) | |
| Sex | ||||||
| Male | 63 801 | 21 211 (33.2) | 21 449 (33.6) | 4759 (7.5) | 16 382 (25.7) | <.001 |
| Female | 106 007 | 23 108 (21.8) | 21 988 (20.7) | 4402 (4.2) | 56 509 (53.3) | |
| Geographic division | ||||||
| Pacific | 24 211 | 6939 (28.7) | 5193 (21.4) | 1184 (4.9) | 10 895 (45.0) | <.001 |
| New England | 9696 | 2867 (29.6) | 1887 (19.5) | 618 (6.4) | 4324 (44.6) | |
| Middle Atlantic | 20 441 | 5051 (24.7) | 4610 (22.6) | 1310 (6.4) | 9470 (46.3) | |
| Northeast central | 38 873 | 9487 (24.4) | 10 966 (28.2) | 2161 (5.6) | 16 259 (41.8) | |
| Northwest central | 7764 | 1977 (25.5) | 1951 (25.1) | 453 (5.8) | 3383 (43.6) | |
| South Atlantic | 31 440 | 7726 (24.6) | 8866 (28.2) | 1529 (4.9) | 13 319 (42.4) | |
| Southeast central | 10 965 | 2522 (23.0) | 3352 (30.6) | 588 (5.4) | 4503 (41.1) | |
| Southwest central | 16 063 | 4793 (29.8) | 4113 (25.6) | 794 (4.9) | 6363 (39.6) | |
| Mountain | 9138 | 2708 (29.6) | 2105 (23.0) | 464 (5.1) | 3861 (42.3) | |
| Comorbidity | ||||||
| 0 | 117 884 | 30 377 (25.8) | 22 943 (19.5) | 5771 (4.9) | 58 793 (49.9) | <.001 |
| 1 | 35 690 | 9349 (26.2) | 13 223 (37.0) | 2331 (6.5) | 10 787 (30.2) | |
| ≥2 | 16 234 | 4593 (28.3) | 7271 (44.8) | 1059 (6.5) | 3311 (20.4) |
Postprocedural Complications
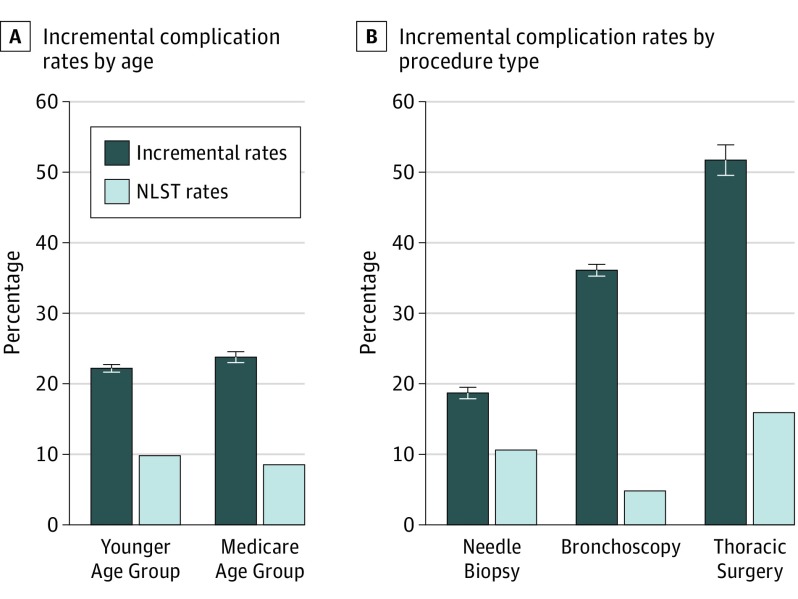
After adjustment (by taking into account the differences in complication rates between the study and control cohorts for the corresponding age groups), individuals in the younger age group had a lower rate of complications than those in the Medicare group (22.2%; 95% CI, 21.7%-22.7% vs 23.8%; 95% CI, 23.0%-24.6%). Compared with the complication rates reported in the NLST (9.8% and 8.5%), the complication rates found in our study were approximately 2 times higher for the younger age group and the Medicare group (Figure 1A).11 The comparison by procedure type showed that the complication rates were higher in our cohort (18.7% after needle biopsy, 36.1% after bronchoscopy, and 51.7% after thoracic surgery) than those reported in the NLST (Figure 1B). The detailed complication rates by age group and procedure type are reported in Table 3. Among these, for patients in the age range of 65 to 69 years who underwent bronchoscopy, the rate of minor complications was 23.6% (95% CI, 22.0%-25.4%); intermediate, 33.6% (31.3%-36.0%); and major, 13.6% (12.3%-14.9%), respectively.
Figure 1. Incremental Complication Rates From Invasive Diagnostic Procedures After False-Positive Screening Results.
A, Incremental complication rates in the younger age group (55-64 years) and the Medicare age group (65-77 years), with the complication rates reported in the National Lung Screening Trial (NLST) for comparison.11 B, Incremental complication rates stratified by the procedure type, with the complication rates reported in the NLST for comparison.2
Table 3. Incremental Complication Rates and Complication Costs Stratified by Procedure Type and Age Group.
| Procedure and Age Group, y | Rate (95% CI) | Cost (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Incremental Complication Rate, %a | Complication Cost, $ | ||||||
| Any | Minor | Intermediate | Major | Minor | Intermediate | Major | |
| Cytology/needle biopsy | |||||||
| All patients | 18.7 (17.9-19.5) | 13.6 (13.1-14.1) | 13.9 (13.1-14.6) | 4.0 (3.6-4.3) | 9501 (8804-10 197) | 15 252 (14 125-16 379) | 38 633 (33 861-43 405) |
| 55-59 | 15.8 (14.6-17.0) | 11.0 (10.3-11.7) | 11.6 (10.6-12.6) | 2.6 (2.2-3.0) | 10 177 (9076-11 278) | 17 860 (15 264-20 457) | 49 062 (35 854-62 270) |
| 60-64 | 18.7 (17.3-20.2) | 13.5 (12.6-14.4) | 13.6 (12.3-14.8) | 4.0 (3.4-4.5) | 12 154 (10 172-14 136) | 17 517 (15 093-19 940) | 51 816 (40 084-63 547) |
| 65-69 | 21.6 (19.1-24.2) | 15.6 (13.9-17.2) | 16.8 (14.5-19.1) | 4.7 (3.6-5.9) | 7159 (5881-8436) | 12 757 (10 334-15 180) | 44 135 (30 127-58 143) |
| 70-77 | 23.5 (21.3-25.6) | 18.7 (17.3-20.1) | 18.0 (16.1-19.8) | 6.6 (5.5-7.7) | 7494 (6575-8412) | 12 290 (10 791-13 789) | 23 050 (19 169-26 932) |
| Bronchoscopy | |||||||
| All patients | 36.0 (35.1-36.9) | 24.0 (23.3-24.6) | 32.1 (31.2-32.9) | 13.4 (12.9-13.9) | 7478 (6617-8340) | 18 985 (16 266-21 703) | 60 838 (50 490-71 187) |
| 55-59 | 37.7 (36.0-39.3) | 24.0 (22.9-25.1) | 32.2 (30.7-33.7) | 12.7 (12.0-13.5) | 7542 (6004-9080) | 20 810 (16 355-25 266) | 86 046 (53 021-119 070) |
| 60-64 | 36.7 (35.0-38.5) | 24.6 (23.4-25.7) | 32.4 (30.8-34.0) | 13.7 (12.9-14.6) | 9795 (7735-11 854) | 22 776 (16 082-29 470) | 57 410 (36 612-78 207) |
| 65-69 | 37.0 (34.5-39.7) | 23.6 (22.0-25.4) | 33.6 (31.3-36.0) | 13.6 (12.3-14.9) | 5573 (3637-7508) | 19 470 (9859-29 081) | 57 893 (37 899-77 888) |
| 70-77 | 33.2 (31.3-35.0) | 23.5 (22.3-24.7) | 30.9 (29.2-32.5) | 13.6 (12.5-14.6) | 6441 (5063-7819) | 14 368 (11 810-16 926) | 52 071 (38 268-65 874) |
| Surgical | |||||||
| All patients | 51.7 (49.6-53.9) | 46.5 (11.8-12.5) | 41.3 (39.6-43.0) | 11.3 (10.3-12.2) | 10 634 (9129-12 139) | 24 841 (22 129-27 552) | 63 034 (52 038-74 031) |
| 55-59 | 54.6 (51.1-58.1) | 46.1 (43.4-48.8) | 42.4 (39.3-45.5) | 10.1 (8.7-11.4) | 12 805 (9323-16 287) | 27 796 (22 311-33 281) | 78 283 (52 313-104 252) |
| 60-64 | 53.1 (49.3-56.9) | 47.8 (44.8-50.8) | 41.4 (38.0-44.8) | 11.3 (9.7-12.9) | 10 564 (8445-12 682) | 25 198 (20 592-29 804) | 75 015 (51 572-98 458) |
| 65-69 | 49.0 (43.5-55.7) | 45.5 (41.0-50.2) | 39.1 (33.9-44.5) | 13.7 (10.7-16.7) | 10 788 (5600-15 976) | 21 420 (15 031-27 809) | 46 544 (27 351-65 738) |
| 70-77 | 47.3 (42.3-51.6) | 46.2 (42.5-49.7) | 40.8 (36.5-44.8) | 11.8 (9.3-14.2) | 8008 (6409-9607) | 22 532 (17 317-27 746) | 52 926 (34 691-71 162) |
| Other | |||||||
| All patients | 13.7 (13.1-14.3) | 12.1 (11.8-12.5) | 6.3 (5.8-6.8) | 2.8 (2.6-3.0) | 6255 (5905-6606) | 10 942 (10 069-11 815) | 31 444 (26 971-35 917) |
| 55-59 | 14.4 (13.4-15.3) | 12.5 (11.9-13.1) | 6.1 (5.3-6.9) | 3.2 (2.9-3.5) | 7115 (6502-7729) | 11 877 (10 023-13 730) | 40 616 (29 680-51 551) |
| 60-64 | 13.9 (12.9-15.0) | 12.4 (11.7-13.0) | 6.1 (5.2-7.0) | 3.0 (2.7-3.4) | 6571 (5942-7199) | 13 882 (11 910-15 853) | 42 854 (31 400-54 309) |
| 65-69 | 13.6 (11.8-15.4) | 11.9 (10.8-13.1) | 6.4 (4.8-7.9) | 3.1 (2.4-3.8) | 6009 (4752-7265) | 9416 (7608-11 224) | 23 020 (16 944-29 097) |
| 70-77 | 12.2 (10.8-13.6) | 11.2 (10.3-12.1) | 6.8 (5.6-8.0) | 1.8 (1.2-2.4) | 4975 (4406-5543) | 7973 (6853-9093) | 20 251 (14 896-25 606) |
The percentages are the incremental rates of complication levels (minor, intermediate, and major) in each age group between the study and control cohorts. For example, in the cytology/needle biopsy section, 13.6 in the cell corresponding to the minor incremental complication rate column and all patients row means that, among all individuals between ages 55 and 77 years who underwent cytology or needle biopsy, the adjusted rate (calculated using the incremental approach) of minor complications was 13.6%. The type of complications are not mutually exclusive, so 1 patient can have more than 1 type/level of complication.
Cost Analysis
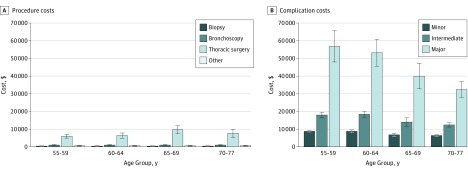
Table 3 also lists the costs of complications stratified by the level of complication and type of diagnostic procedure. Complication costs varied based on the type of procedure as well as the level of complication. Among these, for patients between ages 65 and 69 years, the cost of a minor complication associated with bronchoscopy was $5573 (95% CI, $3637-$7508); intermediate, $19 470 ($9859-$29 081); and major, $57 893 (95% CI, $37 899-$77 888). The cost of procedures was lower for biopsy ($312-$374) and bronchoscopy ($855-$1063) than for thoracic surgery ($5957-$9670) (Figure 2A). Managing postprocedural complications incurred higher costs than the diagnostic procedures, ranging from $6320 (95% CI, $5863-$6777) for minor complications to $56 845 (95% CI, $47 953-$65 737) for major complications (Figure 2B).
Figure 2. Procedure Costs and Complication Costs.
A, Procedure costs stratified by age group and invasive diagnostic procedure group. B, Complication costs stratified by age group and complication type.
Discussion
This study estimated the rates and costs of complications associated with invasive diagnostic procedures that are likely to be received by individuals who have positive findings from thoracic imaging examination. We found that, compared with the rate of complications reported in the NLST, the rate was twice as high in the younger and Medicare age groups, even after adjustment for the underlying rate in the control cohort. In addition, the overall rate of complications in the elderly group was higher than that in younger individuals. For certain complications, such as those associated with surgical diagnosis, the costs can be as high as $56 200.
Although the NLST demonstrated the effectiveness of lung cancer screening, whether a similar magnitude of mortality benefit will be realized outside the trial setting remains uncertain; a similar concern has been raised about complication rates from diagnostic procedures. Tanner and colleagues12 reported that, compared with NLST participants who had stage I non–small cell lung cancer, the 5-year all-cause mortality was comparable for an NLST-eligible cohort extracted from SEER-Medicare data but was worse for elderly patients with stage 1 non–small cell lung cancer who had significant comorbidities or did not undergo surgery. The authors concluded that the benefit of screening could be weakened among sicker elderly patients owing to the competing risk of death. This conclusion was not drawn from non–small cell lung cancer patients who had been screened for lung cancer with LDCT as the analysis used 1998 to 2010 SEER-Medicare data; instead, the authors made their inference by contrasting the characteristics between patients in the NLST and those in the general elderly population captured by SEER-Medicare data.
We followed a similar approach by analyzing complication rates and the associated costs among individuals who had undergone the types of invasive diagnostic procedures likely to be experienced by those who have positive findings identified on LDCT screening. Although our study cohort predated the implementation of lung cancer screening with LDCT, findings from our analysis contribute to the literature by informing policymakers and clinical communities of the potential magnitude of complication rates and the financial burden, at the population level, as the uptake of LDCT is extended to individuals in the general population who meet the screening eligibility criteria.
The complications associated with invasive diagnostic procedures played a role at a meeting that the Medicare Evidence Development & Coverage Advisory Committee convened to discuss Medicare coverage of lung cancer screening.32 Several experts at the meeting expressed concerns that complication rates in settings outside the NLST could be higher than those reported in the NLST. Our findings echoed this concern, showing that the rates of postprocedural complications were more than twice the rates in the NLST (22.2% vs 9.8% among individuals younger than 65 years and 23.8% vs 8.5% among those 65 years or older).11 Another concern voiced at the above meeting was variations in the quality of care across facilities that performed the follow-up diagnostic procedures. This concern is corroborated; the rates of postprocedural complications identified in our study and others showed considerable variation associated with various thoracic procedures.33,34,35,36,37,38
Many factors may have contributed to the wide variation in postprocedural complication rates across studies, such as quality standards, physician proficiency, and clinical infrastructure among community practices in performing diagnostic procedures subsequent to a positive finding. These variations will induce uncertainty regarding the harms of lung cancer screening programs in patients with positive findings determined on LDCT. Therefore, further studies are needed to determine factors that affect the quality of diagnostic procedures. Identifying factors that differentiate between practices with high vs low rates of complications will provide an opportunity to design interventions to lower the risk of postprocedural complications following positive LDCT findings and thereby reduce the physical, psychological, and financial burden associated with lung cancer screening.
The financial burden of postprocedural complications can be great, as our data indicate that considerable costs are associated with the invasive diagnostic procedures and postprocedural complications. Although none of the costs reported in our study should be directly interpreted as costs associated with lung cancer screening, 2 implications can be drawn from our study for future research on the costs and cost-effectiveness of lung cancer screening. First, studies estimating costs associated with lung cancer screening programs should use complication rates observed in community-based clinical practices. Studies that use rates from clinical trials would likely underestimate the real cost; our study showed that the complication rates were higher in community settings than those reported in clinical trials. Second, our findings suggest that age is associated with the clinical and economic outcomes. The positive association between age and complication rates (and consequently costs) implies that future research using age-stratified analyses should yield more accurate estimates of costs and cost-effectiveness.
Limitations
Our study has limitations. We analyzed insurance claims data to estimate rates of complications occurring after invasive diagnostic procedures for lung abnormalities. To avoid overestimating the rates of complications, we applied an incremental approach by constructing a matched control cohort. Although this approach allows a more conservative assessment of postprocedural complication rates, its application to claims data has limitations.
First, our study may have underestimated minor complications because they are less likely to be coded and recorded in administrative data. Second, even with the use of an incremental approach by including a matched control cohort, this method was unlikely to subtract all medical problems that were not related to the invasive procedures in claims data. Third, MarketScan databases do not report information on the dates and causes of deaths; therefore, we were unable to evaluate whether the mortality rates associated with invasive diagnostic procedures were also higher in the community setting compared with the rate reported in the NLST. Fourth, we were not able to determine the extent to which the higher complication rates observed in community settings were owing to lower quality of care in these facilities, less experienced physicians performing these procedures, or unmeasured patient-level factors.
Fifth, as the data used in our study predated the acceptance of LDCT lung cancer screening by payers and the medical community, our study did not assess complication rates among individuals who met the screening eligibility criteria and had received LDCT. Individuals who underwent invasive procedures captured in our study likely either had a lung nodule noted as an incidental finding on imaging or a symptom indicating lung abnormality that prompted further investigations. Sixth, in claims data, it is difficult to determine whether medical events subsequent to an invasive diagnostic procedure were caused by the procedure, so the complication rates estimated in our study are more suggestive than conclusive. Seventh, some unobserved patient-level factors, such as smoking history, were not matched between the study and control cohorts owing to lack of data availability.
Conclusions
The complication rates associated with invasive diagnostic procedures for lung abnormalities estimated from the general population indicate that complication rates from diagnostic procedures subsequent to lung cancer screening in the real-world setting are likely to be significantly higher than those reported from clinical trials. As the number of individuals seeking lung cancer screening with LDCT increases, so too will the number of individuals undergoing invasive diagnostic procedures as a result of abnormal findings.39 Results from this study, while tentative, emphasize the importance of including the risks of subsequent adverse events and downstream costs in the shared decision-making communications between physicians and patients.
eTable 1. Claim Codes Used to Determine Diagnostic Procedures
eTable 2. Claim Codes Used to Determine Complication Outcomes
References
- 1.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2011. Bethesda, MD: National Cancer Institute; 2014. [Google Scholar]
- 2.Aberle DR, Adams AM, Berg CD, et al. ; National Lung Screening Trial Research Team . Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinsky PF, Church TR, Izmirlian G, Kramer BS. The National Lung Screening Trial: results stratified by demographics, smoking history, and lung cancer histology. Cancer. 2013;119(22):3976-3983. doi: 10.1002/cncr.28326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wender R, Fontham ETH, Barrera E Jr, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63(2):107-117. doi: 10.3322/caac.21172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood DE, Eapen GA, Ettinger DS, et al. Lung cancer screening. J Natl Compr Canc Netw. 2012;10(2):240-265. doi: 10.6004/jnccn.2012.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaklitsch MT, Jacobson FL, Austin JHM, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg. 2012;144(1):33-38. doi: 10.1016/j.jtcvs.2012.05.060 [DOI] [PubMed] [Google Scholar]
- 7.Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5)(suppl):e78S-e92S. doi: 10.1378/chest.12-2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307(22):2418-2429. doi: 10.1001/jama.2012.5521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Lung Association Lung Cancer Screening Committee Providing Guidance on Lung Cancer Screening to Patients and Physicians. Chicago, IL: American Lung Association; 2012. [Google Scholar]
- 10.Moyer VA; US Preventive Services Task Force . Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi: 10.7326/M13-2771 [DOI] [PubMed] [Google Scholar]
- 11.Pinsky PF, Gierada DS, Hocking W, Patz EF Jr, Kramer BS. National Lung Screening Trial findings by age: Medicare-eligible versus under-65 population. Ann Intern Med. 2014;161(9):627-633. doi: 10.7326/M14-1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanner NT, Dai L, Bade BC, Gebregziabher M, Silvestri GA. Assessing the generalizability of the National Lung Screening Trial: comparison of patients with stage 1 disease. Am J Respir Crit Care Med. 2017;196(5):602-608. doi: 10.1164/rccm.201705-0914OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woolf SH, Harris RP, Campos-Outcalt D. Low-dose computed tomography screening for lung cancer: how strong is the evidence? JAMA Intern Med. 2014;174(12):2019-2022. doi: 10.1001/jamainternmed.2014.5626 [DOI] [PubMed] [Google Scholar]
- 14.Wiener RS. Balancing the benefits and harms of low-dose computed tomography screening for lung cancer: Medicare’s options for coverage. Ann Intern Med. 2014;161(6):445-446. doi: 10.7326/M14-1352 [DOI] [PubMed] [Google Scholar]
- 15.Silvestri GA. Screening for lung cancer: it works, but does it really work? Ann Intern Med. 2011;155(8):537-539. doi: 10.7326/0003-4819-155-8-201110180-00364 [DOI] [PubMed] [Google Scholar]
- 16.Gould MK. Lung cancer screening and elderly adults: do we have sufficient evidence? Ann Intern Med. 2014;161(9):672-673. doi: 10.7326/M14-2006 [DOI] [PubMed] [Google Scholar]
- 17.Davis AM, Cifu AS. Lung cancer screening. JAMA. 2014;312(12):1248-1249. doi: 10.1001/jama.2014.12272 [DOI] [PubMed] [Google Scholar]
- 18.Courtright K, Manaker S. Counterpoint: should lung cancer screening by chest CT scan be a covered benefit? no. Chest. 2015;147(2):289-292. doi: 10.1378/chest.14-2815 [DOI] [PubMed] [Google Scholar]
- 19.Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399-406. doi: 10.1001/jamainternmed.2016.9022 [DOI] [PubMed] [Google Scholar]
- 20.Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N). Centers for Medicare & Medicaid Services website. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274. Accessed April 21, 2018.
- 21.Roberts SCM, Upadhyay UD, Liu G, et al. Association of facility type with procedural-related morbidities and adverse events among patients undergoing induced abortions. JAMA. 2018;319(24):2497-2506. doi: 10.1001/jama.2018.7675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seamans MJ, Carey TS, Westreich DJ, et al. Association of household opioid availability and prescription opioid initiation among household members. JAMA Intern Med. 2018;178(1):102-109. doi: 10.1001/jamainternmed.2017.7280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van la Parra RFD, Liao K, Smith BD, et al. Incidence and outcome of breast biopsy procedures during follow-up after treatment for breast cancer. JAMA Surg. 2018;153(6):559-568. doi: 10.1001/jamasurg.2017.5572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winn AN, Keating NL, Trogdon JG, Basch EM, Dusetzina SB. Spending by commercial insurers on chemotherapy based on site of care, 2004-2014. JAMA Oncol. 2018;4(4):580-581. doi: 10.1001/jamaoncol.2017.5544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care. 2002;40(8)(suppl):IV-26-IV-35. doi: 10.1097/01.MLR.0000020936.03651.2D [DOI] [PubMed] [Google Scholar]
- 26.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100(9):630-641. doi: 10.1093/jnci/djn103 [DOI] [PubMed] [Google Scholar]
- 27.Chang S, Long SR, Kutikova L, et al. Estimating the cost of cancer: results on the basis of claims data analyses for cancer patients diagnosed with seven types of cancer during 1999 to 2000. J Clin Oncol. 2004;22(17):3524-3530. doi: 10.1200/JCO.2004.10.170 [DOI] [PubMed] [Google Scholar]
- 28.Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Wagner EH. Patient-level estimates of the cost of complications in diabetes in a managed-care population. Pharmacoeconomics. 1999;16(3):285-295. doi: 10.2165/00019053-199916030-00005 [DOI] [PubMed] [Google Scholar]
- 29.Parsons L. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. Paper presented at: Proceedings of the Twenty-Sixth Annual SAS Users Group International Conference; April 22-25, 2001; Cary, NC. [Google Scholar]
- 30.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bureau of Labor Statistics. Measuring Price Change for Medical Care in the CPI. Consumer Price Index 2010. https://www.bls.gov/cpi/home.htm. Accessed February 10, 2015.
- 32.Medicare Evidence Development & Coverage Advisory Committee (MEDCAC) MEDCAC meeting 4/30/2014-lung cancer screening with low dose computed tomography. https://www.cms.gov/medicare-coverage-database/details/medcac-meeting-details.aspx?MEDCACId=68. Published 2014. Accessed July 25, 2018.
- 33.Wiener RS, Schwartz LM, Woloshin S, Welch HG. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med. 2011;155(3):137-144. doi: 10.7326/0003-4819-155-3-201108020-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng YL, Patsios D, Roberts H, et al. CT-guided percutaneous fine-needle aspiration biopsy of pulmonary nodules measuring 10 mm or less. Clin Radiol. 2008;63(3):272-277. doi: 10.1016/j.crad.2007.09.003 [DOI] [PubMed] [Google Scholar]
- 35.Khan MF, Straub R, Moghaddam SR, et al. Variables affecting the risk of pneumothorax and intrapulmonal hemorrhage in CT-guided transthoracic biopsy. Eur Radiol. 2008;18(7):1356-1363. doi: 10.1007/s00330-008-0893-1 [DOI] [PubMed] [Google Scholar]
- 36.Hiraki T, Mimura H, Gobara H, et al. Incidence of and risk factors for pneumothorax and chest tube placement after CT fluoroscopy-guided percutaneous lung biopsy: retrospective analysis of the procedures conducted over a 9-year period. AJR Am J Roentgenol. 2010;194(3):809-814. doi: 10.2214/AJR.09.3224 [DOI] [PubMed] [Google Scholar]
- 37.Cattaneo SM, Park BJ, Wilton AS, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg. 2008;85(1):231-235. doi: 10.1016/j.athoracsur.2007.07.080 [DOI] [PubMed] [Google Scholar]
- 38.Yan TD, Black D, Bannon PG, McCaughan BC. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol. 2009;27(15):2553-2562. doi: 10.1200/JCO.2008.18.2733 [DOI] [PubMed] [Google Scholar]
- 39.Huo J, Shen C, Volk RJ, Shih YT. Use of CT and chest radiography for lung cancer screening before and after publication of screening guidelines: intended and unintended uptake. JAMA Intern Med. 2017;177(3):439-441. doi: 10.1001/jamainternmed.2016.9016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Claim Codes Used to Determine Diagnostic Procedures
eTable 2. Claim Codes Used to Determine Complication Outcomes