Abstract
Importance
Second-line treatment with chemotherapy plus bevacizumab or cetuximab is a valid option for metastatic colorectal cancer.
Objective
To evaluate the progression-free survival (PFS) rate at 4 months with chemotherapy plus bevacizumab vs cetuximab for patients with progression of metastatic colorectal cancer after bevacizumab plus chemotherapy.
Design, Setting, and Participants
A prospective, open-label, multicenter, randomized phase 2 trial was conducted from December 14, 2010, to May 5, 2015. The main eligibility criterion was disease progression after bevacizumab plus fluorouracil with irinotecan or oxaliplatin in patients with wild-type KRAS exon 2 metastatic colorectal cancer. All analyses were performed on the modified intent-to-treat population.
Interventions
Patients were randomized to arm A (FOLFIRI [fluorouracil and folinic acid combined with irinotecan] or modified FOLFOX6 [fluorouracil and folinic acid combined with oxaliplatin] plus bevacizumab) or arm B (FOLFIRI or modified FOLFOX6 plus cetuximab); the second-line chemotherapy regimen was chosen according to first-line treatment (crossover).
Main Outcomes and Measures
The primary end point was the 4-month PFS rate. Secondary end points included safety, objective response rate, overall survival, and PFS.
Results
A total of 132 patients (47 women and 85 men; median age, 63.0 years [range, 33.0-84.0 years]; 74 patients with an Eastern Cooperative Oncology Group performance status of 0, 54 patients with a performance status of 1, and 4 patients with unknown performance status) were included at 25 sites. The 4-month PFS rate was 80.3% (95% CI, 68.0%-88.3%) in arm A and 66.7% (95% CI, 53.6%-76.8%) in arm B. The median PFS was 7.1 months (95% CI, 5.7-8.2 months) in arm A and 5.6 months (95% CI, 4.2-6.5 months) in arm B (hazard ratio, 0.71; 95% CI, 0.50-1.02; P = .06), and the median overall survival was 15.8 months (95% CI, 9.5-22.3 months) in arm A and 10.4 months (95% CI, 7.0-16.2 months) in arm B (hazard ratio, 0.69; 95% CI, 0.46-1.04; P = .08). A central analysis of KRAS (exons 2, 3, and 4), NRAS (exons 2, 3, and 4), and BRAF (V600) was performed for 95 tumor samples. Eighty-one patients had wild-type KRAS and wild-type NRAS tumors.
Conclusions and Relevance
The results of the PRODIGE18 (Partenariat de Recherche en Oncologie DIGEstive) study showed a nonsignificant difference but favored continuation of bevacizumab with chemotherapy crossover for patients with wild-type RAS metastatic colorectal cancer that progressed with first-line bevacizumab plus chemotherapy.
Trial Registration
ClinicalTrials.gov identifier: NCT01442649 and clinicaltrialsregister.eu identifier: EUDRACT 2009-012942-22
This open-label, randomized phase 2 trial evaluates progression-free survival with chemotherapy plus bevacizumab vs cetuximab among patients with progression of metastatic colorectal cancer (mCRC) after bevacizumab plus chemotherapy.
Key Points
Question
Which is the most appropriate treatment for patients with wild-type RAS metastatic colorectal cancer progressing after bevacizumab plus chemotherapy: chemotherapy with bevacizumab or cetuximab?
Findings
In this randomized phase 2 study, the 4-month progression-free survival rate was numerically higher with bevacizumab plus chemotherapy than with cetuximab plus chemotherapy, though the difference was not statistically significant.
Meaning
The present PRODIGE18 (Partenariat de Recherche en Oncologie DIGEstive) study highlights that, after a first progression of metastatic colorectal cancer with bevacizumab plus chemotherapy, continuation of bevacizumab plus a switch of chemotherapy may be the most appropriate option.
Introduction
In metastatic colorectal cancer (mCRC), the available drugs are classified into 3 major therapeutic classes: cytotoxic agents (eg, fluoropyrimidines, irinotecan, and oxaliplatin), angiogenesis inhibitors (eg, bevacizumab), and anti–epidermal growth factor receptor (EGFR) antibodies (eg, cetuximab and panitumumab). The chemotherapy regimen often consists of fluorouracil and folinic acid combined with either oxaliplatin (FOLFOX) or irinotecan (FOLFIRI). A patient’s treatment depends on numerous factors, including the therapy received in earlier treatment lines and the tumor mutation status.1
Three antiangiogenic compounds—bevacizumab, aflibercept, and ramucirumab—are currently validated as second-line treatments for mCRC in combination with the appropriate chemotherapy regimen.2,3,4 Large randomized phase 3 clinical trials have also confirmed a role for the anti-EGFR agents panitumumab and cetuximab for patients with mCRC after failure of first-line treatment. The oldest study, the EPIC (ERBITUX Plus Irinotecan for Metastatic Colorectal Cancer) trial, which was performed before the identification of the RAS mutation as a predictive factor for resistance to anti-EGFR antibodies, showed that irinotecan plus cetuximab increased median progression-free survival (PFS) vs irinotecan alone.5 In a selected population with wild-type (wt) RAS mCRC, FOLFIRI plus panitumumab was superior to FOLFIRI alone in terms of objective response rate, median PFS, and overall survival (OS).6
In the setting of mCRC, anti-EGFR and antiangiogenic antibodies combined with chemotherapy have been compared for the treatment-naive patients with wtKRAS tumors. The CALGB/SWOG (Cancer and Leukemia Group/Southwest Oncology Group) 80405 trial assessed cetuximab or bevacizumab combined with FOLFIRI or FOLFOX.7 The median PFS and the median OS were similar in the treatment arms. Similarly, the FIRE-3 study assessed cetuximab or bevacizumab combined with FOLFIRI.8 The median PFS was similar in the 2 treatment arms, but the median OS was significantly longer with the cetuximab-FOLFIRI combination.
In line with these findings, the management of nonresectable mCRC has progressively integrated the concept of a multiline strategy combined with the determination of some tumor characteristics (ie, KRAS, NRAS, and BRAF mutational status). Maintenance therapy and the reintroduction of chemotherapy regimens make up the therapeutic schedules sequentially offered to patients with nonresectable mCRC. Further progress in elucidating the optimal treatment algorithm in mCRC was made when the TML (Treatment Multiline) study demonstrated that sustained vascular endothelial growth factor inhibition with bevacizumab was beneficial in patients progressing after first-line bevacizumab plus chemotherapy.9 This study showed that continuing bevacizumab with chemotherapy (switched from the first-line chemotherapy regimen) significantly prolonged OS and PFS with second-line treatment compared with chemotherapy alone for patients with mCRC who had received bevacizumab plus standard chemotherapy in the first-line setting. In an exploratory analysis of the TML study, patients with wtKRAS exon 2 tumors achieved a statistically significant PFS and OS benefit from the continuation of bevacizumab beyond disease progression vs chemotherapy alone.10
Based on these data, the present randomized phase 2 clinical trial evaluated standard fluoropyrimidine-based chemotherapy combined with either bevacizumab or cetuximab among patients with wtKRAS exon 2 mCRC who had previously received bevacizumab plus standard chemotherapy in the first-line setting.
Methods
Patients
Patients were enrolled in this trial from December 14, 2010, through May 5, 2015. The trial protocol is available in Supplement 1. Patients were 18 years of age or older, with an Eastern Cooperative Oncology Group performance status of 0 or 1, with histologically or cytologically proven mCRC, and with wtKRAS exon 2 (assessed by local molecular biology platforms in formalin-fixed paraffin-embedded tumor tissue from the primary or metastatic tumor). From September 14, 2014, an amendment restricted inclusion to patients with wtRAS tumors (KRAS exons 2, 3, and 4 and NRAS exons 2, 3, and 4). Documented progressive disease (PD) during or after first-line treatment of mCRC with bevacizumab plus fluoropyrimidines and irinotecan or oxaliplatin, according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1,11 was required. A stop and go strategy and/or maintenance therapy was permitted as first-line treatment for mCRC; an escalating-dose schedule was not allowed. Other main inclusion criteria included adequate bone marrow and adequate hepatic and renal function. The eligibility criteria are described in eTable 1 in Supplement 2. This trial was performed in accordance with the Declaration of Helsinki12 and Good Clinical Practice Guidelines. The study was approved by Comité de Protection des Personnes. All patients provided written informed consent before starting the trial.
Interventions and Randomization
The PRODIGE18 (Partenariat de Recherche en Oncologie DIGEstive) study was an open-label, randomized phase 2 clinical trial assessing 2 standard regimens—bevacizumab and cetuximab—combined with chemotherapy (modified FOLFOX6 [mFOLFOX6] or FOLFIRI) after the failure of first-line treatment containing bevacizumab in mCRC. Patients were randomized in a 1:1 manner to the 2 regimens. The chemotherapy regimen was chosen according to the regimen used in the first-line setting (crossover): patients initially treated with irinotecan plus fluoropyrimidines received mFOLFOX6 (oxaliplatin, 85 mg/m2, folinic acid, 400 mg/m2 [l-folinic acid, 200 mg/m2], a bolus dose of fluorouracil, 400 mg/m2, and a 46-hour infusion of fluorouracil, 2400 mg/m2). Conversely, those initially treated with oxaliplatin plus fluoropyrimidines received FOLFIRI (irinotecan, 180 mg/m2, folinic acid, 400 mg/m2 [l-folinic acid, 200 mg/m2], a bolus dose of fluorouracil, 400 mg/m2, and a 46-hour infusion of fluorouracil, 2400 mg/m2). Bevacizumab, 5 mg/kg (arm A), or cetuximab, 500 mg/m2 (arm B), was administered with mFOLFOX6 or FOLFIRI every 14 days until PD, the occurrence of unacceptable toxic effects, or the patient’s refusal. Randomization was stratified by first-line chemotherapy (fluoropyrimidine with oxaliplatin vs fluoropyrimidine with irinotecan), PFS with the first-line therapy (≤9 months vs >9 months), and the center.
Study End Points and Assessments
The primary end point was the PFS rate at 4 months. Progression-free survival is defined as the time from randomization to documented disease progression or death from any cause, whichever occurred earlier. Secondary end points were the median PFS; OS, defined as the time from randomization to death from any cause; OS from the start of first-line therapy, defined as the time from the start of first-line therapy to death from any cause; the objective response rate, defined as the percentage of complete or partial responses; and safety (assessed by the National Cancer Institute Common Terminology Criteria for Adverse Events [version 3.0]).13 Tumor response evaluations were performed at baseline and every 6 weeks until PD, based on RECIST, version 1.1, using spiral or conventional computed tomography or magnetic resonance imaging. Patients who discontinued treatment before PD and those who completed treatment were followed up every 3 months after treatment for survival data, subsequent anticancer therapy, and study drug–related serious adverse effects (AEs); patients who discontinued treatment before PD were assessed for tumor status until PD.
Molecular Analysis
Tissue sections (10 μm) were prepared from formalin-fixed paraffin-embedded tumor samples, and DNA was extracted using the QIAamp DNA FFPE Tissue Kit (Qiagen) according to the manufacturer’s recommendation. Molecular testing was performed using the KRAS/BRAF Mutation Analysis Kit Panel Kit for Real-Time PCR (KRAS exons 2, 3, 4 and BRAF600; EntroGen) and the NRAS Mutation Analysis Kit (exons 2, 3, and 4; EntroGen). These polymerase chain reaction–based assays use allele-specific probes to identify KRAS (18 mutations), NRAS (11 mutations), and BRAFV600 mutations, with a limit of detection of less than 1%. This assay is approved for in vitro diagnosis.
Statistical Analysis
The trial was not powered for a direct comparison between arms. In both groups, the sample size calculation was based on a 2-step phase II Simon method using the same hypotheses. A 4-month PFS rate of 30% was considered insufficient to warrant further investigation, and a rate of 50% was considered as the minimal target of clinical efficacy. With a 1-sided type 1 error α of 0.05 and a power (1 − β) of 0.90, 59 evaluable patients were required in each arm. In the first step, each treatment arm was considered to have sufficient preliminary efficacy to continue if 7 or more of 20 patients, with 4 months of follow-up, were alive without progression. At the end of the trial, proof of potential efficacy was considered if 24 or more patients were alive without progression at 4 months. Assuming that 10% of patients would be nonevaluable or lost to follow-up, the study required 132 patients.
All analyses were performed on the modified intent-to-treat population (mITT; ie, patients who received at least 1 dose of treatment). Qualitative variables were described as percentages, and quantitative variables were described by their median values, with ranges or mean values with SDs, depending on whether the values were normally distributed. The median PFS and the median OS (with their 95% CIs) were determined using the Kaplan-Meier method. Hazard ratios (HRs) and their 95% CIs were determined using Cox proportional hazards regression models. The proportionality assumption was verified. Log-rank and Cox proportional hazards regression model P values are reported in an exploratory manner. The median follow-up times and their 95% CIs were determined using the reverse Kaplan-Meier method. Analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
Patients
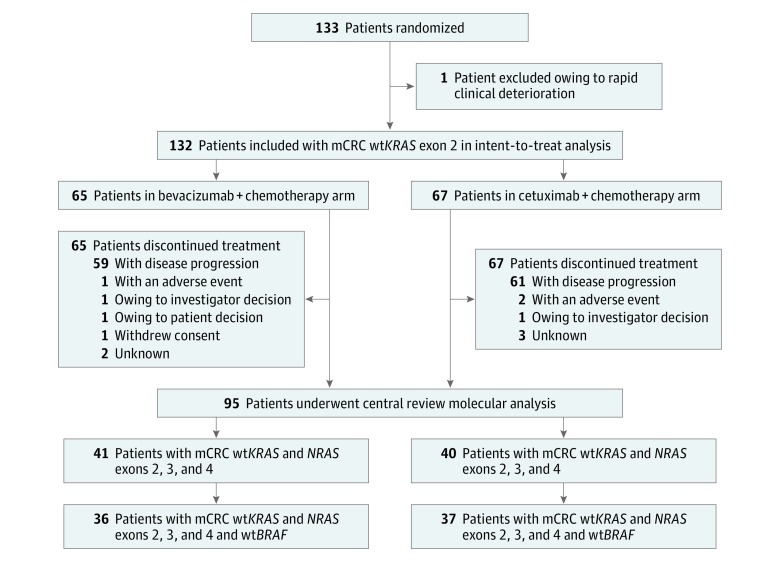
From December 14, 2010, through May 5, 2015, 133 patients were enrolled at 25 centers in France (Figure 1). One patient randomized to arm A did not receive treatment owing to rapid clinical deterioration; consequently, the mITT population comprised 132 patients. Baseline demographic and clinical characteristics were well balanced between treatment arms (Table). During the study, 82 patients (62.1%) who received the FOLFIRI regimen as first-line therapy received the mFOLFOX6 regimen and 50 patients (37.9%) who received the mFOLFOX6 regimen as first-line therapy received the FOLFIRI regimen. The distributions of use of chemotherapy were similar in both study arms (Table).
Figure 1. Patient Enrollment.
mCRC Indicates metastatic colorectal cancer, wt, wild-type.
Table. Baseline Demographic and Clinical Characteristics.
| Characteristic | Patients, No. (%) | |
|---|---|---|
| Bevacizumab + Chemotherapy (n = 65) | Cetuximab + Chemotherapy (n = 67) | |
| Sex | ||
| Male | 41 (63.1) | 44 (65.7) |
| Female | 24 (36.9) | 23 (34.3) |
| Age, median (range), y | 61 (33-83) | 63 (37-84) |
| ECOG performance status | ||
| 0 | 36 (55.4) | 38 (56.7) |
| 1 | 27 (41.5) | 27 (40.3) |
| Unknown | 2 (3.1) | 2 (3.0) |
| Primary tumor location | ||
| Right colon | 14 (21.5) | 10 (14.9) |
| Left colon | 32 (49.2) | 35 (52.2) |
| Rectum | 18 (27.7) | 21 (31.3) |
| Left and right colon | 1 (1.5) | 0 |
| Colon | 0 | 1 (1.5) |
| Surgery of the primary tumor | 36 (55.4) | 41 (61.2) |
| Metastatic sites | ||
| Liver | 52 (80.0) | 53 (79.1) |
| Lung | 30 (46.2) | 31 (46.3) |
| Peritoneal carcinomatosis | 7 (10.8) | 9 (13.4) |
| Other | 22 (33.8) | 32 (47.8) |
| Adjuvant chemotherapy | 11 (16.9) | 17 (25.4) |
| First-line progression-free survival, mo | ||
| ≤9 | 40 (61.5) | 40 (59.7) |
| >9 | 25 (38.5) | 27 (40.3) |
| Bevacizumab chemotherapy partner in first line | ||
| Irinotecan-based | 40 (61.5) | 42 (62.7) |
| Oxaliplatin-based | 25 (38.5) | 25 (37.3) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
All patients included in the study had mCRC typed as wtKRAS exon 2 status. After inclusion of 101 patients, a protocol amendment restricted eligibility to patients with tumors with not only wtKRAS exon 2 but also with wtKRAS exons 3 and 4 and wtNRAS exons 2, 3, and 4. A central analysis of KRAS, NRAS, and BRAF mutations was performed. Of the 133 included patients, 109 provided written informed consent to participate in the biomarker analysis (54 patients in arm A and 55 patients in arm B). Formalin-fixed paraffin-embedded tissue samples were collected from 105 patients. Of these samples, 7 contained no residual tumor cells and 3 had DNA of insufficient quality for analysis; therefore, complete tumor KRAS, NRAS, and BRAF status was obtained for 95 patients. The subgroup with confirmed wtKRAS and wtNRAS status represented 61.4% (n = 81) of the ITT population; of these patients, 73 (55.3% of the ITT population) had tumors that were wild type for all 3 biomarkers (wtKRAS, wtNRAS, and wtBRAF) (Figure 1).
Efficacy
Efficacy data were assessable in the eligible population corresponding to 132 patients with wtKRAS exon 2 mCRC. The median follow-up was 37.4 months (minimum follow-up, 1 month; maximum follow-up, 48 months).
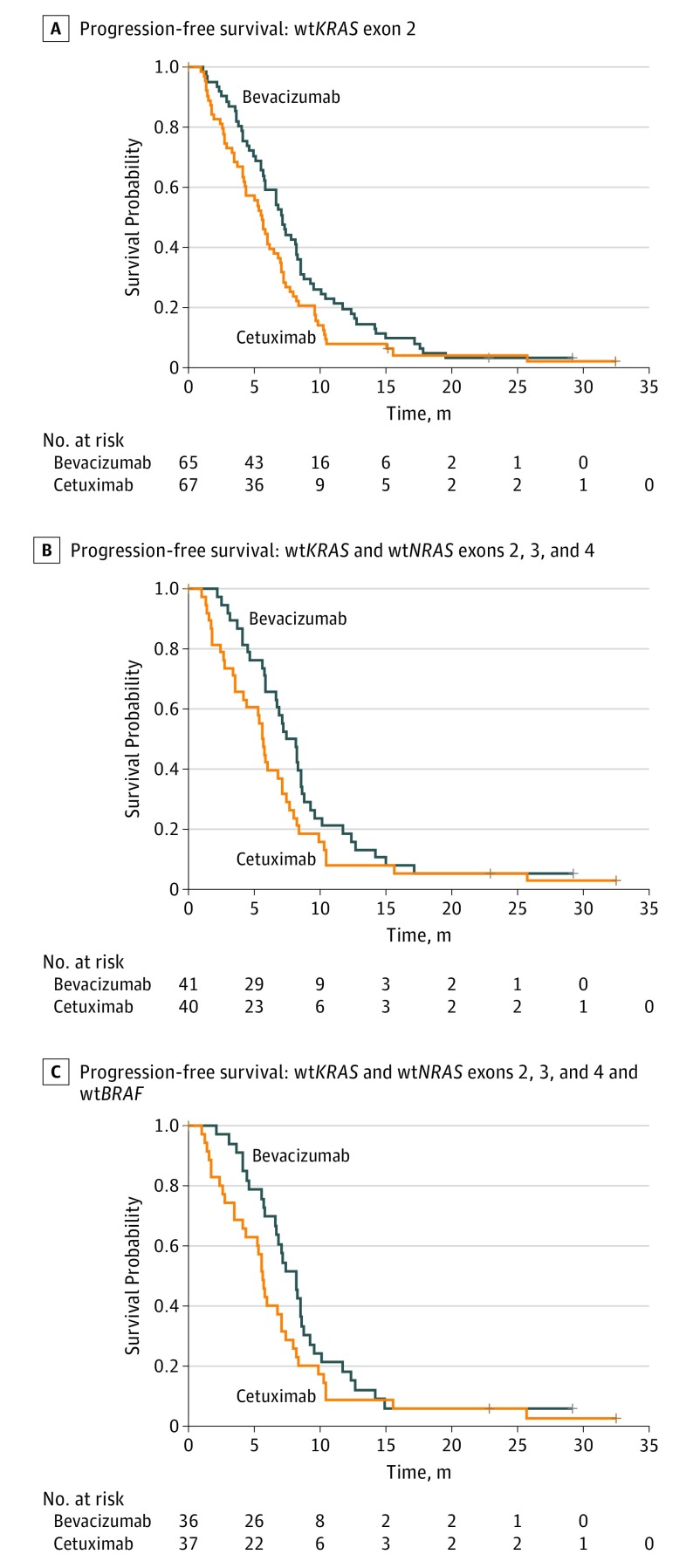
After 4 months of follow-up for each patient, 12 PFS events had occurred in arm A and 21 PFS events had occurred in arm B. The median PFS was 7.1 months (95% CI, 5.7-8.2 months) with bevacizumab plus chemotherapy and 5.6 months (95% CI, 4.2-6.5 months) with cetuximab plus chemotherapy (HR, 0.71; 95% CI, 0.50-1.02; P = .06) (Figure 2A and eTable 2 in Supplement 2).
Figure 2. Kaplan-Meier Estimates of Progression-Free Survival.
A, Progression-free survival among patients with wild-type (wt) KRAS exon 2 (log-rank P = .06). B, Progression-free survival among patients with wtKRAS and wtNRAS exons 2, 3, and 4 (log-rank P = .08). C, Progression-free survival among patients with wtKRAS and wtNRAS exons 2, 3, and 4 and wtBRAF (log-rank P = .10). Plus signs indicate censored patients.
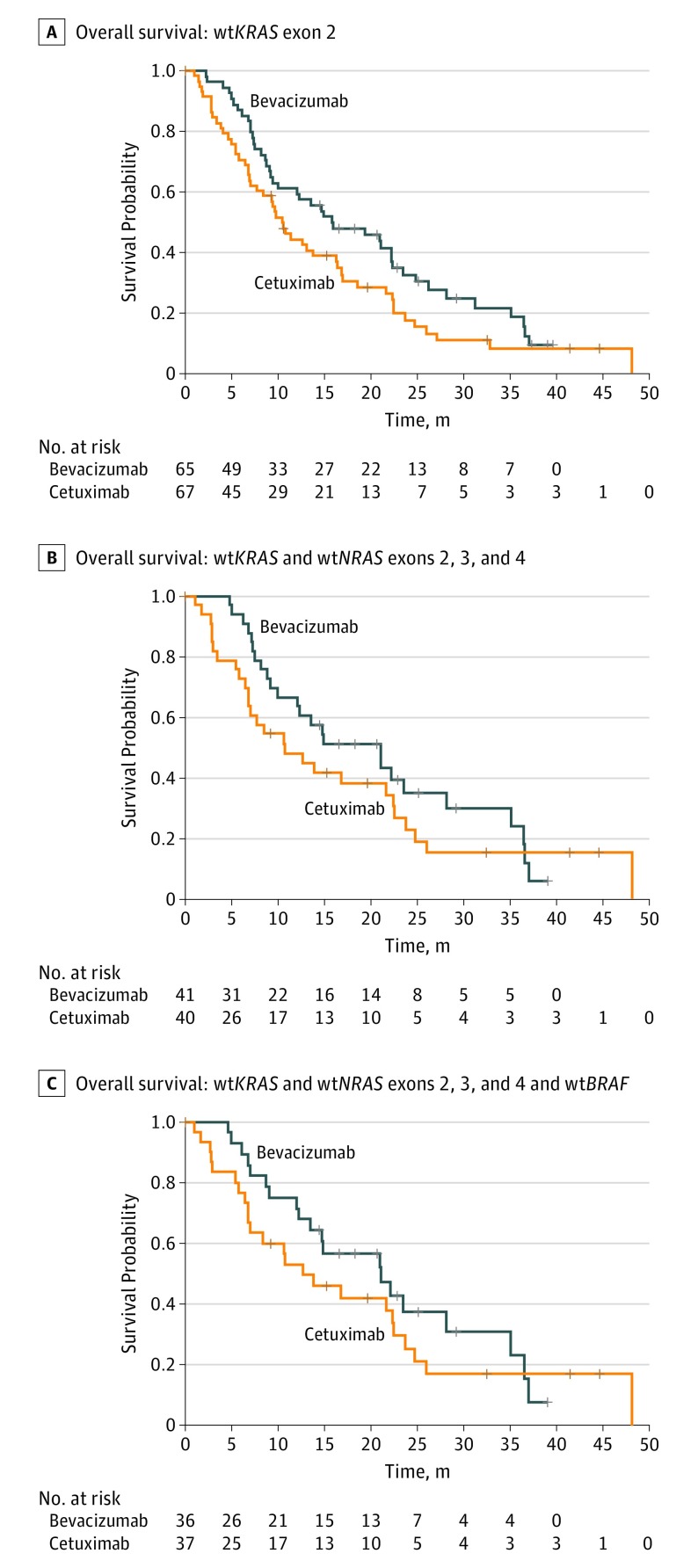
The 4-month PFS rate (primary objective) was 80.3% (95% CI, 68.0%-88.3%) with bevacizumab (arm A) and 66.7% (95% CI, 53.6%-76.8%) with cetuximab (arm B) (eTable 2 in Supplement 2). At the end of the study, 93 of the 132 patients in the mITT population had died (43 in arm A and 50 in arm B). The median duration of OS was 15.8 months (95% CI, 9.5-22.3 months) in the bevacizumab arm and 10.4 months (95% CI, 7.0-16.2 months) in the cetuximab arm (HR, 0.69; 95% CI, 0.46-1.04; P = .08) (Figure 3A and eTable 2 in Supplement 2). Conversely, the objective response rate was 24.6% (95% CI, 14.1%-35.1%) in the bevacizumab arm and 31.8% (95% CI, 20.3%-43.2%) in the cetuximab arm.
Figure 3. Kaplan-Meier Estimates of Overall Survival.
A, Overall survival among patients with wild-type (wt) KRAS exon 2 (log-rank P = .07). B, Overall survival among patients with wtKRAS and wtNRAS exons 2, 3, and 4 (log-rank P = .32). C, Overall survival among patients with wtKRAS and wtNRAS exons 2, 3, and 4 and wtBRAF (log-rank P = .37). Plus signs indicate censored patients.
In the subgroup with confirmed wtKRAS and wtNRAS status, the median follow-up was 29.2 months (minimum follow-up, 0 months; maximum follow-up, 48 months). The median follow-up in the subgroup with wild-type tumors for all 3 biomarkers was also 29.2 months (minimum follow-up, 0 months; maximum follow-up, 48.1 months). Subgroup analysis of the 81 patients with wtKRAS and wtNRAS tumors found a median PFS of 7.8 months (95% CI, 5.8-8.5 months) in arm A and 5.6 months (95% CI, 3.5-7.1 months) in arm B; the median OS was 21.0 months (95% CI, 10.0-28.2 months) in arm A and 10.7 months (95% CI, 6.8-22.4 months) in arm B. Subgroup analysis of the 73 patients with wtBRAF tumors in addition to wtKRAS and wtNRAS found a median PFS of 8.2 months (95% CI, 6.6-8.6 months) in arm A and 5.7 months (95% CI, 4.1-7.1 months) in arm B; the median OS was 21.1 months (95% CI, 12.3-35.1 months) in arm A and 12.6 months (95% CI, 6.8-22.5 months) in arm B. eTable 2 in Supplement 2 shows the efficacy outcomes in these subgroups, which confirm the results obtained in the mITT population (Figure 2 and Figure 3).
After completion of study treatment, 49 of the 65 patients (75.4%) who had received bevacizumab plus chemotherapy went on to receive a third-line systemic therapy. Thirty-one of these patients (63.3%) received an anti-EGFR antibody, with or without chemotherapy, in the third line. In the cetuximab plus chemotherapy arm, 43 of 67 patients (64.2%) received a third-line treatment.
In the mITT population, after a median follow-up of 54.7 months (minimum follow-up, 3.2 months; maximum follow-up, 90.8 months) from the start of first-line therapy (retrospectively documented), OS was 32.7 months (range, 25.4-36.6 months) with bevacizumab plus chemotherapy and 25.5 months (range, 21.8-34.8 months) with cetuximab plus chemotherapy (HR, 0.89; 95% CI, 0.58-1.35; P = .58). Overall survival in the wtKRAS and wtNRAS subgroup of the bevacizumab arm was 36.3 months (95% CI, 24.0-41.0 months) vs 24.8 months (95% CI, 21.0-36.0 months; P = .56) in the same subgroup of the cetuximab arm. Overall survival in the subgroups with wild-type tumors for all 3 biomarkers of the bevacizumab arm was 36.6 months (95% CI, 20.1-45.6 months) and 28.1 months (95% CI, 21.0-36.0 months; P = .71) in the same subgroup of the cetuximab arm.
Tolerability
The median number of treatment cycles was 12 in both arms, with a range of 1 to 38 cycles in the bevacizumab arm and 3 to 36 cycles in the cetuximab arm. However, the median number of chemotherapy cycles was higher in the bevacizumab arm (12 [range, 1-38]) than in the cetuximab arm (9 [range, 1-37]). The median treatment duration was 6.2 months (range, 2.4-20.0 months) in the bevacizumab plus chemotherapy arm and 5.7 months (range, 0.8-20.7 months) in the cetuximab plus chemotherapy arm.
At least 1 AE was reported for all 65 patients (100%) in the bevacizumab plus chemotherapy arm compared with 66 of 67 patients (98.5%) in the cetuximab plus chemotherapy arm. No toxic deaths were reported in either arm. Grade 3 and 4 AEs occurred in 52 of 65 patients (80.0%) in the bevacizumab plus chemotherapy arm and 57 of 67 patients (85.1%) in the cetuximab plus chemotherapy arm (eTable 3 in Supplement 2). The most common grade 3 or 4 AEs in the bevacizumab plus chemotherapy arm were diarrhea (5 [7.7%]), fatigue (7 [10.8%]), and neutropenia (12 [18.5%]). In the cetuximab plus chemotherapy arm, grade 3 or 4 stomatitis (5 [7.5%]), diarrhea (6 [9.0%]), fatigue (7 [10.4%]), anemia (9 [13.4%]), neutropenia (10 [14.9%]), and skin disorders (13 [19.4%]) were most frequently recorded. Febrile neutropenia was reported in 3 patients (4.6%) treated with chemotherapy plus bevacizumab.
In total, chemotherapy was discontinued because of AEs among 3 patients (4.6%) in the bevacizumab arm and for 6 patients (9.0%) in the cetuximab arm. Bevacizumab administration was discontinued owing to AEs for 4 patients (6.2%), and cetuximab administration was discontinued owing to AEs among 17 patients (25.4%).
Discussion
The randomized phase 2 PRODIGE18 clinical trial evaluated standard fluoropyrimidine-based chemotherapy combined with either bevacizumab or cetuximab for patients with wtKRAS exon 2 mCRC who had previously received bevacizumab plus standard chemotherapy as first-line treatment. Continuation of bevacizumab with second-line chemotherapy after PD appeared to confer an advantage for OS and PFS compared with the alternative strategy of switching both the chemotherapy regimen and the targeted therapy. These results were also confirmed for patients with wtKRAS, wtNRAS, and wtBRAF tumors.
In addition to the aforementioned TML study,9 the BEBYP (Bevacizumab Beyond Progression) trial has helped to establish second-line maintenance therapy with bevacizumab after failure of first-line therapy as a standard of care in unresectable mCRC, with significantly longer OS than with the discontinuation of bevacizumab.14 The exploratory analysis of KRAS status in the ML18147 study showed that patients with wtKRAS exon 2 tumors gain a benefit from this multiline strategy, with a PFS of 6.4 months for bevacizumab plus chemotherapy vs 4.5 months for chemotherapy alone (P < .001; HR, 0.61; 95% CI, 0.49-0.77) and an OS of 15.4 months for bevacizumab plus chemotherapy vs 11.1 months for chemotherapy alone (P = .005; HR, 0.69; 95% CI, 0.53-0.90).10
Once the multiline strategy had been established, there was a need to define the optimal therapeutic approach. Trials were performed that compared a switch of only the chemotherapy regimen between treatment lines, while others switched both the chemotherapy regimen and the targeted therapy (bevacizumab or anti-EGFR agents). The randomized phase 2 SPIRITT (Second-Line Panitumumab Irinotecan Treatment Trial) study included 182 patients treated with FOLFIRI plus panitumumab or bevacizumab after PD with oxaliplatin-based chemotherapy plus bevacizumab.15 Although no statistical difference was seen between the 2 arms, the PFS (9.2 vs 7.7 months) and OS (21.4 vs 18.0 months) were numerically higher with bevacizumab vs panitumumab, with similar HRs for these 2 outcomes (1.01 for PFS and 1.06 for OS). An exploratory analysis of data from the FIRE-3 trial examined the effect of subsequent treatment lines after FOLFIRI plus cetuximab or FOLFIRI plus bevacizumab.16 In this analysis, “PFS2nd” was defined as the time from first application of second-line therapy to disease progression or death resulting from any cause. In later treatment lines, 47.1% of patients originally assigned to receive FOLFIRI plus cetuximab subsequently received bevacizumab, and 52.2% of those who originally received FOLFIRI plus bevacizumab were treated with an anti-EGFR antibody (cetuximab or panitumumab). In the wtKRAS exon 2 population, PFS2nd was 6.5 months for patients treated with first-line FOLFIRI plus cetuximab vs 4.7 months for patients treated with first-line FOLFIRI plus bevacizumab (HR, 0.68; 95% CI, 0.54-0.85; P < .001). A similar pattern was seen for OS with second-line treatment (16.3 months with FOLFIRI plus cetuximab vs 13.2 months with FOLFIRI plus bevacizumab; HR, 0.70; 95% CI, 0.55-0.88; P = .002). To corroborate these findings, the randomized phase 3 COMETS trial randomized 110 patients with mCRC who had been treated with first-line FOLFIRI plus bevacizumab to receive second-line irinotecan plus cetuximab or FOLFOX, with crossover as third-line treatment.17 Again, adding cetuximab after bevacizumab did not improve the median PFS or the median OS.
The biological mechanisms that potentially reduced the efficacy of anti-EGFR antibodies when administered after antiangiogenic therapy are not clearly understood. In vitro studies using wtRAS mCRC tumor cells emphasized that an anti–vascular endothelial growth factor may activate the RAS pathway, promoting resistance to anti-EGFR antibodies.18 Moreover, overexpression of the vascular endothelial growth factor A level induced by bevacizumab was involved in the acquired resistance to anti-EGFR antibodies.19,20
Limitations
The results must be interpreted with caution owing to the small number of patients included and the phase 2 study design. Consequently, no statistically significant difference was observed between the 2 arms of this study for efficacy end points.
Conclusions
Our phase 2 randomized clinical trial is in line with the study discussed above.17 The randomized phase 2 PRODIGE 18 clinical trial suggests that cetuximab exhibits only modest activity as second-line treatment after PD with first-line bevacizumab. Overall data obtained from the subgroup analysis of the FIRE-3 trial,16 the SPIRITT,15 and COMETS17 trial reinforce the opportunities of the multiline strategy in wtRAS mCRC. According to the FIRE-3 trial, anti-EGFR antibody plus chemotherapy could be the first choice of treatment followed at progression with bevacizumab plus a chemotherapy switch.8 If bevacizumab is used during first-line treatment in this patient population, there is now a growing body of evidence to recommend that anti-EGFR antibodies (panitumumab or cetuximab) be used in third-line treatment after bevacizumab beyond the first progression of disease.
Trial Protocol
eTable 1. Study Inclusion and Exclusion Criteria
eTable 2. Overall Tumor Response and Efficacy According to RAS and BRAF Status
eTable 3. Overview of Main Adverse Events
References
- 1.National Comprehensive Cancer Network Clinical practice guidelines in oncology (NCCN guidelines): colon cancer: version 2.2017. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed November 6, 2017.
- 2.Giantonio BJ, Catalano PJ, Meropol NJ, et al. ; Eastern Cooperative Oncology Group Study E3200 . Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539-1544. doi: 10.1200/JCO.2006.09.6305 [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30(28):3499-3506. doi: 10.1200/JCO.2012.42.8201 [DOI] [PubMed] [Google Scholar]
- 4.Tabernero J, Yoshino T, Cohn AL, et al. ; RAISE Study Investigators . Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16(5):499-508. doi: 10.1016/S1470-2045(15)70127-0 [DOI] [PubMed] [Google Scholar]
- 5.Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(14):2311-2319. doi: 10.1200/JCO.2007.13.1193 [DOI] [PubMed] [Google Scholar]
- 6.Peeters M, Oliner KS, Price TJ, et al. Analysis of KRAS/NRAS mutations in a phase III study of panitumumab with FOLFIRI compared with FOLFIRI alone as second-line treatment for metastatic colorectal cancer. Clin Cancer Res. 2015;21(24):5469-5479. doi: 10.1158/1078-0432.CCR-15-0526 [DOI] [PubMed] [Google Scholar]
- 7.Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA. 2017;317(23):2392-2401. doi: 10.1001/jama.2017.7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065-1075. doi: 10.1016/S1470-2045(14)70330-4 [DOI] [PubMed] [Google Scholar]
- 9.Bennouna J, Sastre J, Arnold D, et al. ; ML18147 Study Investigators . Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14(1):29-37. doi: 10.1016/S1470-2045(12)70477-1 [DOI] [PubMed] [Google Scholar]
- 10.Kubicka S, Greil R, André T, et al. ; ML18147 study investigators including AIO, GERCOR, FFCD, UNICANCER GI, TTD, BGDO, GEMCAD, and AGMT groups . Bevacizumab plus chemotherapy continued beyond first progression in patients with metastatic colorectal cancer previously treated with bevacizumab plus chemotherapy: ML18147 study KRAS subgroup findings. Ann Oncol. 2013;24(9):2342-2349. doi: 10.1093/annonc/mdt231 [DOI] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 12.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 13.National Cancer Institute, National Institutes of Health, US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Published August 9, 2006. Accessed September 19, 2018.
- 14.Masi G, Salvatore L, Boni L, et al. ; BEBYP Study Investigators . Continuation or reintroduction of bevacizumab beyond progression to first-line therapy in metastatic colorectal cancer: final results of the randomized BEBYP trial. Ann Oncol. 2015;26(4):724-730. doi: 10.1093/annonc/mdv012 [DOI] [PubMed] [Google Scholar]
- 15.Hecht JR, Cohn A, Dakhil S, et al. SPIRITT: a randomized, multicenter, phase II study of panitumumab with FOLFIRI and bevacizumab with FOLFIRI as second-line treatment in patients with unresectable wild type KRAS metastatic colorectal cancer. Clin Colorectal Cancer. 2015;14(2):72-80. doi: 10.1016/j.clcc.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 16.Modest DP, Stintzing S, von Weikersthal LF, et al. Impact of subsequent therapies on outcome of the FIRE-3/AIO KRK0306 trial: first-line therapy with FOLFIRI plus cetuximab or bevacizumab in patients with KRAS wild-type tumors in metastatic colorectal cancer. J Clin Oncol. 2015;33(32):3718-3726. doi: 10.1200/JCO.2015.61.2887 [DOI] [PubMed] [Google Scholar]
- 17.Cascinu S, Rosati G, Nasti G, et al. ; GISCAD investigators . Treatment sequence with either irinotecan/cetuximab followed by FOLFOX-4 or the reverse strategy in metastatic colorectal cancer patients progressing after first-line FOLFIRI/bevacizumab: an Italian Group for the Study of Gastrointestinal Cancer phase III, randomised trial comparing two sequences of therapy in colorectal metastatic patients. Eur J Cancer. 2017;83:106-115. doi: 10.1016/j.ejca.2017.06.029 [DOI] [PubMed] [Google Scholar]
- 18.Zaniboni A, Formica V. The best: first: anti-EGFR before anti-VEGF, in the first-line treatment of RAS wild-type metastatic colorectal cancer: from bench to bedside. Cancer Chemother Pharmacol. 2016;78(2):233-244. doi: 10.1007/s00280-016-3032-8 [DOI] [PubMed] [Google Scholar]
- 19.Viloria-Petit A, Crombet T, Jothy S, et al. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesis. Cancer Res. 2001;61(13):5090-5101. [PubMed] [Google Scholar]
- 20.Viloria-Petit AM, Kerbel RS. Acquired resistance to EGFR inhibitors: mechanisms and prevention strategies. Int J Radiat Oncol Biol Phys. 2004;58(3):914-926. doi: 10.1016/j.ijrobp.2003.09.091 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Study Inclusion and Exclusion Criteria
eTable 2. Overall Tumor Response and Efficacy According to RAS and BRAF Status
eTable 3. Overview of Main Adverse Events