Key Points
Question
Is cerebrospinal fluid neurofilament light (CSF NFL) a general marker of neurodegeneration, and is it associated with disease progression?
Findings
In this study, there was a stepwise increase in CSF NFL protein concentration between control participants, participants with mild cognitive impairment, and those with Alzheimer disease, and a correlation with annual decreases in Mini-Mental State Examination scores in individuals with Alzheimer disease, frontotemporal dementia, and the cohort overall. Levels of CSF NFL were higher in patients with all forms of dementia, with the highest levels in frontotemporal dementia, amyotrophic lateral sclerosis, and atypical parkinsonian disorders.
Meaning
Concentration of CSF NFL is a general marker of neurodegeneration and is associated with cognitive decline in Alzheimer disease and frontotemporal dementia; the most pronounced alterations are seen in frontotemporal dementia, amyotrophic lateral sclerosis, and atypical parkinsonian disorders.
This case-control study investigates cerebrospinal fluid levels of neurofilament light protein in participants with various forms of dementia, motor neuron disease, and movement disorders and controls, as determined by clinical and autopsy-definite criteria.
Abstract
Importance
Neuronal and axonal destruction are hallmarks of neurodegenerative diseases, but it is difficult to estimate the extent and progress of the damage in the disease process.
Objective
To investigate cerebrospinal fluid (CSF) levels of neurofilament light (NFL) protein, a marker of neuroaxonal degeneration, in control participants and patients with dementia, motor neuron disease, and parkinsonian disorders (determined by clinical criteria and autopsy), and determine its association with longitudinal cognitive decline.
Design, Setting, and Participants
In this case-control study, we investigated NFL levels in CSF obtained from controls and patients with several neurodegenerative diseases. Collection of samples occurred between 1996 and 2014, patients were followed up longitudinally for cognitive testing, and a portion were autopsied in a single center (University of Pennsylvania). Data were analyzed throughout 2016.
Exposures
Concentrations of NFL in CSF.
Main Outcomes and Measures
Levels of CSF NFL and correlations with cognition scores.
Results
A total of 913 participants (mean [SD] age, 68.7 [10.0] years; 456 [49.9%] women) were included: 75 control participants plus 114 patients with mild cognitive impairment (MCI), 397 with Alzheimer disease, 96 with frontotemporal dementia, 68 with amyotrophic lateral sclerosis, 41 with Parkinson disease (PD), 19 with PD with MCI, 29 with PD dementia, 33 with dementia with Lewy bodies, 21 with corticobasal syndrome, and 20 with progressive supranuclear palsy. Cognitive testing follow-up occurred for 1 to 18 years (mean [SD], 0.98 [2.25] years); autopsy-verified diagnoses were available for 120 of 845 participants with diseases (14.2%). There was a stepwise increase in CSF NFL levels between control participants (median [range] score, 536 [398-777] pg/mL), participants with MCI (831 [526-1075] pg/mL), and those with Alzheimer disease (951 [758-1261] pg/mL), indicating that NFL levels increase with increasing cognitive impairment. Levels of NFL correlated inversely with baseline Mini-Mental State Examination scores (ρ, −0.19; P < .001) in the full cohort (n = 822) and annual score decline in the full cohort (ρ, 0.36, P < .001), participants with AD (ρ, 0.25; P < .001), and participants with FTD (ρ, 0.46; P = .003). Concentrations of NFL were highest in participants with amyotrophic lateral sclerosis (median [range], 4185 [2207-7453] pg/mL) and frontotemporal dementia (2094 [230-7744] pg/mL). In individuals with parkinsonian disorders, NFL concentrations were highest in those with progressive supranuclear palsy (median [range], 1578 [1287-3104] pg/mL) and corticobasal degeneration (1281 [828-2713] pg/mL). The NFL concentrations in CSF correlated with TDP-43 load in 13 of 17 brain regions in the full cohort. Adding NFL to β-amyloid 42, total tau, and phosphorylated tau increased accuracy of discrimination of diseases.
Conclusions and Relevance
Levels of CSF NFL are associated with cognitive impairments in patients with Alzheimer disease and frontotemporal dementia. In other neurodegenerative disorders, NFL levels appear to reflect the intensity of the neurodegenerative processes.
Introduction
Neurodegenerative diseases are frequent in Western populations, and approximately 5.5 million Americans have Alzheimer disease (AD), which is the most common type of dementia. Beside AD, there are many other neurodegenerative diseases; some result in dementia, such as frontotemporal dementia (FTD) and dementia with Lewy bodies (DLB); some in parkinsonian symptoms with and without dementia, such as Parkinson disease (PD); and others in motor neuron disease, such as amyotrophic lateral sclerosis (ALS).
In the later stages, neurodegenerative diseases are relatively easy to diagnose. However, early on, it is a diagnostic challenge to separate 1 form from another. Biomarkers are an invaluable help in the diagnoses of diseases in other clinical specialties (eg, troponin T in myocardial infarction).1 In the neurodegenerative field, the most examined and best validated biomarkers so far are cerebrospinal fluid (CSF) β-amyloid 42, total tau (T-tau), and phosphorylated tau (P-tau),2 all of which are used clinically in the diagnosis of AD. However, for other forms of neurodegenerative diseases, such as FTD or DLB, there are no specific CSF or blood biomarkers.
Neurofilaments are neuronal-specific intermediate filaments that determine the axonal caliber, which in turn partly determines the conduction velocity along the axon.3 Neurofilaments are composed of the neurofilament light protein (NFL) in addition to the medium and heavy protein counterparts.3 Neurofilament light protein is released into the CSF during axonal damage and has been shown to be elevated in different forms of dementia and ALS.2,4,5
To date and to our knowledge, studies of NFL have been fairly small, covered a limited set of diagnoses, or relied on clinical diagnoses only.6 Thus, the usefulness of CSF NFL in understanding neurodegenerative disease is unclear. To address this, we analyzed NFL levels in CSF from a cohort of individuals who either had different forms of neurodegenerative diseases or were control participants. Furthermore, a number of these also had neuropathologically verified diagnoses, allowing for the evaluation of NFL levels against both clinical criteria and definite diagnoses.
Methods
Participants
This study included patients with clinical diagnoses of neurodegenerative disorders. Eligible individuals were those with mild cognitive impairment (MCI), AD, posterior cortical atrophy, FTD (including the behavioral variant of FTD, the logopenic variant of primary progressive aphasia [PPA], nonfluent/agrammatic PPA, and the semantic form of PPA), ALS, vascular dementia, Parkinson disease with normal cognition (PD), PD with MCI, Parkinson disease dementia (PDD), DLB, corticobasal syndrome (CBS), and progressive supranuclear palsy (PSP). The study also included control participants with no cognitive decline, whose participation came via 2 means: cognitively intact individuals were followed up to autopsy, and individuals died at the University of Pennsylvania Hospital and their family members participated in a retrospective interview assessing their cognitive status.
At University of Pennsylvania, the Alzheimer Disease Core Center, Penn Memory Center, the Penn Frontotemporal Degeneration Center, Penn Comprehensive ALS Center, the Parkinson Disease and Movement Disorder Center, and the Morris K. Udall Parkinson’s Disease Research Center of Excellence each have protocols approved by the institutional review board to recruit patients, along with their clinical data, into research studies. In addition, these centers invite patients to participate in the brain donation program. Written informed consent had been obtained for all patients using a protocol approved by the institutional review board of the University of Pennsylvania.
Recruitment of the patients and diagnostic criteria for the groups have been described in detail previously.7,8,9 The neuropathological data are ordinal ratings (ie, 0-3) obtained at neuropathological examination at time of autopsy using National Institute on Aging–Alzheimer’s Association criteria10 and have been described elsewhere.8,11 The MMSE scores were analyzed every 6 months in most patients. Because some patients had very short follow-up times, only patients that had at least 2 years of follow-up MMSE data were included in calculations of the annual loss in MMSE scores to avoid spurious test-retest variation in scores obtained in close time proximity.
CSF Measurements
The collection, processing, and storage procedures for CSF samples have been described previously.12 In this study, CSF levels of β-amyloid 42, T-tau, and P-tau were measured using the multiplex xMAP Luminex platform (Luminex Corp) with the INNOBIA AlzBio3 kit (Fujirebio), as described previously.12
The CSF NFL concentrations were analyzed by an in-house enzyme-linked immunoabsorbent assay method.13 In short, 2 NFL mouse monoclonal antibodies (NFL21 and NFL23) were generated. Immunoprecipitation and Western blot analysis showed that they only recognized a single band in CSF samples corresponding to full-length NFL. In the enzyme-linked immunoabsorbent assay, NFL21 was used as the capture antibody and NFL23 as the detector, while purified bovine NFL was used as the calibrator (range, 39-5000 pg/mL). This test had no cross-reactivity with neurofilament medium protein or neurofilament heavy protein. Internal quality control samples were run on each plate, showing within-run and between-run coefficients of variation of less than 8% and less than 13%, respectively.
Statistical Analysis
Because biomarker values were nonnormally distributed, the nonparametric Kruskal-Wallis test and the post hoc pairwise Mann-Whitney U tests were used to assess differences between groups. Correction for multiple comparisons was made with the Benjamini-Hochberg procedure. Associations between NFL and T-tau, NFL and the Mini-Mental State Examination (MMSE) score, and between NFL and transactive response DNA binding protein (TDP)-43 pathology were calculated using Spearman rank correlation (ρ), again with correction for multiple comparisons. The NFL values were adjusted for both age and sex prior to analysis of group differences and correlations.
The additional value provided by including NFL in a diagnostic biomarker panel was analyzed by fitting a logistic regression classifier to distinguish between each pair of disorders. The increase in model performance achieved by adding NFL levels to a baseline model that included only β-amyloid 42, T-tau, and P-tau was calculated. To evaluate model performance, we performed 5-fold cross validations with classification accuracy as the performance metric. The cross-validation groups were stratified such that each group contained roughly the same proportion of disorder membership as were found in the overall population. We report a robust estimate of model performance calculated by repeating the cross-validation procedure 100 times and calculating the mean of the classification accuracy over all 100 runs.
Statistical analyses were performed using the R programming language (version 3.4.3; R Foundation for Statistical Computing), while the biomarker index model was developed using Python 3.6 (Python Software Foundation). All tests were 2-sided, and the significance threshold was set at P < .05.
Results
In addition to 75 healthy control participants, this study included 845 patients with clinical diagnoses of neurodegenerative disorders. This group included 114 individuals with mild cognitive impairment (MCI), 397 individuals with AD, 6 individuals with posterior cortical atrophy, 96 individuals with FTD (including 46 with the behavioral variant of FTD, 12 with the logopenic variant of primary progressive aphasia, 20 with nonfluent/agrammatic PPA, and 18 with the semantic form of PPA), 68 individuals with ALS, 1 individual with vascular dementia, 41 individuals with Parkinson disease with normal cognition (PD), 19 individuals with PD with MCI, 29 individuals with Parkinson disease dementia (PDD), 33 individuals with DLB, 21 individuals with corticobasal syndrome (CBS), and 20 individuals with progressive supranuclear palsy (PSP) (Table).
Table. Demographic and Clinical Data.
| Diagnosis | Total Patients, No. | Age, Mean (SD) | Women, No. | Patients Taking MMSE, No. | MMSE Score, Mean (SD) | Median (Interquartile Range) | |||
|---|---|---|---|---|---|---|---|---|---|
| Neurofilament Light | β-Amyloid 42 | Total Tau | Phosphorylated Tau | ||||||
| Control | 75 | 68.3 (9.4) | 50 | 74 | 29.1 (0.2) | 536 (398-777) | 244 (212-298) | 50 (38-68) | 17 (12-22) |
| Mild cognitive impairment | 114 | 71.5 (9.1) | 58 | 111 | 24.8 (0.4) | 831 (526-1075) | 178 (126-230) | 63 (42-100) | 22 (13-41) |
| Alzheimer disease | 397 | 71.2 (9.1) | 236 | 383 | 14.7 (0.4) | 951 (758-1261) | 134 (107-160) | 104 (75-155) | 36 (23-58) |
| Posterior cortical atrophy | 6 | 60.0 (6.2) | 5 | 6 | 19.5 (3.8) | 982 (698-1108) | 260 (124-308) | 53 (42-123) | 26 (10-36) |
| Vascular dementia | 1 | 70.0 (NA) | 1 | 1 | 27.0 (0.0) | 1742 (1742-1742) | 153 (153-153) | 37 (37-37) | 12 (12-12) |
| Behavioral variant of frontotemporal dementia | 46 | 60.5 (8.2) | 13 | 42 | 19.7 (1.1) | 1873 (830-2588) | 1873 (830-2588) | 238 (174-313) | 16 (12-23) |
| Logopenic variant of primary progressive aphasia | 12 | 62.3 (5.5) | 7 | 12 | 17.9 (2.6) | 918 (578-1653) | 138 (121-152) | 130 (89-157) | 39 (26-47) |
| Nonfluent/ agrammatic primary progressive aphasia | 20 | 64.3 (8.7) | 9 | 17 | 11.2 (2.4) | 1221 (860-2240) | 193 (171-280) | 56 (42-87) | 16 (12-29) |
| Semantic form of primary progressive aphasia | 18 | 63.2 (7.8) | 11 | 17 | 17.4 (2.0) | 2228 (1124-2799) | 282 (178-308) | 88 (58-119) | 15 (12-28) |
| Amyotrophic lateral sclerosis | 68 | 58.4 (11.5) | 18 | 21 | 22.0 (2.3) | 4185 (2207-7453) | 266 (213-323) | 50 (36-70) | 11 (8-15) |
| Parkinson disease with normal cognition | 41 | 64.5 (9.8) | 11 | 31 | 28.3 (0.4) | 619 (526-840) | 258 (219-303) | 40 (31-51) | 18 (13-27) |
| Parkinson disease with mild cognitive impairments | 19 | 65.3 (7.6) | 3 | 17 | 27.7 (0.5) | 779 (464-1021) | 232 (202-288) | 39 (32-56) | 18 (13-35) |
| Parkinson disease dementia | 29 | 73.6 (7.6) | 4 | 27 | 22.6 (1.0) | 981 (679-1722) | 214 (161-260) | 48 (36-67) | 22 (12-26) |
| Dementia with Lewy bodies | 33 | 72.2 (9.3) | 14 | 30 | 16.6 (1.3) | 991 (695-2139) | 147 (126-172) | 54 (35-89) | 16 (12-28) |
| Corticobasal syndrome | 21 | 66.0 (7.6) | 12 | 19 | 16.9 (2.4) | 1281 (828-2713) | 225 (166-293) | 71 (62-108) | 21 (16-28) |
| Progressive supranuclear palsy | 20 | 69.5 (9.3) | 10 | 18 | 22.9 (1.1) | 1578 (1287-3104) | 210 (167-260) | 50 (36-75) | 12 (10-19) |
Abbreviations: MMSE, Mini-Mental State Examination; NA, not applicable.
There was only 1 patient diagnosed with vascular dementia and 6 patients diagnosed with posterior cortical atrophy. Therefore, no comparisons were made against vascular dementia and posterior cortical atrophy. A total of 913 participants were therefore included in this analysis (mean [SD] age, 68.7 [10.0] years; 456 [49.9%] women).
A total of 120 of 845 patients (14.2%) had autopsy-verified diagnoses, of whom 75 of 120 patients (62.5%) had had AD, 12 patients (10.0%) had had frontotemporal lobar degeneration with TDP-43, 16 patients (13.3%) had had DLB, 6 patients (5.0%) had had PSP, 7 patients (5.8%) had had ALS, 3 patients (2.5%) had had CBD, and 1 patient (0.8%) had been a healthy control participant.
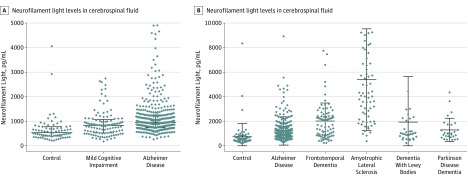
The CSF levels of NFL were significantly increased in participants with MCI compared with control participants (median difference, 216.9 [95% CI, 117.9-321.5] pg/mL; P < .001) and even further increased in participants with AD compared with control participants (median difference, 406.6 [95% CI, 325.6-486.7] pg/mL; P < .001; Figure 1A). Furthermore, compared with the levels in control participants, NFL levels were significantly elevated in participants with other forms of dementia, including FTD (median difference, 1193.7 [95% CI, 778.3-1577.2] pg/mL; P < .001), DLB (median difference, 451.2 [95% CI 267.3-627.6] pg/mL; P < .001), and PDD (median difference, 410.0 [95% CI, 228.3-598.7] pg/mL; P < .001) and the motor neuron disease ALS (median difference, 3549.3 [95% CI, 2924.0-4481.3] pg/mL; P < .001; Figure 1B). The levels of NFL were also significantly higher in participants with FTD than in those with AD (median difference, 686.8 [95% CI, 405.0-1006.8] pg/mL; P < .001), PDD (median difference, 550.4 [95% CI, 168.3-1157.1] pg/mL; P = .01), and DLB (median difference, 451.2 [95% CI, 60.8-1079.4] pg/mL; P = .03, Figure 1B). The highest levels of NFL were observed in patients with ALS, which were significantly increased compared with control participants (median difference, 3549.3 [95% CI, 2924.0-4481.3] pg/mL; P < .001), AD (median difference, 3118.3 [95% CI, 2626.2-3704.5] pg/mL; P < .001), FTD (median difference, 2451.7 [95% CI, 1632.5-3314.7] pg/mL; P < .001), DLB (median difference, 2967.8 [95% CI, 1957.5-4017.9] pg/mL; P < .001), and PDD (median difference, 3066.6 [95% CI, 2124.6-4291.1] pg/mL; P < .001, Figure 1B).
Figure 1. Neurofilament Light Protein in Control Participants and Participants With Mild Cognitive Impairment, Dementias, and Amyotrophic Lateral Sclerosis.
A, Cerebrospinal fluid levels of neurofilament light in control participants, participants with mild cognitive impairment, and participants with Alzheimer disease. The graph omits outlier data points (neurofilament light levels >5000 pg/mL) for 1 control participant, 1 with mild cognitive impairment, and 3 with Alzheimer disease. B, The levels of neurofilament light in cerebrospinal fluid in control participants, participants with forms of dementia, and participants with amyotrophic lateral sclerosis. The graph omits outlier data points (neurofilament light levels >10 000 pg/mL) for 1 participant with dementia with Lewy bodies, 1 with Alzheimer disease, and 7 with amyotrophic lateral sclerosis.
Frontotemporal dementia is a pathologically heterogeneous entity that includes several similar disorders in which progressive degeneration of the frontal and temporal lobes is common.14,15 On the basis of clinical phenotypes, we divided the FTD group into the subgroups of the behavioral variant of FTD and PPA; the second was further divided into nonfluent/agrammatic PPA, the logopenic variant of PPA, and the semantic form of PPA.14 Because the logopenic variant of PPA is associated with AD pathology,16,17 we moved the individuals from this subgroup to the AD group rather than the FTD group when comparing different forms of dementia. However, the only significant difference was between the logopenic variant and semantic form of PPA, with higher NFL levels in the semantic form (median difference, 1114.7 [95% CI, 207.2-1687.2] pg/mL; P = .02).
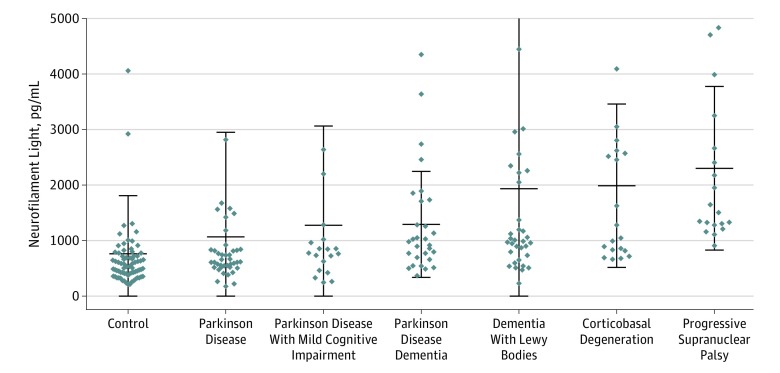
In parkinsonian disorders, we found that multiple groups had significantly higher levels of NFL than control participants, including those with PD (median difference, 149.4 [95% CI, 45.6-257.4] pg/mL; P = .02), PDD (median difference, 410.0 [95% CI, 228.3-598.7] pg/mL; P < .001), DLB (median difference, 451.2 [95% CI, 267.3-627.6] pg/mL; P < .001), CBS (median difference, 675.7 [95% CI, 400.3-1826.2] pg/mL; P < .001), and PSP (median difference, 1027.1 [95% CI, 822.8-1631.9] pg/mL; P < .001). Participants with CBS had higher levels than those with PD (median difference, 675.7 [95% CI, 400.3-1826.2] pg/mL; P < .001) and PD with MCI (median difference, 218.1 [95% CI, 16.9-402.0] pg/mL; P = .03). In addition, patients with PSP had significantly higher levels than participants with PD (median difference, 853.1 [95% CI, 643.0-1566.5] pg/mL; P < .001), PD with MCI (median difference, 880.3 [95% CI, 480.4-1620.0] pg/mL; P < .001), and PDD (median difference, 669.8 [95% CI, 335.0-1280.3] pg/mL; P = .001; Figure 2).
Figure 2. Neurofilament Light (NFL) Protein in Control Participants and Participants With Movement Disorders.
The graph omits outlier data points (neurofilament light levels >5000 pg/mL) for 1 participant with progressive supranuclear palsy, 2 with corticobasal degeneration, 1 with dementia with Lewy bodies, 1 with Parkinson disease, 1 with Parkinson disease with mild cognitive impairment, and 1 control participant.
To estimate the importance of NFL in separating different neurodegenerative disorders from each other and controls, we created a biomarker index based on β-amyloid 42, T-tau, and P-tau. Next, we added NFL and compared the accuracy with or without it. We found that NFL significantly increased the logistic regression model’s ability to discriminate between numerous pairs of disorders. Generally, NFL increased accuracy of discrimination between ALS and most forms of neurodegenerative disorders. Notably, the inclusion of NFL led to a 29% (95% CI, 25.0%-33.3%) increase in accuracy in distinguishing between control participants and ALS (from 62% to 91%), a 17% (95% CI, 14.9%-19.5%) increase between control participants and the behavioral variant of FTD (from 63% to 81%), and a 14% (95% CI, 8.7%-19.1%) increase between PD and PSP (from 62% to 76%; eTable 1 in the Supplement).
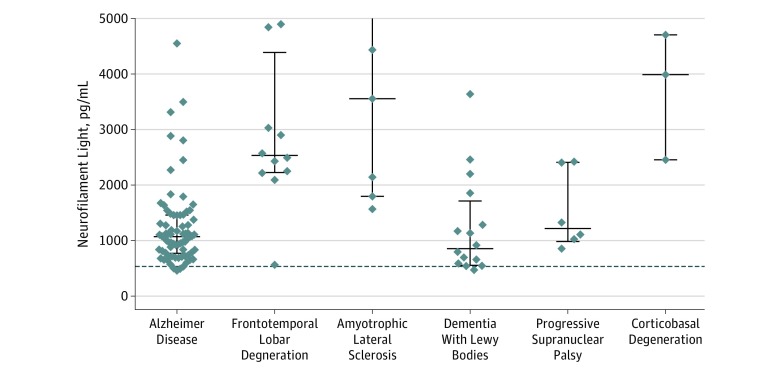
In the 120 patients with autopsy-confirmed neuropathological data, who were diagnosed according to definite criteria, we found that patients with frontotemporal lobar degeneration had significantly higher levels of NFL compared with patients with AD (median difference, 1542.8 [95% CI, 1193.2-1970.1] pg/mL; P = .01) and DLB (median difference, 1792 [95% CI, 1076.1-2369.2] pg/mL; P = .01; Figure 3). Also, patients with ALS had significantly higher levels of NFL than patients with AD (median difference, 2851.9 [95% CI, 866.9-5976.3] pg/mL; P = .003) and DLB (median difference, 2989.0 [95% CI, 982.7-6198.4] pg/mL; P = .01; Figure 3). There was no significant difference between NFL levels in autopsy-verified cases of ALS compared with cases of frontotemporal lobar degeneration (median difference, 1179.8 [ 95% CI, −1231.8 to 4623.1] pg/mL; P = .50; Figure 3).
Figure 3. Neurofilament Light Protein in Participants With Autopsy-Verified Neurodegenerative Diseases.
Neurofilament light levels in clinically diagnosed control participants are added as a dotted line for reference. The graph omits outlier data points (neurofilament light levels >5000 pg/mL) for 1 participant with frontotemporal lobe degeneration and 2 with amyotrophic lateral sclerosis.
Because we found the highest levels of NFL in participants with ALS and FTD, we correlated CSF levels of NFL with TDP-43 load in 17 brain regions in the 60 patients on whom data were available. We found positive, statistically significant correlations between CSF levels of NFL and TDP-43 load in 13 of 17 brain regions (76.4%; eTable 2 in the Supplement).
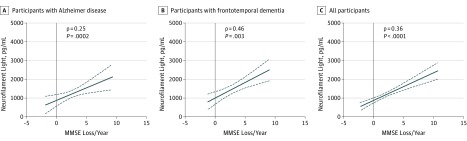
We correlated baseline MMSE scores with CSF levels of NFL in all patients who had MMSE data available (n = 822) and found a significant negative correlation (ρ = −0.19, P < .001). We correlated the magnitude of annual loss in MMSE with CSF levels of NFL and found a significant positive correlation (ρ = 0.25; P < .002; Figure 4A) in patients with AD (n = 219) but not in patients with MCI (n = 53; ρ = 0.15; P = .29) or control participants (n = 58; ρ = 0.02, P = .91). A positive correlation between the magnitude of annual MMSE score loss and NFL was also found in patients with FTD (n = 40; ρ = 0.46; P = .003; Figure 4B). The NFL levels were positively correlated with the magnitude of annual MMSE score loss in individuals in the full cohort, pooled across disorders, with MMSE follow-up of at least 2 years (n = 449; ρ = 0.36, P < .001; Figure 4C).
Figure 4. Correlations Between Neurofilament Light Protein and Annual Mini-Mental State Examination Score Decline.
Correlations between cerebrospinal fluid levels of neurofilament light and the magnitude of annual decline in Mini-Mental State Examination scores in participants with Alzheimer disease (ρ = 0.25; P < .001) (A), frontotemporal dementia (ρ = 0.46; P = .003) (B), and all participants (ρ = 0.36; P < .001) (C).
We were able to obtain longitudinal scores for the Montreal Cognitive Assessment (MoCA) and Dementia Rating Scale (DRS) cognitive tests for 85 individuals with Parkinson disease. There was a significant association between NFL levels and baseline MoCA scores (ρ = −0.33, P = .004) but not between NFL levels and mean change per year in MoCA scores (ρ = −0.1, P = .40). Additionally, we found that there was an association between NFL levels and DRS scores at baseline (ρ = −0.24; P = .03) and a significant association between NFL and mean change per year in DRS scores (ρ = −0.25, P = .03).
Both NFL and T-tau are neuronal markers, and CSF levels of NFL and T-tau correlated in several of the groups: control participants (ρ = 0.33; P = .005), participants with MCI (ρ = 0.27; P = .004), participants with AD (ρ = 0.32; P < .001), participants with ALS (ρ = 0.30; P = .02), participants with the behavioral variant of FTD (ρ = 0.32; P = .04), participants with DLB (ρ = 0.58; P = .004), participants with PD with MCI (ρ = 0.55; P = .02), and participants with the semantic form of PPA (ρ = 0.62; P = .02). However, the 2 neuronal markers were not correlated in participants with PD, PDD, CBS, the logopenic variant of PPA, nonfluent/agrammatic PPA, or PSP.
Discussion
In this analysis, CSF NFL levels were elevated in a stepwise manner, with the lowest levels in control participants, significantly higher levels in participants with MCI, and even higher levels in participants with AD. Although the highest levels were observed in participants with ALS and the second-highest levels in participants with FTD, NFL concentrations were also elevated in participants with PDD, DLB, CBS, and PSP compared with control participants. These results are in agreement with earlier studies,6,18,19,20 but to our knowledge, this is the first time all these diseases are compared with each other in a single cohort in the same study.
It is interesting that CSF NFL levels rose with increasing cognitive impairments as reflected by higher levels in participants with AD and intermediary levels in participants with MCI and lowest in control participants. This was further supported by the negative correlation between baseline MMSE scores and NFL levels, indicating that NFL concentrations increase with cognitive decline. This was also reflected in the positive correlation between NFL levels and the magnitude of annual loss in MMSE scores in the groups with AD and FTD, separately and when the entire cohort was used, but not in participants with MCI or control participants. For participants with PD, we also observed a significant negative correlation between baseline MoCA scores and CSF levels of NFL. Likewise, there were negative correlations between CSF NFL and baseline DRS scores and CSF NFL and annual change in DRS scores, further supporting that NFL is linked to cognitive decline in neurodegenerative disorders.
The highest levels of NFL were observed in participants with ALS. This is not surprising, since the longest axons in the body are found in motor neurons, which are destroyed in ALS.21 Furthermore, ALS is not generally associated with overt dementia, although it has been suggested that up to a third of individuals with ALS may have concurrent FTD22,23 and, as expected, there was no correlation between annual MMSE score loss and NFL levels in these patients. These data are in agreement with previous studies in which increased levels of NFL in CSF have been observed in individuals with ALS.4
We found higher levels of NFL in patients with FTD compared with patients with AD when clinical criteria were used to diagnose the patients, confirming previous studies.22 We could also confirm that this was true using autopsy-confirmed diagnoses, which has never been demonstrated before to our knowledge. We found the highest levels of NFL in the semantic form of PPA among the subtypes of FTD, and they were significantly increased compared with the logopenic variant of PPA. Similar findings have previously been observed.23,24 However, we found no other significant differences between patients with other subtypes of FTD. Interestingly, the NFL levels in patients with ALS were significantly higher than in patients with FTD using clinical criteria. The same trend was observed using autopsy-confirmed diagnoses. This did not, however, reach significance, which was likely owing to the relatively low number of patients in each group.
The CSF levels of NFL were significantly increased in participants with PD, PDD, DLB, CBS, and PSP compared with control participants, whereas CSF NFL was not increased in participants with PD with MCI. The highest levels of NFL in participants with parkinsonian disorders were observed in those with clinical PSP, followed by those with CBS, supporting previous findings.18,19 However, CSF levels of NFL were not correlated with the magnitude of annual MMSE score loss in participants with any of the parkinsonian disorders. This may suggest that NFL levels in these disorders are associated with disease-specific neurodegenerative events, rather than with impaired cognition. Alternatively, MMSE scores may be a less sensitive measure of cognitive change in individuals with parkinsonian disorders. Supporting this is the finding of significant correlations between CSF NFL levels and baseline MoCA scores, baseline DRS scores, and mean change per year in DRS scores in patients with PD.
The CSF levels of NFL correlated with CSF levels of tau in many diagnostic categories but not in all. This is important; otherwise, no additional information would be given by the 2 markers. The usefulness of NFL levels in separating individuals with different forms of neurodegeneration from each other and control participants was also demonstrated by using a biomarker index consisting of β-amyloid 42, T-tau, and P-tau, with or without the addition of NFL. Measuring NFL greatly improved the separation between many forms of neurodegeneration from each other and control participants. It especially separated patients with ALS from patients with other forms of neurodegeneration and control participants and patients with the behavioral variant of FTD from control participants. It also greatly increased the separation of patients with PD from those with PSP. The positive correlation between NFL levels and severity of TDP-43 load in most brain regions further supports the usefulness of NFL measurement in correctly diagnosing ALS and FTD, since both are linked to TDP-43 inclusions.
Limitations
The study has a number of limitations that should be acknowledged. First, the sample size in some subgroups is small, and the risk of false-positive statistical test results warrants replication of the findings. Another limitation is the reliance on Alzheimer disease biomarkers and clinical measures for cross-disease comparisons. The reason for this is simply that biomarkers for the other diseases are lacking. Finally, the MMSE score is not a strong indicator of disease severity for FTD. However, that was the only measure of cognition available in all of the patients with FTD in the study.
Conclusions
In conclusion, NFL is a general marker of neurodegeneration for neurodegenerative diseases across the board. The level of NFL in CSF is associated with progressive cognitive dysfunction in patients with AD and FTD and may have an important role in the clinical workup of patients with cognitive symptoms, to identify, grade, or exclude ongoing neurodegeneration and prognosticate disease progression in AD and FTD.
eTable 1. Biomarker index with and without the addition of CSF NFL.
eTable 2. Correlation between CSF levels of NFL and TDP-43 load in different brain regions.
References
- 1.Westermann D, Neumann JT, Sörensen NA, Blankenberg S. High-sensitivity assays for troponin in patients with cardiac disease. Nat Rev Cardiol. 2017;14(8):472-483. doi: 10.1038/nrcardio.2017.48 [DOI] [PubMed] [Google Scholar]
- 2.Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15(7):673-684. doi: 10.1016/S1474-4422(16)00070-3 [DOI] [PubMed] [Google Scholar]
- 3.Yamasaki H, Itakura C, Mizutani M. Hereditary hypotrophic axonopathy with neurofilament deficiency in a mutant strain of the Japanese quail. Acta Neuropathol. 1991;82(6):427-434. doi: 10.1007/BF00293376 [DOI] [PubMed] [Google Scholar]
- 4.Rosengren LE, Karlsson JE, Karlsson JO, Persson LI, Wikkelsø C. Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J Neurochem. 1996;67(5):2013-2018. doi: 10.1046/j.1471-4159.1996.67052013.x [DOI] [PubMed] [Google Scholar]
- 5.Rosengren LE, Karlsson JE, Sjögren M, Blennow K, Wallin A. Neurofilament protein levels in CSF are increased in dementia. Neurology. 1999;52(5):1090-1093. doi: 10.1212/WNL.52.5.1090 [DOI] [PubMed] [Google Scholar]
- 6.Skillbäck T, Farahmand B, Bartlett JW, et al. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology. 2014;83(21):1945-1953. doi: 10.1212/WNL.0000000000001015 [DOI] [PubMed] [Google Scholar]
- 7.Irwin DJ, Cairns NJ, Grossman M, et al. Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine. Acta Neuropathol. 2015;129(4):469-491. doi: 10.1007/s00401-014-1380-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toledo JB, Van Deerlin VM, Lee EB, et al. A platform for discovery: the University of Pennsylvania Integrated Neurodegenerative Disease Biobank. Alzheimers Dement. 2014;10(4):477-484.e1. doi: 10.1016/j.jalz.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie SX, Baek Y, Grossman M, et al. Building an integrated neurodegenerative disease database at an academic health center. Alzheimers Dement. 2011;7(4):e84-e93. doi: 10.1016/j.jalz.2010.08.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montine TJ, Phelps CH, Beach TG, et al. ; National Institute on Aging; Alzheimer’s Association . National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123(1):1-11. doi: 10.1007/s00401-011-0910-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toledo JB, Brettschneider J, Grossman M, et al. CSF biomarkers cutoffs: the importance of coincident neuropathological diseases. Acta Neuropathol. 2012;124(1):23-35. doi: 10.1007/s00401-012-0983-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403-413. doi: 10.1002/ana.21610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaetani L, Höglund K, Parnetti L, et al. A new enzyme-linked immunosorbent assay for neurofilament light in cerebrospinal fluid: analytical validation and clinical evaluation. Alzheimers Res Ther. 2018;10(1):8. doi: 10.1186/s13195-018-0339-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006-1014. doi: 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(pt 9):2456-2477. doi: 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santos-Santos MA, Rabinovici GD, Iaccarino L, et al. Rates of amyloid imaging positivity in patients with primary progressive aphasia. JAMA Neurol. 2018;75(3):342-352. doi: 10.1001/jamaneurol.2017.4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spinelli EG, Mandelli ML, Miller ZA, et al. Typical and atypical pathology in primary progressive aphasia variants. Ann Neurol. 2017;81(3):430-443. doi: 10.1002/ana.24885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall S, Öhrfelt A, Constantinescu R, et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol. 2012;69(11):1445-1452. doi: 10.1001/archneurol.2012.1654 [DOI] [PubMed] [Google Scholar]
- 19.Magdalinou NK, Paterson RW, Schott JM, et al. A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J Neurol Neurosurg Psychiatry. 2015;86(11):1240-1247. doi: 10.1136/jnnp-2014-309562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skillbäck T, Mattsson N, Blennow K, Zetterberg H. Cerebrospinal fluid neurofilament light concentration in motor neuron disease and frontotemporal dementia predicts survival. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(5-6):397-403. doi: 10.1080/21678421.2017.1281962 [DOI] [PubMed] [Google Scholar]
- 21.Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377(2):162-172. doi: 10.1056/NEJMra1603471 [DOI] [PubMed] [Google Scholar]
- 22.Sjögren M, Rosengren L, Minthon L, Davidsson P, Blennow K, Wallin A. Cytoskeleton proteins in CSF distinguish frontotemporal dementia from AD. Neurology. 2000;54(10):1960-1964. doi: 10.1212/WNL.54.10.1960 [DOI] [PubMed] [Google Scholar]
- 23.Rohrer JD, Woollacott IO, Dick KM, et al. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology. 2016;87(13):1329-1336. doi: 10.1212/WNL.0000000000003154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scherling CS, Hall T, Berisha F, et al. Cerebrospinal fluid neurofilament concentration reflects disease severity in frontotemporal degeneration. Ann Neurol. 2014;75(1):116-126. doi: 10.1002/ana.24052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis: subcommittee on motor neuron diseases/amyotrophic lateral sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994;124(suppl):96-107. doi: 10.1016/0022-510X(94)90191-0 [DOI] [PubMed] [Google Scholar]
- 26.Lomen-Hoerth C, Murphy J, Langmore S, Kramer JH, Olney RK, Miller B. Are amyotrophic lateral sclerosis patients cognitively normal? Neurology. 2003;60(7):1094-1097. doi: 10.1212/01.WNL.0000055861.95202.8D [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Biomarker index with and without the addition of CSF NFL.
eTable 2. Correlation between CSF levels of NFL and TDP-43 load in different brain regions.