Key Points
Questions
Is there an association between migraine and type 2 diabetes in women?
Findings
In this study of 74 247 women in a French national cohort, a lower risk of type 2 diabetes was observed in women with active migraine. We also found a linear decrease of migraine prevalence long before and a plateau long after type 2 diabetes diagnosis.
Meaning
These results may suggest a potential role of both hyperglycemia and hyperinsulinism on migraine occurrence.
This longitudinal analysis of a French national cohort evaluates the association of migraine and type 2 diabetes in women, including changes in the prevalence of active migraine episodes before and after type 2 diabetes diagnosis.
Abstract
Importance
Little is known about the associations between migraine and type 2 diabetes and the temporality of the association between these 2 diseases.
Objective
To evaluate the association between migraine and type 2 diabetes incidence as well as the evolution of the prevalence of active migraine before and after type 2 diabetes diagnosis.
Design, Setting, and Participants
We used data from the E3N cohort study, a French prospective population-based study initiated in 1990 on a cohort of women born between 1925 and 1950. The E3N study participants are insured by a health insurance plan that mostly covers teachers. From the eligible women in the E3N study, we included those who completed the 2002 follow-up questionnaire with information available on migraine. We then excluded prevalent cases of type 2 diabetes, leaving a final sample of women who were followed up between 2004 and 2014. All potential occurrences of type 2 diabetes were identified through a drug reimbursement database. Statistical analyses were performed in March 2018.
Exposures
Self-reported migraine occurrence.
Main Outcomes and Measures
Pharmacologically treated type 2 diabetes.
Results
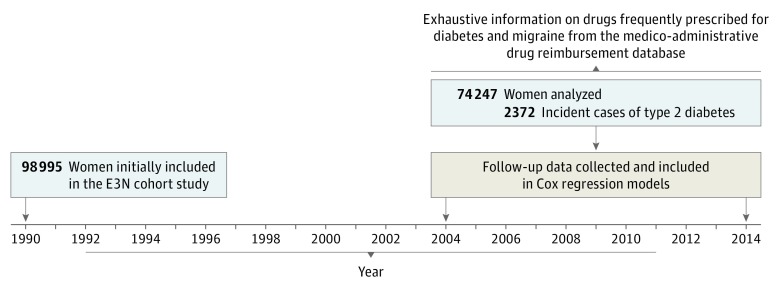
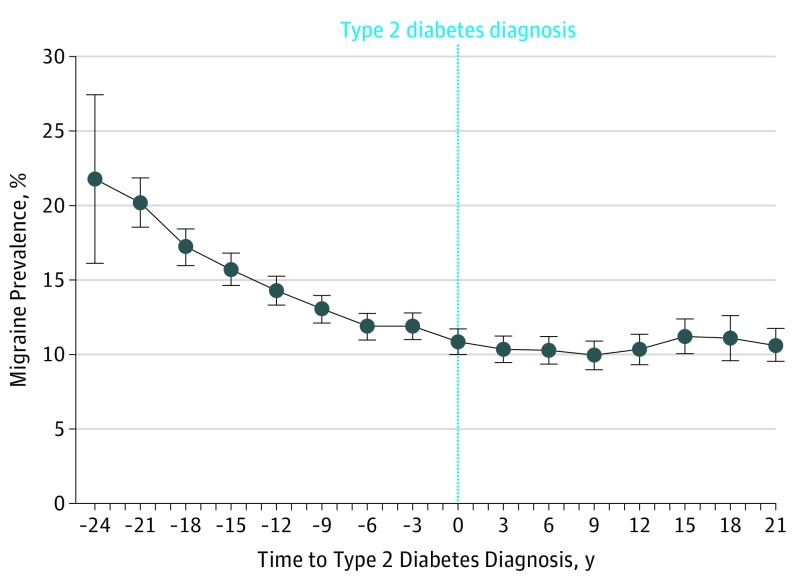
From the 98 995 women in the study, 76 403 women completed the 2002 follow-up survey. Of these, 2156 were excluded because they had type 2 diabetes, leaving 74 247 women. Participants had a mean (SD) age of 61 (6) years at baseline, and all were free of type 2 diabetes. During 10 years of follow-up, 2372 incident type 2 diabetes cases occurred. A lower risk of type 2 diabetes was observed for women with active migraine compared with women with no migraine history (univariate hazard ratio, 0.80 [95% CI, 0.67-0.96], multivariable-adjusted hazard ratio, 0.70 [95% CI, 0.58-0.85]). We also observed a linear decrease in active migraine prevalence from 22% (95% CI, 16%-27%) to 11% (95% CI, 10%-12%) during the 24 years prior to diabetes diagnosis, after adjustment for potential type 2 diabetes risk factors. A plateau of migraine prevalence around 11% was then observed for 22 years after diagnosis.
Conclusions and Relevance
We observed a lower risk of developing type 2 diabetes for women with active migraine and a decrease in active migraine prevalence prior to diabetes diagnosis. Further targeted research should focus on understanding the mechanisms involved in explaining these findings.
Introduction
Migraine is an intermittent painful neurologic headache disorder with an estimated 1-year prevalence of 15% to 18%1,2,3,4,5; it has been shown to be more common in women of reproductive age, with a declining prevalence after menopause.6,7 Previous work has shown that migraine, and especially migraine with aura, is associated with hyperlipidemia, hypertension, and an elevated Framingham Risk Score for coronary heart disease.8,9,10 Migraine has further been associated with increased risk of overall and specific cardiovascular disease events.11,12,13,14,15 Because migraine has also been associated with factors associated with insulin resistance and type 2 diabetes, an association between migraine and diabetes has been hypothesized. However, to our knowledge, data are scarce. Some previous articles have suggested an association between polymorphisms in the insulin receptor gene and migraine,16 impaired insulin sensitivity in individuals with migraine,17 and possibly elevated blood glucose and insulin levels in people with headaches,18 whereas others have shown that the frequency of migraine increased with body mass index,19 a major risk factor for type 2 diabetes. Indeed, it has been previously reported20 that chronic daily headache was increased in adults with obesity and the prevalence of episodic headaches may be increased in adults with obesity who are of reproductive age. However, despite the high prevalence of both diseases, the association between migraine and type 2 diabetes is still unclear.
Results from a population-based study in Norway have shown that people with type 1 or type 2 diabetes had a lower risk of migraine compared with the general population.21 Data from the Women’s Health Study did not find associations between migraine and incident diabetes.22 Moreover, little is known about the temporality of the potential association between migraine and type 2 diabetes.
Therefore, we aimed to evaluate the associations between migraine and the risk of developing type 2 diabetes in the prospective E3N cohort study. We also aimed to determine how the likelihood of migraine changed in association with incidence of type 2 diabetes.
Methods
Study Population
The Etude Epidémiologique Auprès des Femmes de la Mutuelle Générale de l’Education Nationale (E3N) study is a French prospective cohort study, initiated in 1990, of 98 995 women born between 1925 and 1950.23 The E3N study participants are insured by a health insurance plan that mostly covers teachers. The E3N study is the French component of the European Prospective Investigation into Cancer and Nutrition (EPIC) and is part of EPIC-InterAct, a case-cohort study on type 2 diabetes nested within EPIC.24 Participants have completed self-administered questionnaires that have been sent biennially since 1990. The mean response rate to a follow-up questionnaire is 83%, with a total loss to follow-up since 1990 of less than 3%. Furthermore, for each cohort member, the health insurance plan provided data that included all outpatient reimbursements for health expenditure since January 1, 2004; these data included brand names, dosages, and dates of drug purchases.
The study was approved by the French National Commission for Data Protection and Privacy (ClinicalTrials.gov identifier: NCT03285230). All participants gave their written informed consent.
Population for Analysis and Follow-up
Follow-up surveying for this study started on April 1, 2004. Participants contributed person-years of follow-up until the date of diagnosis of type 2 diabetes, the date of the last completed questionnaire, or November 17, 2014 (the date at which the last E3N questionnaire used for this study was sent to participants), whichever occurred first. From the women in the E3N study, we included women who completed the 2002 follow-up questionnaire with information available on migraine. We then excluded prevalent cases of type 2 diabetes to define the final sample for this study.
Assessment of Migraine and Migraine Medications
Information on migraine episodes was asked in the questionnaires sent in 1992, 1993, 1995, 1997, 2000, 2002, 2005, and 2011 (Figure 1), similar to what has been done in other studies.14 We have been able to use all of this information to update the exposure at each questionnaire with 3 main categories: (1) no migraine history; (2) active migraine (ie, all women who self-reported migraine on the current questionnaire cycle); and (3) prior migraine (ie, women who reported experiencing migraine in at least 1 of the past questionnaires but not on the current questionnaire). Information on the presence of aura was unavailable.
Figure 1. Data Available on Migraine and Follow-up Summary.
Years were those in which information on migraine questions was recorded on self-reported questionnaires.
We also controlled for the use of drugs frequently prescribed for migraine. These drugs were identified through the drug reimbursement database. We considered all reimbursements of drugs since April 1, 2004, corresponding to the World Health Organization Anatomical Therapeutic Chemical codes N02C (antimigraine preparations), M01A (nonsteroidal anti-inflammatory drugs and antirheumatic products), N02B (other analgesics and antipyretics), N03A (antiepileptics), N07C (antivertigo preparations), and C07 (β-blocking agents). Exposures to both antimigraine preparations and other drugs potentially used to treat migraine were updated continuously between 2004 and 2014.
Assessment of Type 2 Diabetes Cases
We defined cases of type 2 diabetes if a participating woman was pharmacologically treated with type 2 diabetes–specific medications. All potential type 2 diabetes occurrences were identified through the drug reimbursement database: women reimbursed at least twice for glucose-lowering medications within a sliding period of 1 year were classified as having type 2 diabetes, with the date of diagnosis defined as the date of first reimbursement.25
Statistical Analyses
Baseline characteristics of the study population were described in the overall population and according to migraine history. We used Cox proportional hazards regression models with age as the time scale to estimate hazard ratios (HRs) and 95% CIs to evaluate the association of migraine and the risk of type 2 diabetes. Migraine was considered as a time-dependent variable in the Cox proportional hazards regression models and categorized as (1) no migraine history, (2) prior migraine, or (3) active migraine. During the follow-up surveys, we had 4% missing data on migraine in 2005 and 5% missing data in 2011. If data on migraine were missing, we used the last known data available (last observation carried forward method). Models were univariate and then further adjusted for a list of established type 2 diabetes risk factors or variables leading to potential confounding: level of education (undergraduate or less, graduate, postgraduate or more; at baseline), level of recreational physical activity (metabolic equivalent task–hours per week, as a continuous variable; at baseline), body mass index (calculated as weight in kilograms divided by height in meters squared; <20, 20-25, 25-30, ≥30; time dependent), smoking status (nonsmoker, former smoker, or current smoker; time dependent), history of hypertension (no or yes; time dependent), menopausal status (premenopausal or postmenopausal; time dependent), menopausal hormone therapy use (never or ever; time dependent), use of oral contraceptives (never or ever; time dependent), family history of diabetes (no, yes, or unknown; at baseline), handedness (right-handed, left-handed, mixed, or unknown; at baseline),26 use of antimigraine preparations (current, past, or never; time dependent), and use of drugs other than antimigraine preparations frequently prescribed for migraine (current, past, or never; time dependent).
In a secondary analysis of women who developed type 2 diabetes during the follow-up period, we investigated the evolution of the prevalence of active migraine with respect to the date of type 2 diabetes diagnosis. To do so, we analyzed 4371 women with type 2 diabetes cases that occurred between 1992 and 2014. Analyses were based on a possible 46-year window (from 24 years prior to the diagnosis to 22 years after) with year 0 as the year of diagnosis of type 2 diabetes. We used a repeated-measures logistic regression analysis by the generalized estimating equations method, with an autoregressive correlation structure.27 The method takes the intraindividual correlation between measurements into account and is robust to missing values. To plot the trajectory of self-reported migraine episodes in association with the years before diabetes diagnosis, odds ratios (ORs) and their 95% CIs were estimated each year and then converted into proportions. As for the Cox models, this model was adjusted for age, level of education, family history of diabetes, body mass index, smoking status, hypertension, level of recreational physical activity, use of oral contraceptives, menopausal status, menopausal hormone therapy use, and handedness.
All statistical analyses used SAS version 9.4 (SAS Institute Inc) with the PHREG procedure used for Cox models and the GENMOD procedure for repeated-measures logistic regression. Missing values were less than 5% for all variables and were imputed with the median (quantitative variables) or the mode (qualitative variables) of the study population. All statistical tests were 2-sided, and we considered a P value less than .05 statistically significant. Figure 1 was plotted with the statistical software R version 3.1.0 (Free Software Foundation). Data analysis was completed in March 2018.
Results
From a total of 98 995 women in the E3N study, 76 403 had provided the requisite data and were included. Another 2156 were excluded because of type 2 diabetes diagnoses at baseline, leaving 74 247 women in the study analysis. Participants had a mean (SD) age of 61 (6) years on average at baseline. A total of 2372 participants experienced incident cases of type 2 diabetes between 2004 and 2014.
Study Participant Characteristics
When compared with women with no migraine history (Table 1), women who reported active migraine were younger (mean [SD] age: women with no migraine history, 61.8 [7] years; women with active migraine, 59.9 [6] years), had a lower level of physical activity (mean [SD] metabolic equivalent task–hours per week, 25.05 [21] vs 23.16 [20]), were more likely to have a family history of diabetes (5598 of 49 199 [11.4%] vs 964 of 7839 [12.3%]), were more likely to use oral contraceptives (29 142 of 49 199 [59.2%] vs 5167 of 7839 [65.9%]), were more likely to have a body mass index less than 20(5907 of 49 199 [12.0%] vs 1092 of 7839 [13.9%]), and were more likely to be former smokers (17 359 of 49 199 [35.6%] vs 3086 of 7839 [39.4%]). Compared with women with no migraine history, women with prior migraine were younger (mean [SD] age: women with no history of migraine, 61.8 [7] years; women with prior migraine, 60.9 [6] years) and were more likely to have a family history of diabetes (5598 of 49 199 [11.4%] vs 2133 of 17 209 [12.4%]), have a history of hypertension (22 114 of 49 199 [44.9%] vs 8921 of 17 209 [51.8%]), use oral contraceptives (29 142 of 49 199 [59.2%] vs 10 945 of 17 209 [63.6%]), be overweight (11 621 of 49 199 [23.6%] vs 4218 of 17 209 [24.5%]), and have never smoked (26 721 of 49 199 [54.3%] vs 1595 of 17 209 [9.3%]).
Table 1. Baseline Characteristics of the E3N Study Population (April 2004).
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| All (N = 74 247) | Migraine | |||
| No History (n = 49 199) | Prior (n = 17 209) | Active (n = 7839) | ||
| Age, mean (SD), y | 61.4 (6) | 61.8 (7) | 60.9 (6) | 59.9 (6) |
| Diabetes at the end of follow-up | 2372 (3.2) | 1569 (3.2) | 601 (3.5) | 202 (2.6) |
| Medications ever reimbursed between January and April 2004a | ||||
| Antimigraine preparations | 1264 (2) | 107 (0) | 209 (1) | 948 (12) |
| NSAIDs and antirheumatic products | 1537 (2.1) | 866 (1.8) | 396 (2.3) | 275 (3.5) |
| Other analgesics and antipyretics | 107 (0.1) | 23 (0.0) | 30 (0.2) | 54 (0.7) |
| Antiepileptics | 43 (0.1) | 16 (0.0) | 10 (0.1) | 17 (0.2) |
| Antivertigo preparations | 242 (0.3) | 86 (0.2) | 70 (0.4) | 86 (1.1) |
| β-Blocking agents | 1030 (1.4) | 418 (0.8) | 296 (1.7) | 316 (4.0) |
| Family history of diabetes | 8695 (11.7) | 5598 (11.4) | 2133 (12.4) | 964 (12.3) |
| Hypertension | 34 748 (46.8) | 22 114 (44.9) | 8921 (51.8) | 3713 (47.3) |
| Ever use of oral contraceptives | 45 254 (60.9) | 29 142 (59.2) | 10 945 (63.6) | 5167 (65.9) |
| Postmenopause | 72 465 (97.5) | 48 085 (97.7) | 16 823 (97.7) | 7557 (96.4) |
| Ever use of menopausal hormone therapy | 49 297 (66.3) | 31 983 (65.0) | 11 868 (68.9) | 5446 (69.4) |
| Recreational physical activity MET, mean (SD), h/wk | 24.6 (21) | 25.1 (21) | 24.1 (21) | 23.2 (20) |
| BMI | ||||
| <20 | 8994 (12.1) | 5907 (12.0) | 1995 (11.6) | 1092 (13.9) |
| 20-25 | 43 369 (58.4) | 28 824 (58.6) | 9890 (57.5) | 4655 (59.4) |
| 25-30 | 17 524 (23.6) | 11 621 (23.6) | 4218 (24.5) | 1685 (21.5) |
| ≥30 | 4360 (5.8) | 2847 (5.8) | 1106 (6.4) | 407 (5.2) |
| Handedness | ||||
| Right | 59 739 (80.5) | 39 537 (80.4) | 13 894 (80.7) | 6308 (80.5) |
| Left | 1678 (2.3) | 1111 (2.3) | 386 (2.2) | 181 (2.3) |
| Ambidextrous | 3470 (4.7) | 2212 (4.5) | 867 (5.0) | 391 (5.0) |
| Unknown | 9360 (12.6) | 6339 (12.9) | 2062 (12.0) | 959 (12.2) |
| Level of education | ||||
| Undergraduate and less | 8788 (11.8) | 5730 (11.6) | 2056 (11.9) | 1002 (12.8) |
| Graduate | 39 284 (52.9) | 25 904 (52.7) | 9218 (53.6) | 4162 (53.1) |
| Postgraduate and more | 26 175 (35.3) | 17 565 (35.7) | 5935 (34.5) | 2675 (34.1) |
| Smoking status | ||||
| Current | 7340 (9.9) | 5119 (10.4) | 1595 (9.3) | 626 (8.0) |
| Former | 27 151 (36.6) | 17 359 (35.3) | 6706 (39.0) | 3086 (39.4) |
| Never | 39 756 (53.5) | 26 721 (54.3) | 8908 (51.8) | 4127 (52.6) |
Abbreviations: ATC, anatomic therapeutic chemical; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); MET, metabolic equivalent task; NSAID, nonsteroidal anti-inflammatory drug.
ATC codes: antimigraine preparations, N02C; nonsteroidal anti-inflammatory drugs and antirheumatic products, M01A; other analgesics and antipyretics, N02B; antiepileptics, N03A; antivertigo preparations, N07C; and β-blocking agents, C07.
There was no difference in the baseline distribution of handedness with respect to migraine self-report. However, we found that women with a mixed or ambidextrous handedness were at increased risk of self-reporting migraine during the follow-up period (2004-2014) compared with right-handed women (OR, 1.13 [95% CI, 1.02-1.24]), whereas left-handed women were not (OR, 1.01 [95% CI, 0.88-1.16]).
Migraine and Risk of Incident Type 2 Diabetes
Between 2004 and 2014, a total of 2372 women developed type 2 diabetes (Table 2). In univariate models, we observed a lower risk of incident type 2 diabetes in women with active migraine (HR, 0.80 [95% CI, 0.60-0.96]) than in women with no migraine history. The magnitude of this association increased in multivariable-adjusted models (HR, 0.70 [95% CI, 0.58-0.85]). Prior migraine was not associated with the risk of type 2 diabetes both in univariate models (HR, 1.16 [95% CI, 1.06-1.27]) and multivariable-adjusted models (HR, 1.07 [95% CI, 0.98-1.17]).
Table 2. Hazard Ratios of Type 2 Diabetes Risk According to Self-reported Migraine History (2004-2014).
| Migraine | No Incident Diabetes (n = 71 875) | Incident Type 2 Diabetes (n = 2372) | Hazard Ratio (95% CI) | |
|---|---|---|---|---|
| Model 1a | Model 2b | |||
| No history | 47 191 | 1562 | 1 [Reference] | 1 [Reference] |
| Prior | 21 918 | 681 | 1.16 (1.06-1.27) | 1.07 (0.98-1.17) |
| Active | 2766 | 129 | 0.80 (0.67-0.96) | 0.70 (0.58-0.85) |
Model 1 was age adjusted.
Model 2 was adjusted for myocardial infarction, plus level of education, family history of diabetes, body mass index, smoking status, hypertension, level of recreational physical activity, use of oral contraceptives, menopausal status, use of menopausal hormone therapy, handedness, use of antimigraine preparations, and use of other drugs prescribed for migraine.
Trends in the Proportion of Reported Active Migraine Before and After Type 2 Diabetes Diagnosis
There was a clear linear decrease in the 2-year prevalence of active migraine from 24 years before diagnosis (22% [95% CI, 16%-27%]; Figure 2) to the date of diagnosis (11% [95% CI, 10%-12%]). After type 2 diabetes diagnosis, there was a plateau in the prevalence of active migraine (10% to 11%) that persisted up to 22 years after diagnosis. The magnitudes of the estimates were not sensitive to adjustment for time-varying covariates.
Figure 2. Evolution of the 2-Year Prevalence of Active Migraine Before and After Diagnosis of Type 2 Diabetes.
The graph shows a secondary analysis of data from 1992 to 2014 on 4371 women with type 2 diabetes in the E3N Cohort Study. The prevalences of migraine and their 95% CIs have been estimated from a generalized estimating equations model from 24 years prior to 22 years after type 2 diabetes diagnosis. Year 0 is the date of type 2 diagnosis. Models were adjusted for age, level of education, family history of diabetes, body mass index, smoking status, hypertension, level of recreational physical activity, use of oral contraceptives, menopausal status, menopausal hormone therapy use, and handedness.
Discussion
Based on data from this large prospective cohort of women, we report an inverse association between active migraine and type 2 diabetes incidence. Women with active migraine had an approximate 30% decrease in the risk of developing diabetes. We found a clear linear decrease in the prevalence of active migraine prior to type 2 diabetes diagnosis and a stagnation of migraine prevalence after diagnosis of diabetes.
Comparison With Previous Studies
Despite the high prevalence of both diseases, to our knowledge, little is known about the association of migraine with type 2 diabetes at a population level. Migraine has been associated with factors that are implicated in diabetes; some previous publications have suggested an association between polymorphisms in the insulin receptor gene and migraine,16 an impaired insulin sensitivity in individuals with migraine,17 and possible elevations in blood glucose and insulin levels in people with headaches,18 whereas a cross-sectional study on 27 people19 showed that the frequency of migraine was high in individuals with obesity, a major risk factor for type 2 diabetes. A review20 published in 2010 summarizing 3 prospective cohort studies reported that, in both sexes, chronic daily headache was increased in adults with obesity and the prevalence of episodic headaches may also be increased in adults of reproductive age with obesity.
Potential Mechanisms
It has been proposed that a trigger for migraine could be nutritional, hormonal, or metabolic in some individuals. An association between polymorphisms in the insulin receptor gene and migraine has been shown.16 An elevation in free fatty acid plasma concentration and ketone bodies has also been reported before a migraine attack.28 Therefore, fasting could promote the development of migraine, mostly by favoring hypoglycemia and increased ketone bodies production.29 Hypoglycemia has long been known to be a precipitating factor in migraine onset.30 These biological factors could therefore explain an inverse association between migraine and type 2 diabetes risk. They could also support our observed decreased prevalence of migraine in the years before type 2 diabetes diagnosis, when there is usually a progressively increasing hyperglycemic state. Increased secretion of insulin after intake of carbohydrate and sucrose-rich meals may promote the occurrence of reactive hypoglycemia in some people, which may trigger migraine.31 In this regard, 1 study32 reported a higher level of plasma insulin in women with migraine compared with control participants.
In addition, other mechanisms may be involved: calcitonin gene-related peptide (CGRP), a neuropeptide expressed in sensory nerves, which seems to play an important role in migraine pathophysiology,33 is also associated with glucose metabolism. It has been reported34 that rats with experimentally induced diabetes have a decreased density of CGRP sensory nerve fibers.
In addition, CGRP is a well-established potent vasodilator and has a vascular protective role. In animal models, diabetic impairment of sensory nerves with reduced expression of CGRP has been reported.35 We may speculate that the vasodilation and the nociceptive effects induced by CGRP are impaired after diabetes appears, which may explain the reduced prevalence of active migraine. However, we cannot exclude that a factor associated with migraine pathophysiology may modulate glucose metabolism and have an influence on the appearance of hyperglycemia. The association between CGRP and glucose homeostasis is complex and bidirectional. Studies conducted predominantly in rats with obesity and type 2 diabetes have shown that infusion of pharmacological doses of CGRP induces insulin resistance and decreases peripheral glucose clearance.36,37 Altogether, these findings underscore potential associations between CGRP, migraine pathophysiology, and glucose metabolism.38
In a community-based case-control study39 including 1832 participants in China, authors reported increased insulin resistance in individuals with both migraine and prediabetes and an inverse association between type 2 diabetes and migraine. This also is in line with our findings.
Strengths
This study has numerous strengths. We evaluated the associations between migraine and type 2 diabetes, updating information of migraine and many covariates during the follow-up period. The prospective design reduces a differential bias in the reporting of migraine episodes associated with the incident type 2 diabetes. The large number of participants and type 2 diabetes cases ensured a high statistical power. Incident cases were identified from an extensive medico-administrative database, which reduced the risk of missing or false-positive cases. The long follow-up time, updated information on migraine, and statistical methodology enabled us to study the evolution of migraine 2-year prevalence from long before type 2 diabetes diagnosis to long after diagnosis, with a possible window of observation of 46 years.
Limitations
This study has also some limitations. Migraine was self-reported, and information on the presence of migraine aura was not available. However, the repeated questionnaires over time and the medico-administrative database on drug reimbursements allowed us to isolate associations with both active and prior migraine episodes from associations with antimigraine preparations. However, no information on self-medication was available in this study.
Type 2 diabetes cases who were not treated pharmacologically were considered as noncases, which could have reduced the magnitude of the observed associations between migraine and type 2 diabetes. However, we believe that this would have a minor influence on the results.
The E3N cohort is not representative of the general French population because it includes rather homogeneous, health-conscious women. In addition, we have analyzed mainly women in postmenopause. Although this might reduce the variability of certain characteristics and the possibility to extrapolate to the general population and premenopausal women, it should not bias the estimates. Finally, even though we controlled for most established type 2 diabetes risk factors, potential residual and unmeasurable confounding cannot be ruled out completely because this study is observational.
Conclusions
Both migraine and type 2 diabetes are highly prevalent diseases. Therefore, these results can have substantial implications on the understanding of mechanisms underlying these 2 conditions. Because plasma glucose concentration rises with time up to the point of type 2 diabetes occurrence, the prevalence of migraine symptoms may decrease. Consequently, tracking the evolution and especially the decrease of migraine frequency in individuals with migraine at high risk of diabetes, such as individuals with obesity, irrespective of age could be the sign of an emerging increased blood glucose levels, prediabetes, or type 2 diabetes.
We observed a lower risk of type 2 diabetes in women with active migraine. The linear decrease of migraine prevalence long before and the plateau long after type 2 diabetes diagnosis is novel and the association deserves to be studied in other populations. The potential beneficial role of both hyperglycemia and hyperinsulinism on migraine occurrence needs to be further explored.
References
- 1.Vos T, Flaxman AD, Naghavi M, et al. . Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010 [published correction appears in Lancet. 2013;381(9867):628]. Lancet. 2012;380(9859):2163-2196. doi: 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burch RC, Loder S, Loder E, Smitherman TA. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55(1):21-34. doi: 10.1111/head.12482 [DOI] [PubMed] [Google Scholar]
- 3.Scher AI, Buse DC, Fanning KM, et al. . Comorbid pain and migraine chronicity: the Chronic Migraine Epidemiology and Outcomes study. Neurology. 2017;89(5):461-468. doi: 10.1212/WNL.0000000000004177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goadsby PJ, Lipton RB, Ferrari MD. Migraine—current understanding and treatment. N Engl J Med. 2002;346(4):257-270. doi: 10.1056/NEJMra010917 [DOI] [PubMed] [Google Scholar]
- 5.Silberstein SD. Migraine. Lancet. 2004;363(9406):381-391. doi: 10.1016/S0140-6736(04)15440-8 [DOI] [PubMed] [Google Scholar]
- 6.Pavlovic JM, Vieira JR, Lipton RB, Bond DS. Association between obesity and migraine in women. Curr Pain Headache Rep. 2017;21(10):41. doi: 10.1007/s11916-017-0634-8 [DOI] [PubMed] [Google Scholar]
- 7.Pressman A, Jacobson A, Eguilos R, et al. . Prevalence of migraine in a diverse community—electronic methods for migraine ascertainment in a large integrated health plan. Cephalalgia. 2016;36(4):325-334. doi: 10.1177/0333102415590242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scher AI, Terwindt GM, Picavet HSJ, Verschuren WMM, Ferrari MD, Launer LJ. Cardiovascular risk factors and migraine: the GEM population-based study. Neurology. 2005;64(4):614-620. doi: 10.1212/01.WNL.0000151857.43225.49 [DOI] [PubMed] [Google Scholar]
- 9.Rist PM, Winter AC, Buring JE, Sesso HD, Kurth T. Migraine and the risk of incident hypertension among women. Cephalalgia. 2018;38(12):1817-1824. doi: 10.1177/0333102418756865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rist PM, Tzourio C, Kurth T. Associations between lipid levels and migraine: cross-sectional analysis in the epidemiology of vascular ageing study. Cephalalgia. 2011;31(14):1459-1465. doi: 10.1177/0333102411421682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurth T, Slomke MA, Kase CS, et al. . Migraine, headache, and the risk of stroke in women: a prospective study. Neurology. 2005;64(6):1020-1026. doi: 10.1212/01.WNL.0000154528.21485.3A [DOI] [PubMed] [Google Scholar]
- 12.Spector JT, Kahn SR, Jones MR, Jayakumar M, Dalal D, Nazarian S. Migraine headache and ischemic stroke risk: an updated meta-analysis. Am J Med. 2010;123(7):612-624. doi: 10.1016/j.amjmed.2009.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adelborg K, Szépligeti SK, Holland-Bill L, et al. . Migraine and risk of cardiovascular diseases: Danish population based matched cohort study. BMJ. 2018;360:k96. doi: 10.1136/bmj.k96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener H-C, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA. 2006;296(3):283-291. doi: 10.1001/jama.296.3.283 [DOI] [PubMed] [Google Scholar]
- 15.Kurth T, Winter AC, Eliassen AH, et al. . Migraine and risk of cardiovascular disease in women: prospective cohort study. BMJ. 2016;353:i2610. doi: 10.1136/bmj.i2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarthy LC, Hosford DA, Riley JH, et al. . Single-nucleotide polymorphism alleles in the insulin receptor gene are associated with typical migraine. Genomics. 2001;78(3):135-149. doi: 10.1006/geno.2001.6647 [DOI] [PubMed] [Google Scholar]
- 17.Rainero I, Limone P, Ferrero M, et al. . Insulin sensitivity is impaired in patients with migraine. Cephalalgia. 2005;25(8):593-597. doi: 10.1111/j.1468-2982.2005.00928.x [DOI] [PubMed] [Google Scholar]
- 18.Cavestro C, Rosatello A, Micca G, et al. . Insulin metabolism is altered in migraineurs: a new pathogenic mechanism for migraine? Headache. 2007;47(10):1436-1442. doi: 10.1111/j.1526-4610.2007.00719.x [DOI] [PubMed] [Google Scholar]
- 19.Horev A, Wirguin I, Lantsberg L, Ifergane G. A high incidence of migraine with aura among morbidly obese women. Headache. 2005;45(7):936-938. doi: 10.1111/j.1526-4610.2005.05162.x [DOI] [PubMed] [Google Scholar]
- 20.Peterlin BL, Rapoport AM, Kurth T. Migraine and obesity: epidemiology, mechanisms, and implications. Headache. 2010;50(4):631-648. doi: 10.1111/j.1526-4610.2009.01554.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antonazzo IC, Riise T, Cortese M, et al. . Diabetes is associated with decreased migraine risk: a nationwide cohort study. Cephalalgia. 2018;38(11):1759-1764. [DOI] [PubMed] [Google Scholar]
- 22.Burch RC, Rist PM, Winter AC, et al. . Migraine and risk of incident diabetes in women: a prospective study. Cephalalgia. 2012;32(13):991-997. doi: 10.1177/0333102412453954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clavel-Chapelon F; E3N Study Group . Cohort profile: the French E3N cohort study. Int J Epidemiol. 2015;44(3):801-809. doi: 10.1093/ije/dyu184 [DOI] [PubMed] [Google Scholar]
- 24.Kengne AP, Beulens JWJ, Peelen LM, et al. . Non-invasive risk scores for prediction of type 2 diabetes (EPIC-InterAct): a validation of existing models. Lancet Diabetes Endocrinol. 2014;2(1):19-29. doi: 10.1016/S2213-8587(13)70103-7 [DOI] [PubMed] [Google Scholar]
- 25.Mancini FR, Dow C, Affret A, et al. . Micronutrient dietary patterns associated with type 2 diabetes mellitus among women of the E3N-EPIC (Etude Epidémiologique Auprès de Femmes de l’Education Nationale) cohort study. J Diabetes. 2018;10(8):665-674. doi: 10.1111/1753-0407.12654 [DOI] [PubMed] [Google Scholar]
- 26.Bonnet F, Affret A, Boutron-Ruault M-C, Balkau B, Clavel-Chapelon F, Fagherazzi G. Association between handedness and type 2 diabetes: the E3N study. Diabetes Care. 2015;38(12):e199. doi: 10.2337/dc15-1669 [DOI] [PubMed] [Google Scholar]
- 27.Lipsitz SR, Kim K, Zhao L. Analysis of repeated categorical data using generalized estimating equations. Stat Med. 1994;13(11):1149-1163. doi: 10.1002/sim.4780131106 [DOI] [PubMed] [Google Scholar]
- 28.Hockaday JM, Williamson DH, Whitty CWM. Blood-group levels and fatty-acid metabolism in migraine related to fasting. Lancet. 1971;1(7710):1153-1156. doi: 10.1016/S0140-6736(71)91662-X [DOI] [PubMed] [Google Scholar]
- 29.Marsters JB, Mortimer MJ, Hay KM. Glucose and diet in the fasting migraineur. Headache. 1986;26(5):243-247. doi: 10.1111/j.1526-4610.1986.hed2605243.x [DOI] [PubMed] [Google Scholar]
- 30.Critchley M, Ferguson F. Migraine. Lancet. 1933;221(5708):123-126. doi: 10.1016/S0140-6736(00)84315-9 [DOI] [Google Scholar]
- 31.Dexter JD, Roberts J, Byer JA. The five hour glucose tolerance test and effect of low sucrose diet in migraine. Headache. 1978;18(2):91-94. doi: 10.1111/j.1526-4610.1978.hed1802091.x [DOI] [PubMed] [Google Scholar]
- 32.Kokavec A. Effect of sucrose consumption on serum insulin, serum cortisol and insulin sensitivity in migraine: evidence of sex differences. Physiol Behav. 2015;142:170-178. doi: 10.1016/j.physbeh.2015.02.022 [DOI] [PubMed] [Google Scholar]
- 33.Vollesen ALH, Snoer A, Beske RP, et al. . Effect of infusion of calcitonin gene-related peptide on cluster headache attacks: a randomized clinical trial. JAMA Neurol. 2018;75(10):1187-1197. doi: 10.1001/jamaneurol.2018.1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enríquez-Pérez IA, Galindo-Ordoñez KE, Pantoja-Ortíz CE, et al. . Streptozocin-induced type-1 diabetes mellitus results in decreased density of CGRP sensory and TH sympathetic nerve fibers that are positively correlated with bone loss at the mouse femoral neck. Neurosci Lett. 2017;655:28-34. doi: 10.1016/j.neulet.2017.06.042 [DOI] [PubMed] [Google Scholar]
- 35.Li T-P, Guo Z, Liu C-J, Sun T, Chen L, Zhao X. Association of down-regulation of calcitonin gene-related peptide and substance P with increase of myocardial vulnerability in diabetic neuropathic rats. Peptides. 2017;96:1-7. doi: 10.1016/j.peptides.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 36.Molina JM, Cooper GJ, Leighton B, Olefsky JM. Induction of insulin resistance in vivo by amylin and calcitonin gene-related peptide. Diabetes. 1990;39(2):260-265. doi: 10.2337/diab.39.2.260 [DOI] [PubMed] [Google Scholar]
- 37.Abbasi A, Corpeleijn E, Postmus D, et al. . Plasma procalcitonin is associated with obesity, insulin resistance, and the metabolic syndrome. J Clin Endocrinol Metab. 2010;95(9):E26-E31. doi: 10.1210/jc.2010-0305 [DOI] [PubMed] [Google Scholar]
- 38.Siva ZO, Uluduz D, Keskin FE, et al. . Determinants of glucose metabolism and the role of NPY in the progression of insulin resistance in chronic migraine. Cephalalgia. 2018;38(11):1773-1781. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Li X, Diao Y, et al. . Are glucose and insulin metabolism and diabetes associated with migraine? a community-based, case-control study. J Oral Facial Pain Headache. 2017;31(3):240-250. doi: 10.11607/ofph.1843 [DOI] [PubMed] [Google Scholar]