Key Points
Question
What are the clinical and radiologic features of myelin oligodendrocyte glycoprotein autoantibody (MOG-IgG) myelitis, and what distinguishes it from other myelitis etiologies?
Findings
In this observational study, MOG-IgG myelitis may present with an acute flaccid myelitis phenotype. Longitudinally extensive T2-hyperintense lesions are typical, but most patients have more than 1 lesion, and an H-shaped axial T2 pattern confined to gray matter and lack of enhancement are discriminating features from aquaporin-4–IgG and MS myelitis.
Meaning
Recognition of the clinical and radiologic characteristics of MOG-IgG myelitis and its discriminators from other etiologies will help clinicians identify patients with myelitis in whom MOG-IgG should be tested.
Abstract
Importance
Recognizing the characteristics of myelin oligodendrocyte glycoprotein autoantibody (MOG-IgG) myelitis is essential for early accurate diagnosis and treatment.
Objective
To evaluate the clinical, radiologic, and prognostic features of MOG-IgG myelitis and compare with myelitis with aquaporin-4–IgG (AQP4-IgG) and multiple sclerosis (MS).
Design, Setting, and Participants
We retrospectively identified 199 MOG-IgG–positive Mayo Clinic patients from January 1, 2000, through December 31, 2017, through our neuroimmunology laboratory. Fifty-four patients met inclusion criteria of (1) clinical myelitis; (2) MOG-IgG positivity; and (3) medical records available. We excluded 145 patients without documented myelitis. Myelitis of AQP4-IgG (n = 46) and MS (n = 26) were used for comparison.
Main Outcomes and Measures
Outcome variables included modified Rankin score and need for gait aid. A neuroradiologist analyzed spine magnetic resonance imaging of patients with MOG-IgG and control patients blinded to diagnosis.
Results
Of 54 included patients with MOG-IgG myelitis, the median age was 25 years (range, 3-73 years) and 24 were women (44%). Isolated transverse myelitis was the initial manifestation in 29 patients (54%), and 10 (19%) were initially diagnosed as having viral/postviral acute flaccid myelitis. Cerebrospinal fluid–elevated oligoclonal bands occurred in 1 of 38 (3%). At final follow-up (median, 24 months; range, 2-120 months), 32 patients (59%) had developed 1 or more relapses of optic neuritis (n = 31); transverse myelitis (n = 7); or acute disseminated encephalomyelitis (n = 1). Clinical features favoring MOG-IgG myelitis vs AQP4-IgG or MS myelitis included prodromal symptoms and concurrent acute disseminated encephalomyelitis. Magnetic resonance imaging features favoring MOG-IgG over AQP4-IgG or MS myelitis were T2-signal abnormality confined to gray matter (sagittal line and axial H sign) and lack of enhancement. Longitudinally extensive T2 lesions were of similar frequency in MOG-IgG and AQP4-IgG myelitis (37 of 47 [79%] vs 28 of 34 [82%]; P = .52) but not found in MS. Multiple spinal cord lesions and conus involvement were more frequent with MOG-IgG than AQP4-IgG but not different from MS. Wheelchair dependence at myelitis nadir occurred in one-third of patients with MOG-IgG and AQP4-IgG but never with MS, although patients with MOG-IgG myelitis recovered better than those with AQP4-IgG.
Conclusions and Relevance
Myelitis is an early manifestation of MOG-IgG–related disease and may have a clinical phenotype of acute flaccid myelitis. We identified a variety of clinical and magnetic resonance imaging features that may help clinicians identify those at risk in whom MOG-IgG should be tested.
This study evaluates the clinical, radiologic,al and prognostic features of of myelin oligodendrocyte glycoprotein autoantibody myelitis and compares with myelitis with aquaporin-4–IgG and multiple sclerosis.
Introduction
Transverse myelitis (TM) is a potentially disabling neurologic syndrome with a variety of causes; single or recurrent episodes may result in permanent wheelchair dependence.1 In 1 study, 70% of patients referred to a tertiary referral center with a diagnosis of idiopathic transverse myelitis were found to have a specific etiology for their myelopathy,2 highlighting that the causes of transverse myelitis are poorly recognized among the neurology community.2 The discovery of the serum diagnostic biomarkers, aquaporin-4 autoantibody (AQP4-IgG) targeting astrocytes, and myelin oligodendrocyte glycoprotein autoantibody (MOG-IgG) targeting oligodendrocytes has aided in the ability to classify patients with idiopathic TM into a specific disease category.3,4,5,6 Accurate diagnosis of these disorders has important treatment and prognostic implications. The clinical, radiologic, and prognostic features of myelitis seropositive for AQP4-IgG have been well characterized,7,8,9,10 but information about the features accompanying myelitis associated with MOG-IgG is much more limited.11,12,13 Prior reports suggest that patients with MOG-IgG may have either a monophasic or relapsing course and that the myelitis may occur in isolation, as a component of acute disseminated encephalomyelitis (ADEM), or concurrently with optic neuritis.12 Accumulating evidence has suggested that detection of MOG-IgG defines a clinical syndrome distinct from both multiple sclerosis (MS) and AQP4-IgG seropositive neuromyelitis optica spectrum disorder.11,13,14,15,16 In this study, we sought to determine the clinical, radiologic, and laboratory characteristics and outcome of myelitis seropositive for MOG-IgG and compare this disease with myelitis associated with AQP4-IgG and MS.
Methods
Identification of Patients
The study was approved by the institutional review board of Mayo Clinic, Rochester, Minnesota. All patients gave written consent to the passive use of their medical records for research purposes. We identified 199 patients through the neuroimmunology laboratory from January 1, 2000, to December 31, 2017, who were MOG-IgG seropositive and seen at the Mayo Clinic for inflammatory demyelinating diseases of the central nervous system. Inclusion criteria for this study were (1) clinical myelitis episode; (2) MOG-IgG seropositivity by live cell-based assay17; and (3) medical records available. We excluded 145 patients who did not have documented myelitis.
Collection of Clinical and Laboratory Data
Medical records of patients with MOG-IgG seropositive myelitis were reviewed by 3 neurologists (D.D., E.P.F., and E. Shosha) for demographic, clinical, and laboratory data. Outcome was measured using the need of a gait aid and modified Rankin score at the last clinic follow-up. Change in modified Rankin score by at least 1 was considered to be a favorable response to immunotherapy.
MOG-IgG1 Detection
All patients were evaluated serologically for MOG-IgG1 seropositivity in the Mayo Clinic Neuroimmunology Laboratory as described previously.6,17 Samples are screened at 1/20 dilution and, if positive, titrated at 1:20, 1:40, 1:100, and then in 10-fold dilution steps up to 1:100 000. The farthest dilution with a positive result was recorded as the end point of positivity.
Comparative Groups
Forty-six AQP4-IgG seropositive patients with TM (longitudinally extensive or short lesions) who were identified from our TM database were used as a comparative group; 40 were included in previous studies.7,18 All 13 AQP4-IgG seropositive patients with available samples were seronegative for MOG-IgG. Twenty-six patients with MS with myelitis from a population-based cohort of inflammatory demyelinating diseases of the central nervous system and whose sera were negative for both MOG-IgG and AQP4-IgG were also included as a comparison group.19
Disease Control Individuals With Acute Flaccid Myelitis
We tested 6 patients from a prior series of enterovirus D68-associated acute flaccid myelitis (AFM) in Colorado in 2014 given the similarity in radiologic appearance with MOG-IgG myelitis.20 All 6 were negative for MOG-IgG. Patients with enterovirus D68 AFM consented as part of a previous study approved by the Colorado Multiple institutional review board and met the Council of State and Territorial Epidemiologists case definition for AFM.20,21
Magnetic Resonance Imaging Review
Magnetic resonance imaging (MRI) images within 4 weeks of TM episodes were available for review in 51 of 54 patients (included in the analysis of radiologic characteristics), and the remaining 3 had MRI reports documenting their abnormalities. The MRI images of AQP4-IgG positive myelitis and MS myelitis were used for comparison. Cases in all 3 groups (MOG-IgG myelitis, AQP4-IgG myelitis, and MS myelitis) were dispersed equitably during the 15-year period. Spine and brain MRI at Mayo Clinic were performed with 1.5- and 3-T MRI Siemens and General Electric machines. Gadolinium was administered as 0.1 mmol/kg intravenous, and imaging was performed without delay following gadolinium administration. Cervical spine MRI was performed as follows: (1) T1: echo time (TE), 11 milliseconds; repetition time (TR), 510 milliseconds; slice thickness, 3.5 mm; interspace, 1.5 mm; (2) T2: TE, 107 milliseconds; TR, 2600 to 6000 milliseconds; slice thickness, 4 mm; interspace, 0 mm; and (3) inversion recovery: TE, 74 milliseconds; TR, 3500 milliseconds; TI, 110 milliseconds; slice thickness, 3.5 mm; interspace, 0.5 mm. Thoracic spine imaging was performed as follows: (1) T1: TE, 9.4 milliseconds; TR, 400 to 700 milliseconds; slice thickness, 3.5 mm; and (2) T2: TE, 105 milliseconds; TR, 2600 to 6000 milliseconds; slice thickness, 3.5 mm; skip, 0.5. Lumbar spine imaging was performed as follows: (1) T1: TE, 9.4 milliseconds; TR 400 to 700 milliseconds; slice thickness, 4; skip, 1; and (2) T2 with fat saturation TE, 105 milliseconds; TR, 2600 to 6000 milliseconds; slice thickness, 4; skip, 1. Neuroradiologic images from diagnostic centers outside of Mayo Clinic were also directly reviewed. Available spine MRI images were reviewed by a neurologist (E.P.F.) and neuroradiologist (P.P.M.) in an unblinded fashion to determine radiologic features suggestive of MOG-IgG myelitis. Using this initial review and reports from prior studies, we enlisted a neuroradiologist (K.N.K.) to review MRI results blinded to the clinical data and antibody serostatus and to evaluate for the following features: longitudinally extensive T2 lesion at least 3 vertebral segments on sagittal sequences; single or multiple spinal cord T2-hyperintense lesions; sagittal T2-hyperintense line combined with axial T2-hyperintensity confined to gray matter in an H pattern, involvement of the conus medullaris, and gadolinium enhancement. The MRI head results were taken from the neuroradiology report of a concomitant head MRI (within 4 weeks of spine MRI) or, if no detailed report was available, were reviewed by a neurologist (E.P.F.).
Statistical Analysis
Categorical and continuous variables were analyzed and compared by Pearson χ2 and Mann-Whitney U test, respectively (SPSS, version 23; IBM). The P value was 2-sided, and values of less than .05 were considered significant.
Results
Demographics and Clinical Characteristics of Patients With MOG-IgG
Fifty-four patients with MOG-IgG accompanied by myelitis were included. Demographics and clinical details are summarized in Table 1. Thirty-one patients (57%) had prodromal symptoms (rhinorrhea, sore throat, low-grade fever, malaise, and cough) owing to presumed (n = 28) or confirmed infection (Epstein Barr virus, n = 3), and 2 occurred 1 to 2 weeks after influenza vaccination. The MOG-IgG myelitis episode was the initial manifestation of the disease in 41 patients (76%) occurring in isolation in 29 patients (54%) or as a component of a multifocal presentation in 12 patients (22%; 9 with ADEM22,23 and 3 with optic neuritis). In 13 patients (24%), the MOG-IgG myelitis occurred as a relapse, preceded by 1 or more episodes (median, 2; range, 1-5) of optic neuritis. Ten patients (19%) initially met criteria for AFM with presumed viral/postviral cause. The most frequent clinical features of MOG-IgG myelitis were neurogenic bladder, weakness, and numbness, and one-third were wheelchair dependent at nadir (Table 1).
Table 1. Clinical Characteristics and Outcomes of MOG-IgG Myelitis.
| Demographics and Clinical Features | No./Total No. (%) |
|---|---|
| Age at myelitis onset (range), y | 25 (3-73) |
| Female | 24/54 (44) |
| Children (<18 y) | 16/54 (30) |
| White | 50/54 (93) |
| Weakness | 45/54 (83) |
| Numbness/sensory level | 48/54 (89) |
| Bowel/bladder dysfunction | 45/54 (83) |
| Erectile dysfunction, adult men | 13/24 (54) |
| Spasticity/hyperreflexia/positive babinski | 39/54 (72) |
| Flaccid areflexia | 10/54 (19) |
| Lhermitte phenomenon | 9/54 (17) |
| Ambulated independently at attack nadir | 14/54 (26) |
| Cane/walker dependent at attack nadir | 22/54 (41) |
| Wheelchair dependent at attack nadir | 18/54 (33) |
| Clinical outcomes | |
| Response to initial immunotherapy | 48/52 (92) |
| Duration of follow-up, median (range), mo | 24 (2-120) |
| Gait aid at last follow-up | 3/54 (6) |
| Bowel/bladder dysfunction at last follow-up | 24/54 (44) |
| Erectile dysfunction at last follow-up | 8/24 (33) |
| mRS at last follow-up, median (range) | 1 (0-4) |
Abbreviations: MOG, myelin oligodendrocyte glycoprotein; mRS, modified Rankin score.
Laboratory Characteristics of Patients WIth MOG-IgG
The cerebrospinal fluid (CSF) results are detailed in Table 2. Eight patients who met criteria for AFM had negative CSF viral studies, including West Nile virus serology and polymerase chain reactions for enterovirus, cytomegalovirus, and varicella zoster virus.
Table 2. Comparison of Clinical and MRI Characteristics of MOG-IgG, AQP4-IgG, and MS Myelitis.
| Demographics | Myelitis, No./Total No. (%) | MOG-IgG vs AQP4-IgG P Valuea | MS Myelitis, No./Total No. (%) | MOG-IgG vs MS P Valueb | |
|---|---|---|---|---|---|
| MOG-IgG | AQP4-IgG | ||||
| Age (range), y | 25 (3-73) | 49.5 (15-75) | <.001 | 35 (18-59) | .007 |
| Children (<18 y) | 16/54 (30) | 2/46 (4) | .001 | 0/26 | .002 |
| Female | 24/54 (44) | 39/46 (85) | <.001 | 20/26 (77) | .006 |
| White | 50/54 (93) | 32/46 (70) | .003 | 26/26 (100) | .15 |
| Clinical features | |||||
| Preceding viral-like prodrome or vaccination | 33/54 (61) | 3/46 (7) | <.001 | 0/26 | <.001 |
| ADEM with myelitis | 9/54 (17) | 0/46 | .004 | 0/26 | .03 |
| History of intractable nausea and vomiting | 5/54 (9) | 9/46 (20) | .14 | 0/26 | .11 |
| Neurogenic bowel/bladder | 45/54 (83) | 32/46 (69) | .10 | 8/26 (31) | <.001 |
| Erectile dysfunction | 13/24 (54) | 1/7 (14) | .06 | 0/6 | .06 |
| Wheelchair dependent at attack nadir | 18/54 (33) | 15/46 (33) | .94 | 0/26 | <.001 |
| CSF findings | |||||
| CSF elevated white blood cell count, >5 cells/μL | 30/42 (71)c | 21/24 (88) | .13 | 13/18 (72) | .81 |
| Markedly elevated CSF white blood cell count, >50 cells/μL | 22/42 (52) | 6/24 (25) | .03 | 0/18 | <.001 |
| Elevated CSF protein, >50 mg/dL | 30/42 (71) | 16/24 (67) | .69 | 7/18 (39) | .02 |
| Elevated (≥4) oligoclonal bands | 1/38 (3) | 3/27 (11) | .16 | 16/18 (89) | <.001 |
| MRI spine features | |||||
| Longitudinally extensive sagittal T2 lesion (>3 vertebral segments) | 37/47 (79) | 28/34 (82) | .52 | 0/26 | <.001 |
| ≥2 cord lesions | 29/47 (62) | 0/34 | <.001 | 17/26 (65) | .76 |
| Gadolinium enhancement | 14/54 (26) | 31/40 (78) | <.001 | 19/26 (73) | <.001 |
| Concurrent H sign and linear sagittal hyperintensity | 15/51 (29) | 3/39 (8) | .007 | 0/26 | .002 |
| Involvement of conus | 21/51 (41) | 5/38 (13) | .004 | 5/15 (33) | .59 |
| MRI head features | |||||
| Infratentorial lesions | 19/46 (41) | 8/29 (28) | .23 | 9/25 (36) | .66 |
| Deep gray matter lesions | 14/46 (30) | 3/29 (10) | .04 | 1/25 (4) | .009 |
| Inferior temporal periventricular lesions | 3/43 (7) | 2/29 (7) | .95 | 6/25 (24) | .03 |
| Ovoid periventricular | 8/46 (17) | 4/29 (14) | .76 | 9/25 (36) | .08 |
| Gadolinium enhancement | 8/44 (18) | 3/24 (13) | .37 | 13/25 (52) | .003 |
| Clinical outcomes | |||||
| Gait aid at last follow-up | 3/54 (6) | 17/46 (37) | <.001 | 1/26 (4) | .74 |
| mRS at last follow-up, median (range) | 1 (0-4) | 2 (0-6) | <.001 | 1 (0-4) | .61 |
| Duration of follow-up, median (range), mo | 24 (2-120) | 34 (1-118) | .39 | 90 (1-166) | <.001 |
Abbreviations: ADEM, acute disseminated encephalomyelitis; AQP4, aquaporin-4; CSF, cerebrospinal fluid; MOG, myelin oligodendrocyte glycoprotein; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; MS, multiple sclerosis.
SI conversion factor: To convert protein to grams per liter, multiply by 0.01; white blood cell count to ×109/L, multiply by 0.001.
Statistical comparison between MOG IgG myelitis and AQP4 IgG myelitis cases.
Statistical comparison between MOG IgG myelitis and MS myelitis cases.
Median value of 55 cells/μL (range, 0-430: lymphocytic predominance [96%]).
MOG-IgG Serologic Characteristics
The median MOG-IgG titer was 100 (range, 20-10 000 [normal, <20]). Twenty-four patients had persistent MOG-IgG seropositivity. This included 16 with a single MOG-IgG positive result more than a year after onset (with the presumption that if tested at disease onset, the patient would be seropositive) and 8 patients with serial samples persistently positive for more than 12 months. Among those with persistent seropositivity, 21 of 24 (88%) relapsed by the median follow-up interval of 26 months (range, 14-84 months), while just 1 of 5 (20%) with transient seropositivity relapsed by a median follow-up 35 months (range, 15-120) (P = .001). All subsequent attacks included optic neuritis in isolation (n = 16), concurrently with TM (n = 5) or ADEM (n = 1).
MRI Characteristics of MOG-IgG Myelitis
The MRI characteristics of patients with MOG-IgG seropositive myelitis are summarized in Table 2. The myelitis was accompanied by a longitudinally extensive lesion (≥3 vertebral segment) in 37 of 47 patients (79%) with details available. The median vertebral length of the sagittal T2-hyperintense lesions was 4 vertebral segments (range, 1-15). Twenty-nine of 47 patients (62%) had at least 2 noncontiguous lesions (23 patients had at least 1 long lesion in combination with another long/short lesion; 6 patients had 2 or more short lesions). In the 51 patients with details available, the lesions involved thoracic and cervical cord in 36 patients (71%); cervical cord only in 9 patients (18%); and thoracic cord only in 6 patients (12%). Fifteen of 51 patients had extension to the posterior medulla/area postrema region (29%). A minority of patients had enhancement (Table 2), and the pattern was typically patchy and faint.
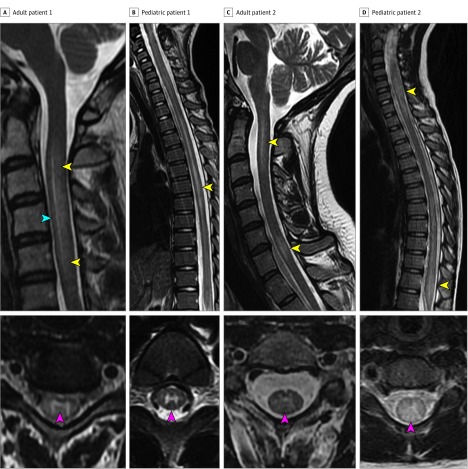
Notably, MRI spine demonstrated T2 hyperintensity restricted to spinal cord gray matter resulting in a characteristic T2-hyperintense line running in the superior-inferior direction on sagittal images (Figure 1A and C; Figure 2A) surrounded by more hazy T2-hyperintense signal of the anterior and posterior gray matter horns (n = 15 of 51; 29%). This manifested as a distinctive H pattern in the axial plane (Figure 1A-C; Figure 2A). On follow-up MRIs (n = 35), only 1 of 35 patients (3%) had appreciable spinal cord atrophy. Sixteen of 53 patients with brain MRI (30%) had lesions consistent with ADEM, despite only 9 patients meeting clinical criteria for ADEM.
Figure 1. Examples of Magnetic Resonance Imaging (MRI) Findings in Myelin Oligodendrocyte Glycoprotein Autoantibody (MOG-IgG) Myelitis.
A, An adult patient with a sagittal T2-hyperintense line (blue arrowhead) that is surrounded by fainter T2 hyperintensity (yellow arrowheads), with a corresponding T2 hyperintensity highly restricted to the gray matter on axial sequences forming an H sign (red arrowhead). B, A pediatric patient with a sagittal line of T2 hyperintensity (yellow arrowhead) confined to gray matter on axial images forming an H sign (red arrowhead). C, An adult patient with 2 noncontiguous T2-hyperintense lesions in the cervical cord extending 2.5 and 1 vertebral segments (yellow arrowhead); the upper lesion has a vague appearance of a central line. The axial sequences show central T2 hyperintensity most prominent at the central canal and fainter signal surrounding that is confined to gray matter vaguely resembling an H sign (red arrowhead). D, A pediatric patient is shown with 2 longitudinally extensive T2 hyperintense lesions on sagittal sequences, 1 in the cervical cord and 1 in the conus (yellow arrowheads). On axial sequences, this lesion shows central predominant signal abnormality that is more diffuse and not confined to gray matter (red arrowhead).
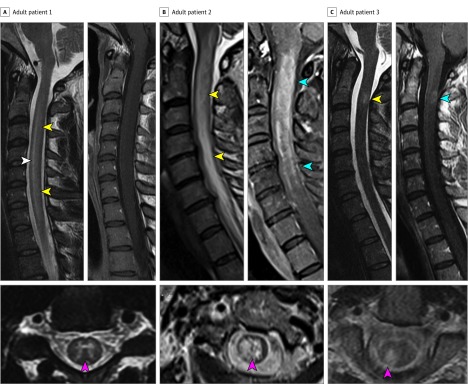
Figure 2. Comparison of Myelitis With Myelin Oligodendrocyte Glycoprotein Autoantibody (MOG-IgG), Aquaporin-4–IgG (AQP4-IgG), and Multiple Sclerosis (MS) Myelitis.
A, An adult patient with MOG-IgG myelitis is shown in which a sagittal T2-hyperintense line (white arrowhead) is present surrounded by hazier T2 hyperintensity (yellow arrowheads) forming a longitudinally extensive spinal cord lesion accompanied by mild spinal cord swelling. On axial sequences, the T2 hyperintensity is highly restricted to the gray matter forming an H sign (red arrowhead). Postgadolinium sagittal images demonstrate no gadolinium enhancement. B, In contrast, an adult patient with AQP4-IgG positive neuromyelitis optica spectrum disorder (NMOSD) and a longitudinally T2 hyperintense extensive lesion with prominent swelling (yellow arrowheads) and an axial T2 lesion shows diffuse central signal abnormality not confined to gray matter (red arrowhead). The lesion has accompanying gadolinium enhancement (blue arrowheads). C, An adult patient with MS has a short T2-hyperintense lesion at the C2 level extending one vertebral segment in length (yellow arrowhead) with accompanying faint ring enhancement (blue arrowhead). On axial sequences, the lesion is located in the periphery of the cord in the right dorsal column (red arrowhead).
Response to Immunotherapy and Long-term Clinical Outcome
Short-term treatments were administered in 52 of 54 patients (96%) and included 1 or more of high-dose intravenous methylprednisolone (n = 51); plasmapheresis (n = 10); and intravenous immunoglobulin (n = 8). Improvement with short-term treatment was reported in 48 (92%). Twenty-eight patients (52%) received long-term maintenance immunotherapy for attack prevention including 1 or more of rituximab (n = 16), mycophenolate mofetil (n = 12), and azathioprine (n = 9). At final follow-up (median, 24 months; range, 2-120 months), 32 patients (59%) had developed 1 or more relapses of optic neuritis (n = 31), TM (n = 7), or ADEM (n = 1). The clinical outcomes are summarized in Table 1.
Comparison of MOG-IgG Myelitis With AQP4-IgG and MS Myelitis
We compared the myelitis seropositive for MOG-IgG (n = 54) vs AQP4-IgG (n = 46) and MS myelitis (n = 26) (Table 2). There was no difference in the median time from attack symptom onset to MRI spine between MOG-IgG myelitis (median, 7 days; interquartile range [IQR], 4-15 days), AQP4-IgG myelitis (median, 9 days; IQR, 5-17.5 days), and MS myelitis (median, 12 days; IQR, 6.5-28 days) (P = .16). Of the 126 patients, just 2 (1.5%) had received immunotherapy for short-term attack treatment prior to MRI (MS, 1; AQP4-IgG, 1). The clinical features favoring MOG-IgG myelitis vs AQP4-IgG and MS myelitis were male sex, younger age, a prodromal infectious syndrome, and myelitis occurring as a component of ADEM (Table 2). On MRI of the spine, radiologic features more common in MOG-IgG than AQP4-IgG and MS myelitis were T2 signal confined to gray matter (sagittal T2-hyperintense line combined with axial H sign), and absence of gadolinium enhancement (Table 2). The frequency of longitudinally extensive lesions among MOG-IgG myelitis and AQP4-IgG myelitis were similar, but MOG-IgG more often had a multiplicity of spinal cord lesions. Conus involvement was more frequent with MOG-IgG than AQP4-IgG but did not differ from MS myelitis.
Discussion
In this study, we identified unique clinical and radiologic features of MOG-IgG–associated myelitis that are distinct from AQP4-IgG and MS myelitis. We showed that patients with MOG-IgG myelitis may meet clinical criteria for AFM, and its identification in this setting has important therapeutic and prognostic implications. The clinical, CSF, and MRI findings that we report with MOG-IgG myelitis may provide insight into the biologic mechanisms involved and help clinicians select those patients in whom MOG IgG should be tested. The selection of appropriate patients to undergo MOG-IgG testing is crucial because serologic testing in low-probability situations substantially increases the risk of false-positives.24
Myelitis in MOG-IgG–related disease most frequently occurs as the initial manifestation and may occur in isolation or concurrently with optic neuritis and/or cerebral involvement with a neuromyelitis optica spectrum disorder/ADEM-phenotype, and such presentations should prompt consideration of MOG-IgG testing. Similar to prior studies, we found urinary retention/incontinence and erectile dysfunction common myelitis accompaniments, fitting with conus involvement radiologically.11 There were prominent motor deficits, and one-third of patients were wheelchair dependent at nadir. Nonetheless, most recovered well with treatment, and long-term wheelchair dependence was rare. Cerebrospinal fluid oligoclonal bands were found in fewer than 15% of patients, similar to prior studies.11,13 In those with subsequent attacks, optic neuritis episodes predominated.
The novel T2 hyperintensity confined to gray matter in a sagittal T2-hyperintense line and forming axial H sign (Figures 1 and 2) was a radiologic clue to MOG-IgG myelitis. Notably, 10 of the MOG myelitis cases were suspected to have an infectious/postinfectious etiology and met clinical criteria for AFM. Among these cases, 8 met the definite AFM criteria (acute flaccid limb weakness and T2 hyperintensity involving spinal cord central gray matter), and 2 met probable AFM criteria (acute flaccid limb weakness and CSF pleocytosis).21 All 6 patients with AFM associated with the enterovirus D68 outbreak in Colorado in 2014 were negative for MOG-IgG. In contrast to the outcomes of the infectious/postinfectious AFM cases25,26 most MOG-IgG myelitis cases responded well to short-term treatment, highlighting that distinction of these disorders has important treatment and prognostic value. Viral myelitis (West Nile and poliomyelitis) and spinal cord infarct may also result in signal abnormalities similarly confined to gray matter, although cord infarct is more common in older adults and presents more acutely.27,28 Care is needed when gradient echo sequences (rather than standard axial T2) sequences are used because the gray matter can appear hyperintense/bright on these sequences in normal people; all patients in our study had definite signal abnormality on regular axial T2 sequences similar to Figures 1 and 2. We used a stringent definition requiring signal strictly confined to the central gray matter as depicted in Figure 1A-C and Figure 2A because the small size of the spinal cord (particularly thoracic cord) can make distinction from combined gray and white matter signal change challenging (Figure 1D and 2B). However, it should also be noted that many MOG-IgG cases had diffuse central T2 hyperintensity (Figure 1D) indistinguishable from AQP4-IgG myelitis.
The etiology of spinal cord and brain deep gray matter T2 hyperintensity in MOG-IgG–related disease is unclear. Oligodendrocytes containing MOG are found in spinal cord gray matter,29 and transcriptome profiles have demonstrated expression of MOG in the gray matter of the brain.30,31 The location of MRI abnormalities in anterior horn cells and the conus recapitulate lesion locations with experimental autoimmune encephalomyelitis animal models immunized with MOG 35-55 peptide.32 We suspect that the MRI findings reflect gray matter demyelination in MOG-IgG–related disease. The variability in lesion location across the demyelinating diseases that we found may reflect differences in expression of MOG, AQP4, and the targets of MS inflammation in the regions involved. Human pathologic studies of biopsied or autopsied patients with MOG-IgG are needed to provide further insight into the pathobiology of these MRI findings.
In Table 3, we summarize the similarities and differences between MOG-IgG and AQP4-IgG myelitis. Clinically, both had similar myelitis severity at nadir, but MOG-IgG myelitis recovered more completely and had better long-term outcomes. The CSF findings and brain MRI findings were broadly similar, although deep gray matter lesions were more common with MOG-IgG. Longitudinally extensive lesions with axial central signal abnormality were common to both, but MOG-IgG myelitis lesions more frequently were confined to gray matter (sagittal line/H sign) and more often had 2 noncontiguous lesions, the latter similar to prior reports.12,13 Involvement of the conus in 21 of 51 patients with MOG-IgG (41%) was also a useful distinguisher from AQP4-IgG, although prior reports have varied from 75%12 to 4%.13 In addition, MOG-IgG myelitis had associated enhancement less frequently than AQP4-IgG and tended to have less cord edema (Figure 2).
Table 3. Summary of the Clinical, Cerebrospinal Fluid, and MRI Characteristics of Myelitis With MOG-IgG, AQP4-IgG, and MS.
| Characteristics | MOG-IgG | AQP4-IgG | MS |
|---|---|---|---|
| Clinical/demographic | |||
| Female | Frequent | Very frequent | Frequent |
| Children | Frequent | Infrequent | Infrequent |
| ADEM phenotype | Frequent | Infrequent | Rare |
| Severe myelitis (wheelchair dependent) | Frequent | Frequent | Rare |
| Excellent recovery | Frequent | Infrequent | Very frequent |
| CSF | |||
| White blood cell >50/μL | Frequent | Infrequent | Rare |
| Positive oligoclonal bands | Infrequent | Infrequent | Very frequent |
| MRI spine | |||
| Longitudinally extensive T2 lesion(s) | Frequent | Very frequent | Rare |
| Short T2 lesion(s) | Frequent | Infrequent | Very frequent |
| Gray matter restricted (H sign/sagittal line) | Frequent | Infrequent | Rare |
| Axial T2 central | Very frequent | Very frequent | Rare |
| Axial peripheral (dorsal/lateral column) | Rare | Rare | Very frequent |
| Multiple lesions | Frequent | Rare | Frequent |
| Enhancement of myelitis lesion(s) | Infrequent | Frequent | Frequent |
| MRI head | |||
| Inferior temporal periventricular lesion(s) | Rare | Rare | Frequent |
| Deep gray matter lesion(s) | Frequent | Infrequent | Rare |
| Gadolinium enhancing lesions | Infrequent | Infrequent | Frequent |
Abbreviations: ADEM, acute disseminated encephalomyelitis; AQP4, aquaporin-4; CSF, cerebrospinal fluid; MOG, myelin oligodendrocyte glycoprotein; MRI, magnetic resonance imaging; MS, multiple sclerosis.
SI conversion factor: To convert white blood cell count to ×109/L, multiply by 0.001.
In Table 3, we also summarize a comparison of MOG-IgG and MS myelitis. The MOG-IgG myelitis is clinically more severe than MS myelitis.7 The low frequency of 3% with oligoclonal bands compared with MS myelitis where it occurred in 89% in our study and 88% in a prior meta-analysis33 highlights it as a useful discriminator. While short lesions (<3 vertebral segments) can occur in both MOG-IgG myelitis and MS, the short lesion will often have a second longitudinally extensive lesion with MOG-IgG myelitis. In addition, on axial sequences, MS lesions are typically peripheral in the cord located in the dorsal or lateral columns in contrast to the central predominance of MOG-IgG myelitis (Figure 2). At the time of acute relapse, MOG-IgG myelitis lesions are frequently nonenhancing, but most MS lesions enhance. On brain MRI, deep gray matter lesions and lack of enhancement was associated with MOG-IgG, while inferior temporal periventricular lesions was associated with MS; a more detailed comparison of brain MRI findings between these diseases has been reported previously.34
Limitations
Our study has certain limitations, including the retrospective design. Also, we had variable follow-up and thus we could not assess the long-term effects in all patients. Our comparison is limited by MOG-IgG myelitis having a greater tendency to affect children than AQP4-IgG, and thus, our MOG-IgG group had a higher proportion of children, some of whom may have reflected our selection of these cases and may have contributed to the higher ADEM rates.35,36,37 However, the clinical and imaging feature of myelitis that we report with MOG-IgG remained consistent for adults (Figure 1A and C; Figure 2A) and children (Figure 1B and D). The patients with MS myelitis were population based, which could affect the comparison, but our findings are consistent with the general clinical observations MS myelitis (mild, accompanied by oligoclonal bands and short MRI lesions). Serial samples were only available in a few patients, and thus, the predictive value of persistence of MOG-IgG could not be assessed fully in this study.
Conclusions
Our study provides practical, clinical, CSF, and MRI clues to MOG-IgG myelitis that are outlined in Table 3. This will be helpful for clinicians encountering patients with myelitis to help better recognize those likely to be MOG-IgG seropositive.
References
- 1.Greenberg BM. Treatment of acute transverse myelitis and its early complications. Continuum (Minneap Minn). 2011;17(4):733-743. [DOI] [PubMed] [Google Scholar]
- 2.Zalewski NL, Flanagan EP, Keegan BM. Evaluation of idiopathic transverse myelitis revealing specific myelopathy diagnoses. Neurology. 2018;90(2):e96-e102. [DOI] [PubMed] [Google Scholar]
- 3.Jiao Y, Fryer JP, Lennon VA, et al. Updated estimate of AQP4-IgG serostatus and disability outcome in neuromyelitis optica. Neurology. 2013;81(14):1197-1204. doi: 10.1212/WNL.0b013e3182a6cb5c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202(4):473-477. doi: 10.1084/jem.20050304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reindl M, Di Pauli F, Rostásy K, Berger T. The spectrum of MOG autoantibody-associated demyelinating diseases. Nat Rev Neurol. 2013;9(8):455-461. doi: 10.1038/nrneurol.2013.118 [DOI] [PubMed] [Google Scholar]
- 6.López-Chiriboga AS, Majed M, Fryer J, et al. Association of MOG-IgG serostatus with relapse after acute disseminated encephalomyelitis and proposed diagnostic criteria for MOG-IgG-associated disorders. JAMA Neurol. 2018. doi: 10.1001/jamaneurol.2018.1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flanagan EP, Weinshenker BG, Krecke KN, et al. Short myelitis lesions in aquaporin-4-IgG-positive neuromyelitis optica spectrum disorders. JAMA Neurol. 2015;72(1):81-87. doi: 10.1001/jamaneurol.2014.2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zalewski NL, Morris PP, Weinshenker BG, et al. Ring-enhancing spinal cord lesions in neuromyelitis optica spectrum disorders. J Neurol Neurosurg Psychiatry. 2017;88(3):218-225. doi: 10.1136/jnnp-2016-314738 [DOI] [PubMed] [Google Scholar]
- 9.Weinshenker BG, Wingerchuk DM, Vukusic S, et al. Neuromyelitis optica IgG predicts relapse after longitudinally extensive transverse myelitis. Ann Neurol. 2006;59(3):566-569. doi: 10.1002/ana.20770 [DOI] [PubMed] [Google Scholar]
- 10.Wingerchuk DM, Banwell B, Bennett JL, et al. ; International Panel for NMO Diagnosis . International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-189. doi: 10.1212/WNL.0000000000001729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140(12):3128-3138. doi: 10.1093/brain/awx276 [DOI] [PubMed] [Google Scholar]
- 12.Kitley J, Woodhall M, Waters P, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. 2012;79(12):1273-1277. doi: 10.1212/WNL.0b013e31826aac4e [DOI] [PubMed] [Google Scholar]
- 13.Jarius S, Ruprecht K, Kleiter I, et al. ; in cooperation with the Neuromyelitis Optica Study Group (NEMOS) . MOG-IgG in NMO and related disorders: a multicenter study of 50 patients, part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13(1):280. doi: 10.1186/s12974-016-0718-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reindl M. Clinical course of MOG antibody-associated recurrent demyelinating diseases. J Neurol Neurosurg Psychiatry. 2018;89(2):118. [DOI] [PubMed] [Google Scholar]
- 15.Bouzar M, Daoudi S, Hattab S, et al. Neuromyelitis optica spectrum disorders with antibodies to myelin oligodendrocyte glycoprotein or aquaporin-4: clinical and paraclinical characteristics in Algerian patients. J Neurol Sci. 2017;381:240-244. doi: 10.1016/j.jns.2017.08.3254 [DOI] [PubMed] [Google Scholar]
- 16.Hyun JW, Woodhall MR, Kim SH, et al. Longitudinal analysis of myelin oligodendrocyte glycoprotein antibodies in CNS inflammatory diseases. J Neurol Neurosurg Psychiatry. 2017;88(10):811-817. doi: 10.1136/jnnp-2017-315998 [DOI] [PubMed] [Google Scholar]
- 17.Jitprapaikulsan J, Chen JJ, Flanagan EP, et al. Aquaporin-4 and myelin oligodendrocyte glycoprotein autoantibody status predict outcome of recurrent optic neuritis. Ophthalmology. 2018;125(10):1628-1637. doi: 10.1016/j.ophtha.2018.03.041 [DOI] [PubMed] [Google Scholar]
- 18.Flanagan EP, Kaufmann TJ, Krecke KN, et al. Discriminating long myelitis of neuromyelitis optica from sarcoidosis. Ann Neurol. 2016;79(3):437-447. doi: 10.1002/ana.24582 [DOI] [PubMed] [Google Scholar]
- 19.Flanagan EP, Cabre P, Weinshenker BG, et al. Epidemiology of aquaporin-4 autoimmunity and neuromyelitis optica spectrum. Ann Neurol. 2016;79(5):775-783. doi: 10.1002/ana.24617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messacar K, Schreiner TL, Maloney JA, et al. A cluster of acute flaccid paralysis and cranial nerve dysfunction temporally associated with an outbreak of enterovirus D68 in children in Colorado, USA. Lancet. 2015;385(9978):1662-1671. doi: 10.1016/S0140-6736(14)62457-0 [DOI] [PubMed] [Google Scholar]
- 21.Environment CDoPHa. Standardized case definition for acute flaccid myelitis. https://wwwn.cdc.gov/nndss/conditions/acute-flaccid-myelitis/case-definition/2018/. Accessed November 14, 2018.
- 22.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404. doi: 10.1016/S1474-4422(15)00401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krupp LB, Banwell B, Tenembaum S; International Pediatric MS Study Group . Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68(16)(suppl 2):S7-S12. doi: 10.1212/01.wnl.0000259422.44235.a8 [DOI] [PubMed] [Google Scholar]
- 24.Jarius S, Paul F, Aktas O, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation. 2018;15(1):134. doi: 10.1186/s12974-018-1144-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin JA, Messacar K, Yang ML, et al. Outcomes of Colorado children with acute flaccid myelitis at 1 year. Neurology. 2017;89(2):129-137. doi: 10.1212/WNL.0000000000004081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messacar K, Schreiner TL, Van Haren K, et al. Acute flaccid myelitis: a clinical review of US cases 2012-2015. Ann Neurol. 2016;80(3):326-338. doi: 10.1002/ana.24730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masson C, Pruvo JP, Meder JF, et al. ; Study Group on Spinal Cord Infarction of the French Neurovascular Society . Spinal cord infarction: clinical and magnetic resonance imaging findings and short term outcome. J Neurol Neurosurg Psychiatry. 2004;75(10):1431-1435. doi: 10.1136/jnnp.2003.031724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maramattom BV, Philips G, Sudheesh N, Arunkumar G. Acute flaccid paralysis due to West Nile virus infection in adults: a paradigm shift entity. Ann Indian Acad Neurol. 2014;17(1):85-88. doi: 10.4103/0972-2327.128561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang SH, Li Y, Fukaya M, et al. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci. 2013;16(5):571-579. doi: 10.1038/nn.3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mills JD, Kavanagh T, Kim WS, et al. Unique transcriptome patterns of the white and grey matter corroborate structural and functional heterogeneity in the human frontal lobe. PLoS One. 2013;8(10):e78480. doi: 10.1371/journal.pone.0078480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinman L. The gray aspects of white matter disease in multiple sclerosis. Proc Natl Acad Sci U S A. 2009;106(20):8083-8084. doi: 10.1073/pnas.0903377106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bannerman PG, Hahn A, Ramirez S, et al. Motor neuron pathology in experimental autoimmune encephalomyelitis: studies in THY1-YFP transgenic mice. Brain. 2005;128(pt 8):1877-1886. doi: 10.1093/brain/awh550 [DOI] [PubMed] [Google Scholar]
- 33.Dobson R, Ramagopalan S, Davis A, Giovannoni G. Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: a meta-analysis of prevalence, prognosis and effect of latitude. J Neurol Neurosurg Psychiatry. 2013;84(8):909-914. doi: 10.1136/jnnp-2012-304695 [DOI] [PubMed] [Google Scholar]
- 34.Jurynczyk M, Geraldes R, Probert F, et al. Distinct brain imaging characteristics of autoantibody-mediated CNS conditions and multiple sclerosis. Brain. 2017;140(3):617-627. doi: 10.1093/brain/aww350 [DOI] [PubMed] [Google Scholar]
- 35.Hennes EM, Baumann M, Schanda K, et al. ; BIOMARKER Study Group . Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology. 2017;89(9):900-908. doi: 10.1212/WNL.0000000000004312 [DOI] [PubMed] [Google Scholar]
- 36.Ramanathan S, Mohammad S, Tantsis E, et al. ; Australasian and New Zealand MOG Study Group . Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry. 2018;89(2):127-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cobo-Calvo Á, Ruiz A, D’Indy H, et al. MOG antibody-related disorders: common features and uncommon presentations. J Neurol. 2017;264(9):1945-1955. doi: 10.1007/s00415-017-8583-z [DOI] [PubMed] [Google Scholar]