Key Points
Question
What is the association between long-term ambient black carbon exposure and intraocular pressure?
Findings
In this analysis of 911 follow-up visits by 419 older men enrolled in a longitudinal cohort study, the association of long-term black carbon exposure with intraocular pressure was greater in individuals with a high oxidative stress allelic score when compared with individuals with a low score.
Meaning
Ambient black carbon exposure may be a risk factor for increased intraocular pressure, particularly in individuals susceptible to other biological oxidative stressors.
Abstract
Importance
Elevated intraocular pressure is a major risk factor for glaucoma, a leading cause of irreversible blindness worldwide. Environmental air pollution has been suggested as a potential contributor to elevated intraocular pressure; however, no studies have demonstrated such an association to date.
Objective
To investigate the association of long-term ambient black carbon exposure with intraocular pressure in community-dwelling older adults.
Design, Setting, and Participants
This population-based analysis, conducted from October 18, 2017, through March 22, 2018, used data from the all-male, New England–based Normative Aging Study of the US Department of Veterans Affairs. The analysis included 419 older men with a total of 911 follow-up study visits between January 1, 2000, and December 30, 2011. Intraocular pressure was measured by Goldmann applanation tonometry during the study visits. Validated spatiotemporal models were used to generate 1-year black carbon exposure levels at the addresses of the participants.
Main Outcomes and Measures
An independently developed genetic score approach was used to calculate allelic risk scores for 3 pathways associated with black carbon toxicity: endothelial function, oxidative stress, and metal processing. The associations among black carbon exposure, allelic risk scores, and intraocular pressure were explored using linear mixed-effects models.
Results
All 419 participants were men with a mean (SD) age of 75.3 (6.9) years. The mean (SD) 1-year black carbon exposure was 0.51 (0.18) μg/m3, and the mean (SD) intraocular pressure for the left eye was 14.1 (2.8) mm Hg and for the right eye was 14.1 (3.0) mm Hg. Of the 911 visits, 520 (57.1%) had a high endothelial function allelic risk score, 644 (70.7%) had a high metal-processing allelic risk score, and 623 (68.4%) had a high oxidative stress allelic risk score. In fully adjusted linear mixed-effects models, the association of black carbon with intraocular pressure was greater in individuals with a high oxidative stress allelic score (β = 0.36; 95% CI, 0.003-0.73) compared with individuals with a low score (β = −0.35; 95% CI, −0.86 to 0.15).
Conclusions and Relevance
Ambient black carbon exposure may be a risk factor for increased intraocular pressure in individuals susceptible to other biological oxidative stressors. If additional studies confirm these results, monitoring ambient black carbon exposure and physiological oxidative stress may prevent the development and progression of intraocular pressure–related disease.
This study examines follow-up visit data from the all-male Normative Aging Study to assess the association of environmental exposures with changes in intraocular pressure among older men.
Introduction
Black carbon (BC), a byproduct of combustion processes, is among the most pervasive ambient particles in the world.1 Exposure to BC has a well-documented association with numerous adverse health outcomes, but the association of BC with increased systemic blood pressure (BP) is among the most extensively studied.2,3,4 Existing studies both replicate the BC-BP association and provide evidence suggesting that biological processes, such as endothelial function, metal processing, and oxidative stress, may explain elements of the association.5,6,7,8
Intraocular pressure (IOP) is a major modifiable risk factor for ocular diseases, including glaucoma, a leading cause of blindness in the world.9,10 Evidence suggests that elevated systemic BP and elevated IOP share some physiologic mechanisms.11,12,13,14,15 To our knowledge, studies of the association of ambient BC with IOP in a population-based sample have not yet been performed. Understanding this association might provide another reason for reducing the contribution of BC to environmental pollution, especially if subsequent studies add adequate strength to such an association.16,17
We hypothesized that BC exposure is associated with increased IOP and that biological processes involved in the BC-BP association are also implicated in the BC-IOP association. The latter hypothesis was evaluated by testing if genetic variants in 3 biological pathways implicated in ambient particle–related disease (endothelial function, metal processing, and oxidative stress) could modify the association of BC with IOP.5 The present study was conducted from October 18, 2017, to March 22, 2018. We tested these hypotheses by using longitudinal data from the all-male cohort of the US Department of Veterans Affairs (VA) Normative Aging Study (NAS). Since its establishment in 1963, the NAS has collected a multitude of health-related variables throughout participants’ lifetimes with the aim of conducting analyses to better understand the aging process.18 The present study is in line with that NAS aim.
Methods
Study Population
The NAS is an ongoing longitudinal study of aging in male volunteers. In 1963, it began to recruit male participants from the Greater Boston area who were free of any chronic disease.18 Although now a closed cohort, the NAS participants return for follow-up study visits every 3 to 5 years since recruitment. These on-site, follow-up visits involve comprehensive physical examinations and detailed collection of data on lifestyle factors that may affect health, including physical activity, diet, smoking habits, alcohol intake, and medications. In this cohort, dropout has been less than 1% per year and predominantly occurs when participants move out of the study area. In the present study, the sample (419 men with 911 study visits) consisted of NAS participants who had all available exposure, outcome, sociodemographic, genetic, and clinical data. Of these 419 participants, 124 (29.6%) had 1 study visit, 135 (32.2%) had 2 study visits, 123 (29.4%) had 3 study visits, and 37 (8.8%) had 4 study visits between January 1, 2000, and December 30, 2011. At recruitment, all NAS participants provided written informed consent to the VA Institutional Review Board and were at least aged 18 years; this initial consent covered subsequent studies such as ours. For this current study, human participant approval was granted by the VA and Harvard T. H. Chan School of Public Health Institutional Review Boards.
Intraocular Pressure Assessment
In the morning of each study visit, predilated IOP of right and left eyes was measured by an ophthalmologist using Goldmann applanation tonometry (the current criterion standard).19 Measurements could range from 0 to 30 mm Hg, but our study sample had a range of 5 to 28 mm Hg. For each participant, we calculated the IOP between both eyes to obtain a mean IOP.
BC Exposure Assessment
We focused on long-term (1-year) BC exposure because it has previously been associated with BP and has consistently been associated with other aging-related diseases.20,21,22,23 We began by using a validated spatiotemporal land-use regression model to generate daily BC exposure estimates (in micrograms per cubic meter) at the 1 × 1-km area resolution on the basis of participants’ residences.24 The spatiotemporal model was based on information from daily BC concentrations at a central monitor, daily BC concentrations at 83 monitoring sites throughout the Greater Boston area, land use (eg, traffic density), meteorological conditions (eg, wind speed), and other descriptors (eg, day of the week). In the training data set, the prediction model had a high multivariate coefficient of determination (R2) of 0.83, and the mean correlation between estimated values and observed BC levels in 4 out-of-sample validation samples was moderate (R2 = 0.59). Mean daily exposures for the 365 days prior to the day of each participant’s NAS visit were calculated to obtain the 1-year exposure estimate.
Allelic Risk Scores
We used genotyping data from the NAS data set and a novel allelic risk score method that was independently developed by Bind et al5 to investigate interactions between environmental exposures and the biological pathways of endothelial function, metal processing, and oxidative stress. Bind et al5 developed the scores using the known biological functionality of genetic polymorphisms25 and independent outcomes representative of each pathway (augmentation index for endothelial function, patella lead concentration for metal processing, and 8-hydroxy-2′-deoxyguanosine for oxidative stress). Each score represents a pathway-specific allelic risk profile, and scores are dichotomized as high or low allelic risk according to score distributions in the study sample of interest (ie, high: ≥median; low: <median). Further details on the allelic scores can be found in the original publication.5
Statistical Analysis
We used linear mixed-effects models with data from all patient visits to determine the association of 1-year BC levels with mean IOP. All covariates were determined a priori on the basis of existing literature regarding BC and IOP.3,26,27,28 Next, we used a tiered framework to build the final model. Tier 1 adjusted for chronological age (continuous) and year of visit (continuous). Tier 2 made additional adjustments for mean 1-year temperature (continuous address-specific satellite measurements29), cumulative cigarette pack-years (continuous), smoking status (current, former, or never), season of visit (spring [March-May], summer [June-August], fall [September-November], and winter [December-February]), body mass index (calculated as weight in kilograms divided by height in meters squared; lean [<25], overweight [25-30], or obese [>30]), alcohol intake (yes or no; ≥2 drinks daily), systolic BP (continuous), diastolic BP (continuous), fasting blood glucose level (continuous), C-reactive protein level (continuous), and total serum cholesterol level (continuous). Tier 3 paralleled tier 2 but replaced fasting blood glucose level, systolic and diastolic BP, and cholesterol level with the dichotomized disease variables of diabetes, hypertension, and ischemic heart disease. To examine the robustness of tier 3, we created a fourth tier of covariates. Tier 4 used the tier 3 covariates but made an additional adjustment for glaucoma defined as follows:
either eye’s cup-disc ratio of 0.7 or higher;
asymmetric cup-disc ratio (the difference of 2 eyes’ cup-disc ratio ≥0.2);
any eye’s cup-disc ratio of 0.6 or higher, with either disc hemorrhage or visual field defect; or
vision loss resulting from nerve fiber layer loss.
Tier 3 resulted in the best model fit (lowest Akaike information criterion) and was used as the final fully adjusted model.
For descriptive purposes, we ran a preliminary model of BC and the 3 allelic risk scores as joint predictors of mean IOP using the fully adjusted framework. We tested whether any of the allelic risk scores substantially modified the association of BC with mean IOP. False discovery rate correction was conducted with the Benjamini-Hochberg procedure.30 To further examine the robustness of our effect modification findings, we ran sensitivity analyses using the tier 4 covariates without alterations and then with the glaucoma and hypertension disease status replaced with glaucoma eyedrops and any antihypertensive medication.
All statistical analyses were performed using R, version 3.4.1 (R Core Team). Linear mixed-effects models were run using the lme function of the nlme R package31 and included a random participant-specific intercept to account for the correlation between repeated outcome measures (ie, multiple visits for a participant). The lme function uses a t distribution/statistic to calculate a 2-sided P value. P < .05 was considered to be statistically significant.
Results
Descriptive Statistics
The demographic and clinical characteristics of participants across all study visits are presented in Table 1. All 419 participants were men, with a mean (SD) age of 75.3 (6.9) years. The mean (SD; interquartile range [IQR]) 1-year BC exposure was 0.51 (0.18; 0.22) μg/m3, and the mean (SD) IOP for the left eye was 14.1 (2.8) mm Hg and for the right eye was 14.1 (3.0) mm Hg. Most participants had completed at least 12 years of formal education (as indicated at 674 visits [74.0%]), were overweight or obese (711 visits [78.0%]), and were former smokers (595 visits [65.3%]). In this study sample, the prevalence of diabetes was 183 participant visits (20.1%); glaucoma, 86 (9.4%); hypertension; 700 (76.8%), and ischemic heart disease, 297 (32.6%).
Table 1. Characteristics of Study Participants .
| Variable | All Study Visits (N = 911) |
|---|---|
| Main Variables | |
| Age, mean (SD), y | 75.3 (6.94) |
| 1-y BC exposure, mean (SD), μg/m3 | 0.51 (0.18) |
| IOP in left eye/right eye, mean (SD), mm Hg | 14.1 (2.81)/14.1 (2.98) |
| Pathway-specific allelic risk scores, No. (%) | |
| Endothelial function, low/high | 391 (42.9)/520 (57.1) |
| Metal processing, low/high | 267 (29.3)/644 (70.7) |
| Oxidative stress, low/high | 288 (31.6)/623 (68.4) |
| Lifestyle and Environmental Variables | |
| Pack-years, mean (SD) | 19.8 (23.6) |
| Temperature, mean (SD), °C | 11.5 (0.98) |
| Alcohol consumption, No. (%) | |
| <2 Drinks/d | 727 (79.8) |
| ≥2 Drinks/d | 184 (20.2) |
| BMI, No. (%) | |
| Healthy/lean | 200 (21.9) |
| Overweight | 484 (53.1) |
| Obese | 227 (24.9) |
| Years of education completed, No. (%) | |
| ≤12 | 237 (26.0) |
| 12-16 | 460 (50.5) |
| >16 | 214 (23.5) |
| Smoking status, No. (%) | |
| Current | 31 (3.4) |
| Former | 595 (65.3) |
| Never | 285 (31.3) |
| Season, No. (%) | |
| Spring | 206 (22.6) |
| Summer | 221 (24.3) |
| Fall | 300 (32.9) |
| Winter | 184 (20.2) |
| Diseases and serum measures, mean (SD) | |
| Cholesterol, mg/dL | 184 (38.6) |
| C-reactive protein, mg/L | 3.37 (8.85) |
| Fasting blood glucose, mg/dL | 107 (24.2) |
| Blood pressure, mean (SD), mm Hg | |
| Diastolic | 71.1 (10.3) |
| Systolic | 128 (17.9) |
| Ischemic heart disease, yes/no, No. (%) | 297 (32.6)/614 (67.4) |
| Diabetes, yes/no, No. (%) | 183 (20.1)/728 (79.9) |
| Glaucoma, yes/no, No. (%) | 86 (9.4)/825 (90.6) |
| Hypertension, yes/no, No. (%) | 700 (76.8)/211 (23.2) |
Abbreviations: BC, black carbon; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IOP, intraocular pressure.
SI conversion factors: To convert blood glucose level to millimoles per liter, multiply by 0.0555; cholesterol level to millimoles per liter, multiply by 0.0259; C-reactive protein level to nanomoles per liter, multiply by 9.524.
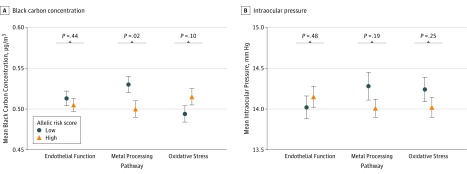
Among the 911 visits, 520 (57.1%) showed evidence that the participant had a high endothelial function allelic risk score; 644 (70.7%), a high metal-processing allelic risk score; and 623 (68.4%), a high oxidative stress allelic risk score. The pathway-specific genetic variants used to generate the allelic risk scores are presented in Table 2. Participants with a low endothelial function or a low oxidative stress allelic risk score did not differ in mean 1-year BC exposures from their counterparts with high allelic risk scores (Figure 1A). Participants with a low metal-processing allelic risk score had a higher mean 1-year BC exposure (mean [SE], 0.53 [0.01] μg/m3; P = .02) when compared with individuals with a high metal-processing allelic risk score (mean [SE], 0.50 [0.007] μg/m3). No differences in mean IOP were found between individuals with low and those with high allelic risk scores across the 3 pathways (Figure 1B).
Table 2. Pathway-Specific Genetic Polymorphisms for Allelic Risk Scores.
| Allelic Variant by Pathway | Gene | Chromosome | Variation | Type | Direction of Model Coefficient |
|---|---|---|---|---|---|
| Endothelial function pathway | |||||
| rs12944039 | NOS2A | 17 | A/G | Intron | + |
| rs2072324 | NOS2A | 17 | A/C | Intron | + |
| rs2255929 | NOS2A | 17 | A/T | Intron | + |
| rs1137933 | NOS2A | 17 | C/T | Coding sequence nonsynonymous | − |
| Metal-processing pathway | |||||
| rs224572 | SLC11A2 | 12 | A/G | Intron | − |
| rs422982 | SLC11A2 | 12 | A/T | Intron | + |
| rs12227734 | SLC11A2 | 12 | A/G | Intron | − |
| rs1005559 | SLC11A2 | 12 | A/T | Intron | − |
| rs1799945 | HFE | 6 | C/G | Exon | − |
| Oxidative stress pathway | |||||
| rs1001179 | CAT | 11 | A/G | Promoter | + |
| rs480575 | CAT | 11 | C/T | Intron | − |
| rs2071746 | HMOX1 | 22 | A/T | Promoter | + |
| rs5995098 | HMOX1 | 22 | C/G | Intron | + |
| rs1800566 | NQO1 | 16 | C/T | Coding sequence nonsynonymous | + |
| rs2282679 | GC | 4 | A/C | Intron | − |
| rs3170633 | GCLM | 1 | A/G | 3′ End | − |
Abbreviations: +, positive; −, negative.
Figure 1. Black Carbon Levels and Intraocular Pressure Across Allelic Risk Scores.
Mean 1-year black carbon concentration (A) and mean intraocular pressure (B) across allelic risk scores for endothelial function, metal processing, and oxidative stress pathways. Error bars show SEs.
Direct Associations
Table 3 presents the results of the tiered framework we used to examine the direct associations of 1-year BC exposure with mean IOP. We did not observe a direct association of IQR increases in 1-year BC exposure with mean IOP in the tier 1 model, which was adjusted for age and year of visit (β = 0.07; 95% CI, −0.23 to 0.37; P = .64). In addition, we did not observe an association after adjusting for important covariates and confounders in the tier 2 model (β = 0.13; 95% CI, −0.18 to 0.45; P = .40), the final fully adjusted tier 3 model (β = 0.14; 95% CI, −0.18 to 0.45; P = .40), or the tier 4 model (β = 0.13; 95% CI, −0.19 to 0.44; P = .42). Again, tier 3 was selected as the final fully adjusted framework because it reflected the best model fit (ie, lowest Akaike information criterion, including important lifestyle and disease covariates). No association with BC was observed when we modeled BC exposure and the 3 allelic risk scores as joint predictors of mean IOP. Moreover, none of the 3 allelic risk scores had direct associations with mean IOP.
Table 3. One-Year Black Carbon Exposure and Allelic Risk Scores .
| Framework | Difference in IOP (95% CI) | P Value | AIC |
|---|---|---|---|
| Independent Model | |||
| Tier 1a | 0.07 (−0.23 to 0.37) | .64 | 4277 |
| Tier 2b | 0.13 (−0.18 to 0.45) | .40 | 4345 |
| Tier 3c | 0.14 (−0.18 to 0.45) | .40 | 4319 |
| Tier 4d | 0.13 (−0.19 to 0.44) | .42 | 4320 |
| Joint Modelc | 4326 | ||
| Black carbon (IQR) | 0.13 (−0.19 to 0.44) | .44 | NA |
| Endothelial function pathway allelic risk score | NA | ||
| Low | 1 [Reference] | .48 | NA |
| High | 0.18 (−0.33 to 0.69) | NA | |
| Metal-processing pathway allelic risk score | NA | ||
| Low | 1 [Reference] | .16 | NA |
| High | −0.39 (−0.94 to 0.15) | NA | |
| Oxidative stress pathway allelic risk score | NA | ||
| Low | 1 [Reference] | .62 | NA |
| High | −0.13 (−0.66 to 0.40) | NA | |
Abbreviations: AIC, Akaike information criterion; BMI, body mass index; CRP, C-reactive protein; IOP, intraocular pressure; IQR, interquartile range; NA, not applicable.
Tier 1 adjusted for chronological age and year of study visit.
Tier 2 adjusted for chronological age, year of study visit, temperature, pack-years, smoking status, season of visit, BMI, alcohol consumption, educational level, CRP C-reactive protein level, systolic blood pressure, diastolic blood pressure, fasting blood glucose level, and cholesterol level.
Tier 3 adjusted for chronological age, year of study visit, temperature, pack-years, smoking status, season of visit, BMI, alcohol consumption, educational level, CRP level, hypertension, diabetes, and ischemic heart disease.
Tier 4 adjusted for tier 3 covariates and glaucoma.
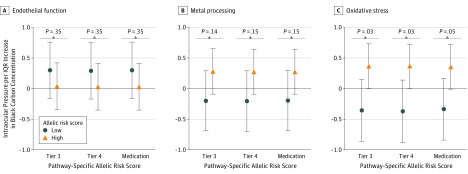
Effect Modification
Figure 2 depicts the modifying association of the 1-year BC exposure with mean IOP. Of all of the allelic risk scores for each of the 3 pathways (endothelial function, metal processing, and oxidative stress), only the oxidative stress allelic score changed the association of BC with IOP (false discovery rate–corrected P = .03 for interaction). Specifically, the association was greater in individuals who had high oxidative stress allelic risk scores (β = 0.36; 95% CI, 0.003-0.73) when compared with individuals who had low oxidative stress allelic risk scores (β = −0.35; 95% CI, −0.86 to 0.15). Our results remained unchanged in the sensitivity analysis using the tier 4 model (ie, glaucoma added as a variable in the fully adjusted tier 3 model) (Figure 2). In the sensitivity analysis, in which the variables for glaucoma eyedrops and antihypertensive medications were used instead of glaucoma and hypertension disease status, again high oxidative stress allelic risk scores (β = 0.36; 95% CI, –0.008 to 0.72) were compared with low oxidative stress allelic risk scores (β = −0.34; 95% CI, –0.84 to 0.17).
Figure 2. Effect Modification of the Black Carbon and Intraocular Pressure Association by Allelic Risk Scores.
Difference in mean intraocular pressure for a 1–interquartile range (IQR) increase (1 IQR = 0.22 μg/m3) in 1 year of black carbon exposure was compared between participants with low pathway-specific allelic risk scores and those with high scores in 3 model frameworks. Tier 3 indicates the main fully adjusted mixed-effects model; tier 4, sensitivity analysis model using the tier 3 model adjusted for glaucoma disease status; and medication, sensitivity analysis using the tier 3 model adjusted for glaucoma eyedrops and hypertension disease status replaced with antihypertension medication. P values were corrected for false discovery rate.
Discussion
In the present study, we describe the novel association among long-term BC exposure, oxidative stress polymorphisms, and IOP in a cohort of community-dwelling men. These associations persisted even after adjusting for important disease states, such as inflammation, glaucoma, obesity, ischemic heart disease, and diabetes. These results suggest that ambient BC exposure may be a risk factor for elevated IOP in individuals at risk for elevated oxidative stress that is independent of discernable disease diagnoses. To our knowledge, this is the first study to report the association of an ambient air pollutant with intraocular pressure on a population level. As such, this study may point to the potential need to broaden the factors considered when evaluating and managing elevated IOP.
It has long been postulated that environmental air pollution may affect eye health; however, evidence supporting this theory is limited. The present study addresses this research gap. Although we considered genetic polymorphisms from 3 different pathways (endothelial function, metal processing, and oxidative stress), we observed findings only in oxidative stress genetic risk. In addition, the polymorphisms used to generate these genetic scores were selected using the independent outcomes of pathways related to the polymorphisms. In the case of the oxidative stress allelic risk score, this score was developed from polymorphisms associated with serum levels of 8-hydroxy-2′-deoxyguanosine,5 which is one of the most common and quantifiable DNA adducts that reflects cellular oxidative stress.32 Thus, the oxidative stress score, although still a measure of genetic risk, is associated with a tangible, physiologic measure of oxidative stress.
When individuals with high or low oxidative stress allelic risk scores were compared, we detected a moderate difference in mean IOP (0.73 mm Hg) for an IQR increase in 1 year of BC exposure. These results suggest that BC exposure susceptibility may be biologically important. Independent of ambient air pollution, increased oxidative stress or reduced antioxidant capacity has been linked to elevated IOP and glaucoma pathologic findings.33,34 For example, a 2017 study compared blood and aqueous humor samples in 96 patients with glaucoma with samples from 64 age-matched healthy controls. The researchers observed higher levels of protein carbonyls and other oxidative stress–related molecular measures in the blood and more prominently in the aqueous humor from individuals with glaucoma.35 Another study of 531 individuals reported an association between systemic oxidative stress and IOP. The researchers first quantified biological antioxidant potential in the participants’ serum and then observed that individuals with the lowest biological antioxidant potential were more likely to have higher IOP.36 The conclusions reached by these observational studies are further supported by experiments in animal models, which provide a greater mechanistic understanding of the association of oxidative stress with IOP physiologic features.37,38 Additional support for these conclusions can be derived from human studies that suggest that oxidative stress–reducing activities (eg, diet, exercise) may curtail some IOP-related conditions.39,40 Exposure to BC has been identified as a source of localized and systemic oxidative stress8; thus, BC may be acting through some of the same oxidative stress physiologic functions already associated with glaucoma and IOP. Still, further research is necessary to confirm our findings and to better establish this association.
We found a novel gene-environment interaction associated with IOP, which is consistent with findings from other studies investigating gene-environment interactions and smoking. A 2011 study reported interactions between the NOS3 gene rs7830 single-nucleotide polymorphism and smoking, which can be viewed as a personal form of air pollution. Specifically, CC homozygous individuals who were past or current smokers were at a greater risk of developing primary open-angle glaucoma when compared with CC homozygous individuals who were never smokers. However, no association with smoking was found in carriers of the single-nucleotide polymorphism A variant.41 Like the present study, the study by Kang et al41 revealed an association that may have been missed if gene-environment interactions had not been considered. Moreover, it also exposed a more nuanced but important point: Not all environmental exposures may be directly associated with IOP-related eye diseases. Future research into why some exposures are directly, indirectly, or not associated with eye diseases will be useful for better understanding disease pathogenesis and potentially safeguarding vulnerable populations from disease onset.42
Strengths and Limitations
The strengths of the present study include the use of novel genetic pathway tools and a longitudinal cohort as well as the repeated measures of ambient pollutant exposures, IOP, covariates, and potential confounders. However, the study has some limitations. First, we used an allelic risk score approach that does not provide genome-wide resolution of the 3 biological pathways and that allots polymorphisms equal weights when they may have different functional magnitudes.5 Still, the variants we used were developed in an independent study and have been used in other studies since they were first described.43,44 Moreover, the variants are representative of their respective biological pathways. Second, we used a validated spatiotemporal model to estimate 1-year BC levels at the participant home. Given that most participants are retired and spend most of their time at home, these estimates were believed to be good proxies of the participants’ personal exposure. Moreover, any nondifferential misclassification bias from this approach of estimating personal exposures is likely to attenuate statistical associations rather than steer them away from the null.45,46 Last, this study cohort consisted of older white men residing in a lightly polluted environment, the lower bound of the 95% CI for the observed association in the high oxidative stress genetic risk group was close to zero, and the design did not reflect causality. These final points reflect the need for additional studies involving other environments, other demographics, and larger groups to confirm our findings more broadly.
Conclusions
This study highlights the potential contributions of gene-environment interactions to the complex physiologic functions of IOP-related disease. This finding could be particularly important because most of the global burden of eye disease is in the developing world.47 The differences in the global prevalence of eye disease are often attributed to genetics alone or the difficulties with health care access that affect early disease detection, management, and treatment.48 These factors are highly relevant but also note that the developing world often faces a larger burden of environmental pollution.49 Annual BC exposure levels greater than 5 times the levels observed in our study have been reported in India and China.50,51 Of importance, the present associations, although novel, do not prove causality and should not affect policy in isolation. Whether these findings persist in more diverse populations experiencing greater pollution and in study designs that can demonstrate causality will be interesting to see. If these future studies substantiate this association, integrated initiatives (ie, combining environmental improvement, socioeconomic outreach, and targeted pharmaceutical interventions) may prove useful for future policy or public health initiatives aimed at addressing the global burden of eye disease.
References
- 1.Ni M, Huang J, Lu S, Li X, Yan J, Cen K. A review on black carbon emissions, worldwide and in China. Chemosphere. 2014;107:83-93. doi: 10.1016/j.chemosphere.2014.02.052 [DOI] [PubMed] [Google Scholar]
- 2.Zhong J, Cayir A, Trevisi L, et al. Traffic-related air pollution, blood pressure, and adaptive response of mitochondrial abundance. Circulation. 2016;133(4):378-387. doi: 10.1161/CIRCULATIONAHA.115.018802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magalhaes S, Baumgartner J, Weichenthal S. Impacts of exposure to black carbon, elemental carbon, and ultrafine particles from indoor and outdoor sources on blood pressure in adults: a review of epidemiological evidence. Environ Res. 2018;161:345-353. doi: 10.1016/j.envres.2017.11.030 [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner J, Zhang Y, Schauer JJ, Huang W, Wang Y, Ezzati M. Highway proximity and black carbon from cookstoves as a risk factor for higher blood pressure in rural China. Proc Natl Acad Sci U S A. 2014;111(36):13229-13234. doi: 10.1073/pnas.1317176111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bind MA, Coull B, Suh H, et al. A novel genetic score approach using instruments to investigate interactions between pathways and environment: application to air pollution. PLoS One. 2014;9(4):e96000. doi: 10.1371/journal.pone.0096000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Chen Y, Wei H, et al. Ultrafine carbon black attenuates the antihypertensive effect of captopril in spontaneously hypertensive rats. Inhal Toxicol. 2014;26(14):853-860. doi: 10.3109/08958378.2014.965558 [DOI] [PubMed] [Google Scholar]
- 7.Weichenthal S, Hatzopoulou M, Goldberg MS. Exposure to traffic-related air pollution during physical activity and acute changes in blood pressure, autonomic and micro-vascular function in women: a cross-over study. Part Fibre Toxicol. 2014;11:70. doi: 10.1186/s12989-014-0070-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niranjan R, Thakur AK. The toxicological mechanisms of environmental soot (black carbon) and carbon black: focus on oxidative stress and inflammatory pathways. Front Immunol. 2017;8:763. doi: 10.3389/fimmu.2017.00763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262-267. doi: 10.1136/bjo.2005.081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-2090. doi: 10.1016/j.ophtha.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 11.Zhao D, Cho J, Kim MH, Guallar E. The association of blood pressure and primary open-angle glaucoma: a meta-analysis. Am J Ophthalmol. 2014;158(3):615-627.e9. doi: 10.1016/j.ajo.2014.05.029 [DOI] [PubMed] [Google Scholar]
- 12.Ricca AM, Morshedi RG, Wirostko BM. High intraocular pressure following anti-vascular endothelial growth factor therapy: proposed pathophysiology due to altered nitric oxide metabolism. J Ocul Pharmacol Ther. 2015;31(1):2-10. doi: 10.1089/jop.2014.0062 [DOI] [PubMed] [Google Scholar]
- 13.Kanadani FN, Figueiredo CR, Miranda RM, Cunha PL, M Kanadani TC, Dorairaj S. Ocular perfusion pressure and pulsatile ocular blood flow in normal and systemic hypertensive patients. J Curr Glaucoma Pract. 2015;9(1):16-19. doi: 10.5005/jp-journals-10008-1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung HJ, Hwang HB, Lee NY. The association between primary open-angle glaucoma and blood pressure: two aspects of hypertension and hypotension. Biomed Res Int. 2015;2015:827516. doi: 10.1155/2015/827516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein BE, Klein R, Knudtson MD. Intraocular pressure and systemic blood pressure: longitudinal perspective: the Beaver Dam Eye Study. Br J Ophthalmol. 2005;89(3):284-287. doi: 10.1136/bjo.2004.048710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klopfer J. Effects of environmental air pollution on the eye. J Am Optom Assoc. 1989;60(10):773-778. [PubMed] [Google Scholar]
- 17.Fullerton DG, Bruce N, Gordon SB. Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Trans R Soc Trop Med Hyg. 2008;102(9):843-851. doi: 10.1016/j.trstmh.2008.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell B, Rose CL, Damon A. The Veterans Administration longitudinal study of healthy aging. Gerontologist. 1966;6(4):179-184. doi: 10.1093/geront/6.4.179 [DOI] [PubMed] [Google Scholar]
- 19.Okafor KC, Brandt JD. Measuring intraocular pressure. Curr Opin Ophthalmol. 2015;26(2):103-109. doi: 10.1097/ICU.0000000000000129 [DOI] [PubMed] [Google Scholar]
- 20.Colicino E, Giuliano G, Power MC, et al. Long-term exposure to black carbon, cognition and single nucleotide polymorphisms in microRNA processing genes in older men. Environ Int. 2016;88:86-93. doi: 10.1016/j.envint.2015.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colicino E, Power MC, Cox DG, et al. Mitochondrial haplogroups modify the effect of black carbon on age-related cognitive impairment. Environ Health. 2014;13(1):42. doi: 10.1186/1476-069X-13-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nwanaji-Enwerem JC, Colicino E, Trevisi L, et al. Long-term ambient particle exposures and blood DNA methylation age: findings from the VA Normative Aging Study. Environ Epigenet. 2016;2(2):dvw006. doi: 10.1093/eep/dvw006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz J, Alexeeff SE, Mordukhovich I, et al. Association between long-term exposure to traffic particles and blood pressure in the Veterans Administration Normative Aging Study. Occup Environ Med. 2012;69(6):422-427. doi: 10.1136/oemed-2011-100268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gryparis A, Coull BA, Schwartz J, Suh HH. Semiparametric latent variable regression models for spatiotemporal modelling of mobile source particles in the greater Boston area. J R Stat Soc Ser C Appl Stat. 2007;56(2):183-209. doi: 10.1111/j.1467-9876.2007.00573.x [DOI] [Google Scholar]
- 25.Safran M, Dalah I, Alexander J, et al. GeneCards version 3: the human gene integrator. Database (Oxford). 2010;2010:baq020. doi: 10.1093/database/baq020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tham YC, Cheng CY. Associations between chronic systemic diseases and primary open angle glaucoma: an epidemiological perspective. Clin Exp Ophthalmol. 2017;45(1):24-32. doi: 10.1111/ceo.12763 [DOI] [PubMed] [Google Scholar]
- 27.Grahame TJ, Klemm R, Schlesinger RB. Public health and components of particulate matter: the changing assessment of black carbon. J Air Waste Manag Assoc. 2014;64(6):620-660. doi: 10.1080/10962247.2014.912692 [DOI] [PubMed] [Google Scholar]
- 28.Luben TJ, Nichols JL, Dutton SJ, et al. A systematic review of cardiovascular emergency department visits, hospital admissions and mortality associated with ambient black carbon. Environ Int. 2017;107:154-162. doi: 10.1016/j.envint.2017.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nwanaji-Enwerem JC, Dai L, Colicino E, et al. Associations between long-term exposure to PM2.5 component species and blood DNA methylation age in the elderly: the VA Normative Aging Study. Environ Int. 2017;102:57-65. doi: 10.1016/j.envint.2016.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strimmer K. fdrtool: a versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics. 2008;24(12):1461-1462. doi: 10.1093/bioinformatics/btn209 [DOI] [PubMed] [Google Scholar]
- 31.Pinheiro J, Bates D, DebRoy S, et al. ; R Core Team Linear and nonlinear mixed effects models. R package version 3.1-137. https://CRAN.R-project.org/package=nlme. Accessed October 18, 2017.
- 32.Valavanidis A, Vlachogianni T, Fiotakis C. 8-Hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27(2):120-139. doi: 10.1080/10590500902885684 [DOI] [PubMed] [Google Scholar]
- 33.Pinazo-Duran MD, Shoaie-Nia K, Zanon-Moreno V, Sanz-Gonzalez SM, Del Castillo JB, Garcia-Medina JJ. Strategies to reduce oxidative stress in glaucoma patients. Curr Neuropharmacol. 2018;16(7):903-918. doi: 10.2174/1570159X15666170705101910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ung L, Pattamatta U, Carnt N, Wilkinson-Berka JL, Liew G, White AJR. Oxidative stress and reactive oxygen species: a review of their role in ocular disease. Clin Sci (Lond). 2017;131(24):2865-2883. doi: 10.1042/CS20171246 [DOI] [PubMed] [Google Scholar]
- 35.Hondur G, Göktas E, Yang X, et al. Oxidative stress-related molecular biomarker candidates for glaucoma. Invest Ophthalmol Vis Sci. 2017;58(10):4078-4088. doi: 10.1167/iovs.17-22242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanito M, Kaidzu S, Takai Y, Ohira A. Correlation between systemic oxidative stress and intraocular pressure level. PLoS One. 2015;10(7):e0133582. doi: 10.1371/journal.pone.0133582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chidlow G, Wood JPM, Casson RJ. Investigations into hypoxia and oxidative stress at the optic nerve head in a rat model of glaucoma. Front Neurosci. 2017;11:478. doi: 10.3389/fnins.2017.00478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu P, Zhang M, Shoeb M, et al. Metal chelator combined with permeability enhancer ameliorates oxidative stress-associated neurodegeneration in rat eyes with elevated intraocular pressure. Free Radic Biol Med. 2014;69:289-299. doi: 10.1016/j.freeradbiomed.2014.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braakhuis A, Raman R, Vaghefi E. The association between dietary intake of antioxidants and ocular disease. Diseases. 2017;5(1):E3. doi: 10.3390/diseases5010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kruk J, Kubasik-Kladna K, Aboul-Enein HY. The role oxidative stress in the pathogenesis of eye diseases: current status and a dual role of physical activity. Mini Rev Med Chem. 2015;16(3):241-257. doi: 10.2174/1389557516666151120114605 [DOI] [PubMed] [Google Scholar]
- 41.Kang JH, Wiggs JL, Rosner BA, Haines J, Abdrabou W, Pasquale LR. Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: interactions with hypertension, alcohol intake, and cigarette smoking. Arch Ophthalmol. 2011;129(6):773-780. doi: 10.1001/archophthalmol.2011.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee SH, Kang EM, Kim GA, et al. Three toxic heavy metals in open-angle glaucoma with low-teen and high-teen intraocular pressure: a cross-sectional study from South Korea. PLoS One. 2016;11(10):e0164983. doi: 10.1371/journal.pone.0164983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai L, Bind MA, Koutrakis P, et al. Fine particles, genetic pathways, and markers of inflammation and endothelial dysfunction: analysis on particulate species and sources. J Expo Sci Environ Epidemiol. 2016;26(4):415-421. doi: 10.1038/jes.2015.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nwanaji-Enwerem JC, Bind MA, Dai L, et al. Editor’s highlight: modifying role of endothelial function gene variants on the association of long-term PM2.5 exposure with blood DNA methylation age: the VA Normative Aging Study. Toxicol Sci. 2017;158(1):116-126. doi: 10.1093/toxsci/kfx077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kioumourtzoglou MA, Spiegelman D, Szpiro AA, et al. Exposure measurement error in PM2.5 health effects studies: a pooled analysis of eight personal exposure validation studies. Environ Health. 2014;13(1):2. doi: 10.1186/1476-069X-13-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weisskopf MG, Webster TF. Trade-offs of personal versus more proxy exposure measures in environmental epidemiology. Epidemiology. 2017;28(5):635-643. doi: 10.1097/EDE.0000000000000686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kyari F, Abdull MM, Bastawrous A, Gilbert CE, Faal H. Epidemiology of glaucoma in sub-Saharan Africa: prevalence, incidence and risk factors. Middle East Afr J Ophthalmol. 2013;20(2):111-125. doi: 10.4103/0974-9233.110605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bucolo C, Drago F. Carbon monoxide and the eye: implications for glaucoma therapy. Pharmacol Ther. 2011;130(2):191-201. doi: 10.1016/j.pharmthera.2011.01.013 [DOI] [PubMed] [Google Scholar]
- 49.Landrigan PJ, Fuller R, Acosta NJR, et al. The Lancet Commission on pollution and health. Lancet. 2018;391(10119):462-512. doi: 10.1016/S0140-6736(17)32345-0 [DOI] [PubMed] [Google Scholar]
- 50.Sanchez M, Ambros A, Milà C, et al. Development of land-use regression models for fine particles and black carbon in peri-urban South India. Sci Total Environ. 2018;634:77-86. doi: 10.1016/j.scitotenv.2018.03.308 [DOI] [PubMed] [Google Scholar]
- 51.Ji D, Li L, Pang B, et al. Characterization of black carbon in an urban-rural fringe area of Beijing. Environ Pollut. 2017;223:524-534. doi: 10.1016/j.envpol.2017.01.055 [DOI] [PubMed] [Google Scholar]